Chapter 14
A Synopsis of Cranial Nerves of the Brainstem
Sensory Cell Columns and Nuclei
Cranial Nerves of the Medulla Oblongata
Syndromes of the Jugular Foramen
Cranial Nerves of the Pons-Medulla Junction
Lesions in the Cerebellopontine Angle
Although the brainstem is small, comprising only about 2.6% of total brain weight, the size of this structure belies its importance. First, all ascending and descending tracts linking the spinal cord and the forebrain traverse the brainstem. Second, there are important ascending fibers (e.g., spinoreticular, spinoperiaqueductal gray) and descending fibers (e.g., rubrospinal, vestibulospinal, reticulospinal) that interconnect the brainstem with the spinal cord. These tracts are essential to the successful function of the nervous system. Third, the nuclei and the exit and entrance points of 10 of the 12 cranial nerves are associated with the brainstem.
Lesions of the brainstem, regardless of their origin (vascular, tumor, trauma), frequently involve cranial nerves. Indeed, in patients with brainstem lesions who have long tract signs, the accompanying cranial nerve deficits usually represent excellent localizing signs.
OVERVIEW
In general, the exit of a cranial nerve from the brainstem (“exit” is used here with reference to both efferent and afferent fibers of the nerve) is associated with the same brainstem area in which the nuclei of that nerve are found. The obvious exception is the trigeminal nerve, the sensory nuclei of which form a continuous cell column from rostral regions of the midbrain to the spinal cord–medulla interface.
This chapter reviews cranial nerves of the brainstem from caudal (hypoglossal) to rostral (oculomotor) and presents a number of clinical examples. The goal here is not simply to review the information covered in the last three chapters but to consider the cranial nerves in a somewhat broader perspective. Structure, function, and dysfunction are described in an integrated manner because this is how cranial nerves are evaluated in the clinical setting.
MOTOR CELL COLUMNS AND NUCLEI
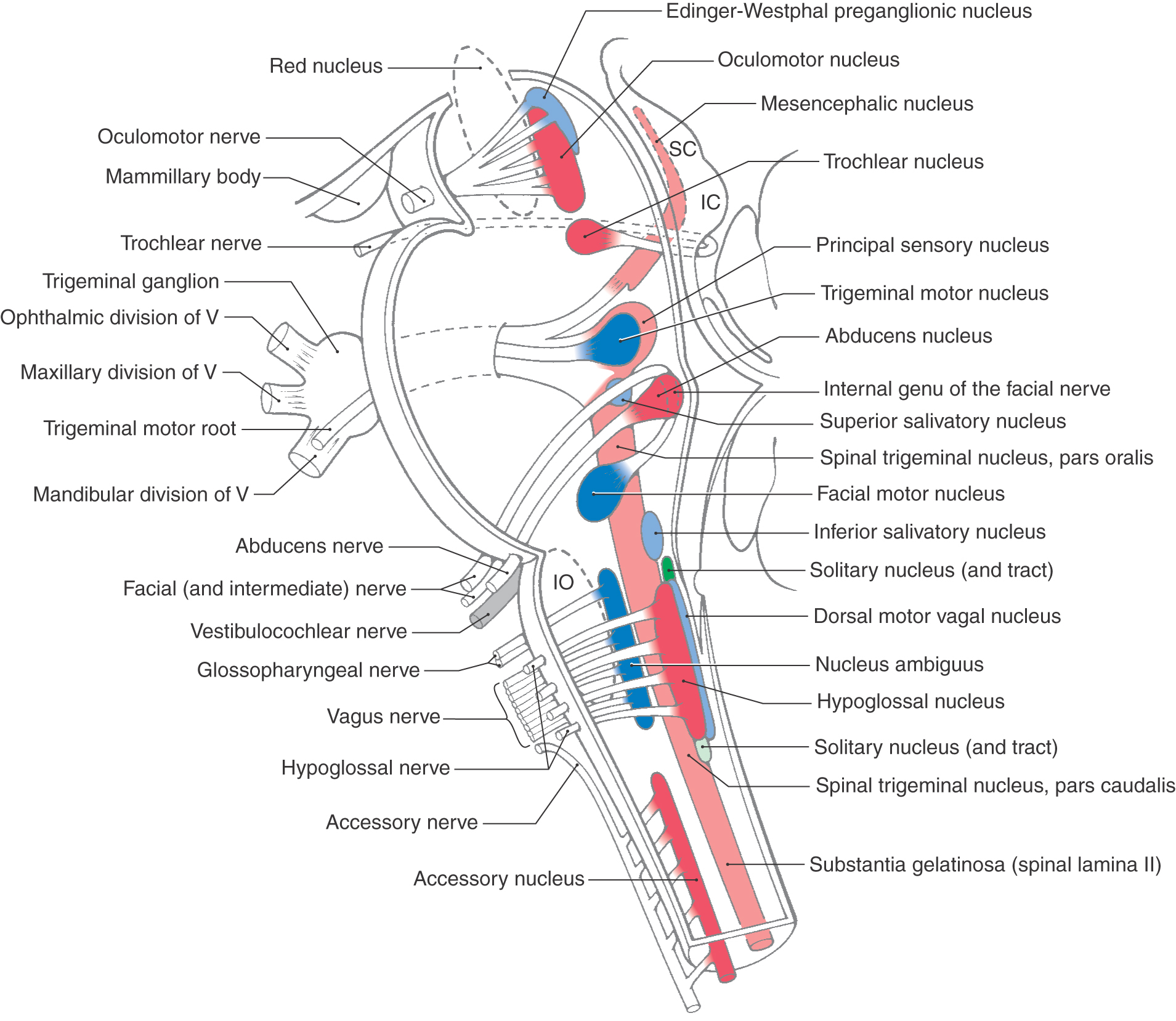
Early in development, the derivatives of the basal plate that form motor nuclei of the cranial nerves in the brainstem tend to form rostrocaudally oriented cell columns. As the brainstem enlarges, these cell columns become discontinuous. That is, they are in line with but are separated from each other in the adult brain (see Figs. 10-5, and 10-7). As we have seen and shall review here, those nuclei that are in line with each other and that have arisen from the same original cell column have developmental, structural, and functional characteristics in common.
The most medial cranial nerve motor nuclei in the brainstem are the hypoglossal (XII), abducens (VI), trochlear (IV), and oculomotor (III) nuclei (Fig. 14-1). These nuclei share three characteristics. First, they are located adjacent to the midline and anterior to the ventricular space of their particular brain division. Second, the motor neurons in these nuclei innervate skeletal muscle that originates from paraxial mesoderm that migrated into the occipital region (tongue muscles) and into the area of the orbit (extraocular muscles). Third, the functional component of the lower motor neurons in these nuclei is somatic efferent (SE), reflecting the fact that these motor neurons innervate skeletal muscles that originate from paraxial mesoderm not located in a pharyngeal arch.
Laterally adjacent to the SE cell column are the nuclei that collectively constitute the cranial part of the craniosacral division (parasympathetic) of the visceromotor nervous system. These nuclei, with the cranial nerves on which the preganglionic fibers travel, are (1) the dorsal motor vagal nucleus—vagus nerve; (2) the inferior salivatory nucleus—glossopharyngeal nerve; (3) the superior salivatory nucleus—the facial nerve, intermediate part; and (4) the Edinger-Westphal pg (preganglionic cells) nucleus—oculomotor nerve (Fig. 14-1; see also Fig. 10-7). These nuclei share the following characteristics. First, they form a discontinuous column located slightly lateral to the SE nuclei. Second, the neurons in these nuclei give rise to preganglionic axons that terminate in a peripheral ganglion, the cells of which give rise to postganglionic fibers that innervate a visceral structure. Third, because these motor neurons are part of a pathway that innervates a visceral structure (tissue composed of smooth muscle, glandular epithelium, or cardiac muscle or a combination of these), they are classified as visceral efferent (VE). They can most accurately be identified as VE preganglionic parasympathetic as this term completely identifies their structural and functional relationships.
The most lateral motor cell column in the medulla and in the pontine tegmentum is formed by the nucleus ambiguus, the efferents of which travel on the vagus and glossopharyngeal nerves, and by the facial motor nucleus and the trigeminal motor nucleus, related to facial and trigeminal nerves, respectively (Fig. 14-1; see also Fig. 10-7). These motor nuclei also share common characteristics. First, they form a discontinuous column in the more lateral part of the medulla and pontine tegmentum. Their position, as is the case for the SE and VE cell columns, reflects the differentiation of the basal plate in the brainstem (see Fig. 10-8). Second, the muscles innervated by these lower motor neurons originate from paraxial mesoderm that initially migrates into the pharyngeal arches. The muscles of mastication (trigeminal nerve innervation) originate through arch I; the muscles of facial expression (facial nerve innervation), through arch II; the stylopharyngeus muscle (glossopharyngeal nerve innervation), through arch III; and the constrictors of the pharynx, intrinsic laryngeal muscles, palatine muscles (except the tensor veli palatini), and the vocalis (vagal nerve innervation), through arch IV. Third, owing to the fact that these lower motor neurons innervate skeletal muscles that also arose from paraxial mesoderm (see Fig. 10-8), they are classified as somatic efferent (SE).
SENSORY CELL COLUMNS AND NUCLEI
The derivatives of the alar plate that give rise to cranial nerve sensory nuclei of the brainstem are located lateral to the sulcus limitans (see Fig. 10-5 and Fig. 10-6). In contrast to the motor nuclei, which form rostrocaudally oriented but discontinuous cell columns, all three of the sensory nuclei in the brainstem form what can arguably be described as continuous cell columns in the adult. These sensory nuclei–cell columns are located in the lateral aspects of the brainstem.
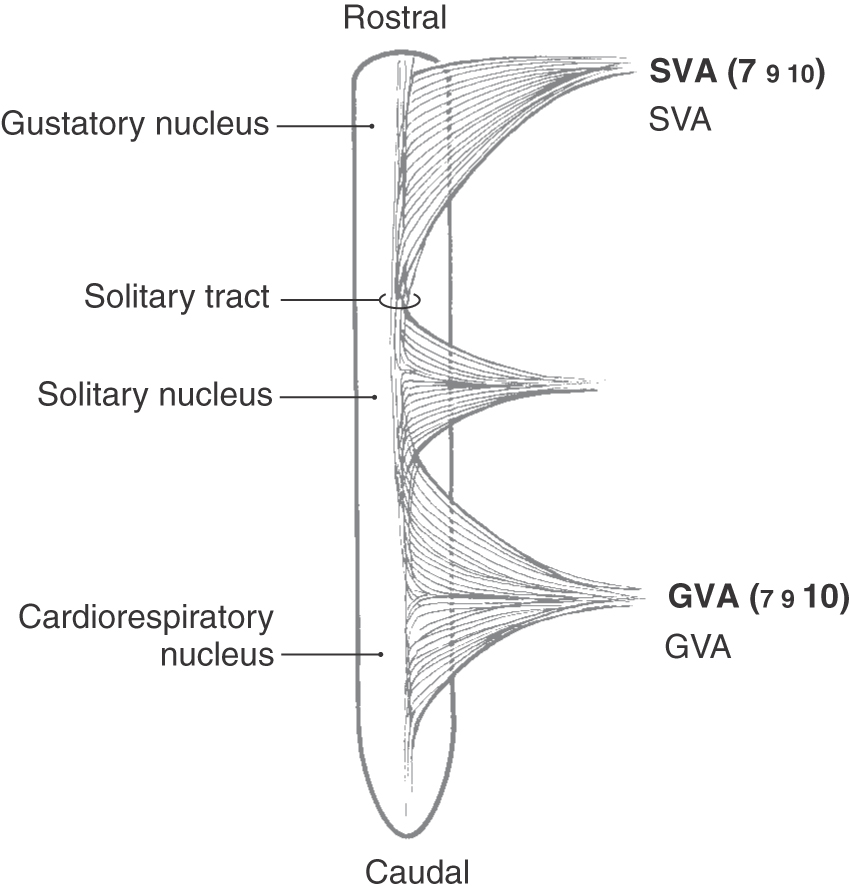
The most medial of these cell columns is the solitary tract and nucleus, which is the visceral afferent center of the brainstem (Fig. 14-1; see also Fig. 10-7). No matter what cranial nerve returns visceral afferent information to the brainstem, the central processes of these primary afferent fibers contribute to the solitary tract, the fibers of which terminate in the solitary nucleus (Fig. 14-2). Visceral afferent (VA) information is conveyed centrally on the facial, glossopharyngeal, and vagus nerves and consists of taste fibers and fibers conveying visceral sensations from salivary glands and viscera of the thorax and abdomen. The majority of taste input reaches rostral portions of the solitary nucleus (sometimes called the gustatory nucleus), whereas most other visceral sensation enters the caudal portion of the solitary nucleus (sometimes referred to as the cardiorespiratory nucleus) (Fig. 14-2). Because the most rostral cranial nerve that contributes to the solitary tract and nucleus is the facial nerve (a nerve of the pons-medulla junction), the solitary tract and its nucleus are found throughout the medulla but do not extend rostrally beyond the pons-medulla junction.
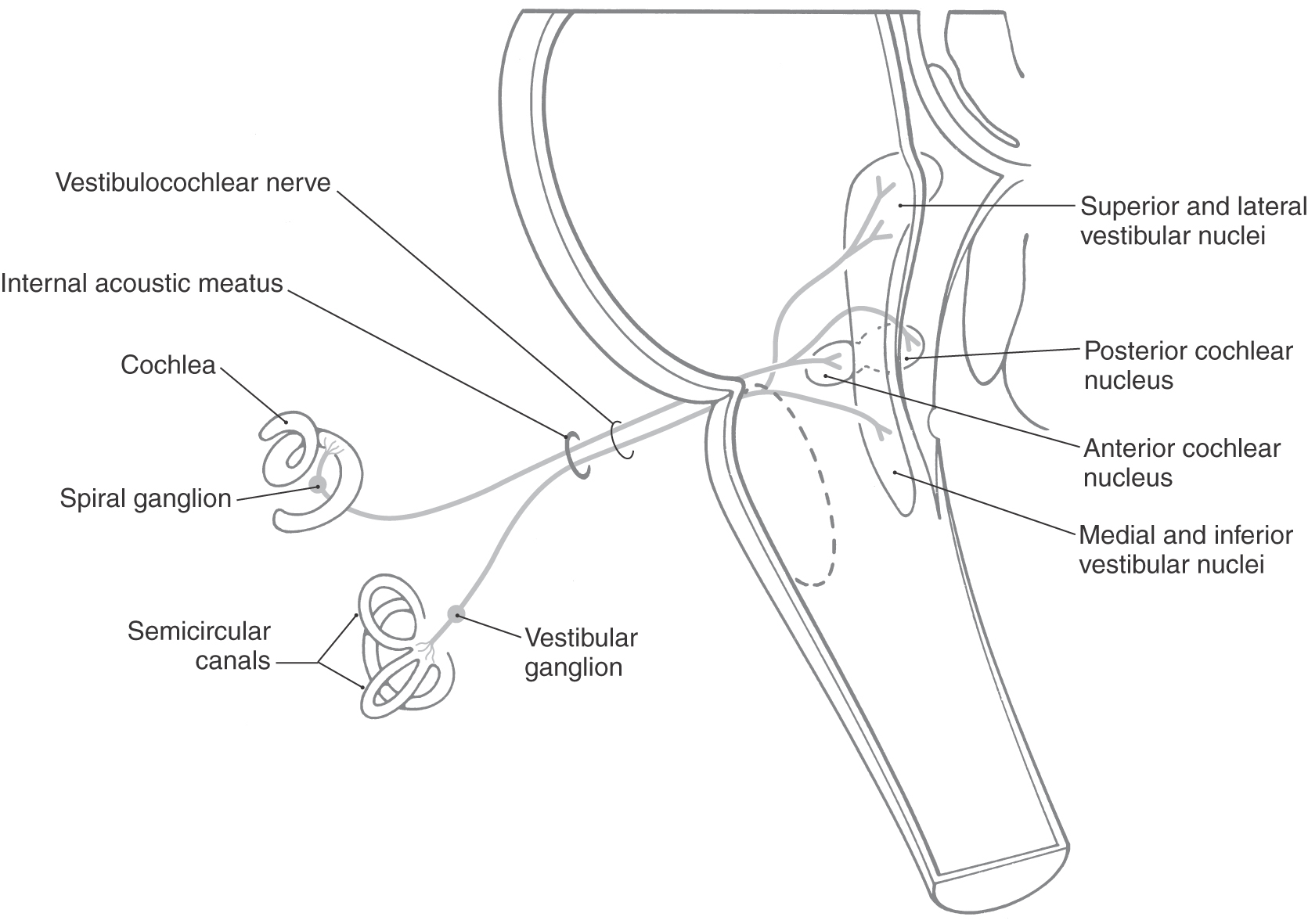
Immediately and posteriorly adjacent to the solitary tract and nucleus are the medial and spinal vestibular nuclei. These continue rostrally, are joined by the anterior and posterior cochlear nuclei at the pons-medulla junction, and interface in the caudal pons with the superior and lateral vestibular nuclei (Fig. 14-9). This cell column receives sensory input from the vestibulocochlear nerve (cranial nerve VIII) only and subserves the sense of hearing (SA, exteroceptive functional component) and balance and equilibrium (SA, proprioceptive functional component).
The nuclei of the trigeminal sensory system form a continuous cell column extending from the spinal cord–medulla junction to the rostral midbrain (Fig. 14-1; see also Fig. 10-7). The trigeminal sensory nuclei are divided into (1) the spinal trigeminal nucleus (consisting of a pars caudalis, pars interpolaris, and pars oralis), located in the lateral medulla and extending into the caudal pons; (2) the principal sensory nucleus, located at the midpontine level; and (3) the mesencephalic nucleus, extending rostrally into the midbrain at the lateral aspect of the periaqueductal gray (Fig. 14-1; see also Fig. 13-8). As is the case for the solitary tract and nucleus (the visceral receiving center of the brainstem), the principal sensory nucleus and especially the spinal trigeminal nucleus constitute the somatic sensory receiving center of the brainstem. Although somatic afferent (SA) pain and thermal sensations enter the brainstem on four different cranial nerves (trigeminal, facial, glossopharyngeal, and vagus), the central processes of these primary afferent fibers enter the spinal trigeminal tract and terminate in the medially adjacent spinal trigeminal nucleus.
CRANIAL NERVES OF THE MEDULLA OBLONGATA
The cranial nerves that are commonly identified as exiting the medulla are the hypoglossal nerve (cranial nerve XII) through the abducens nerve (cranial nerve VI) (Figs. 14-3 and 14-4). However, in the subsequent discussion, the abducens (VI), facial (VII), and vestibulocochlear (VIII) nerves are considered the nerves of the pons-medulla junction. Consequently, the cranial nerves that are generally associated with only the medulla are the hypoglossal (XII), accessory (XI), vagus (X), and glossopharyngeal (IX) nerves (Figs. 14-3 and 14-4). The unique situation of the accessory nerve is addressed further on.
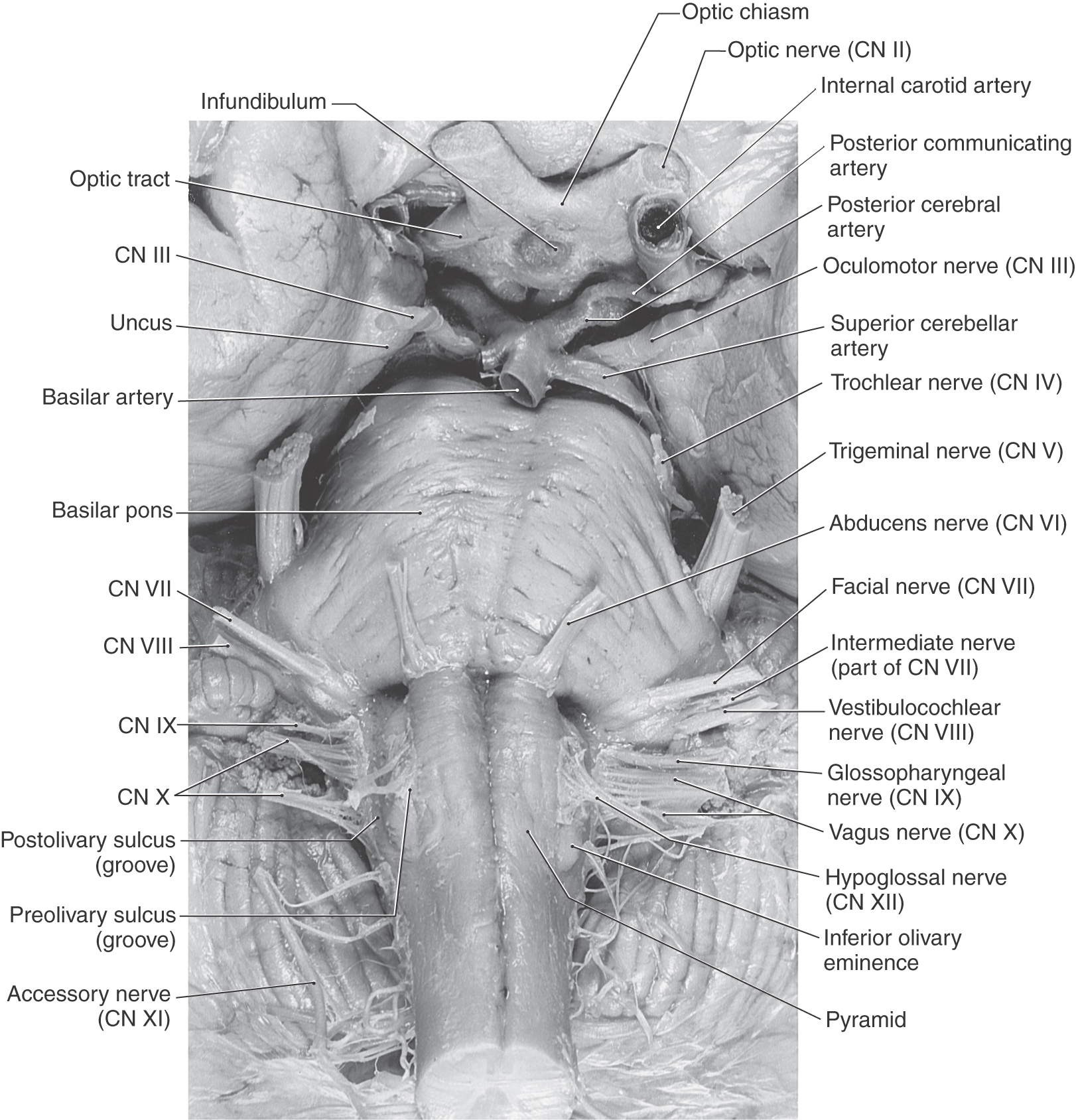
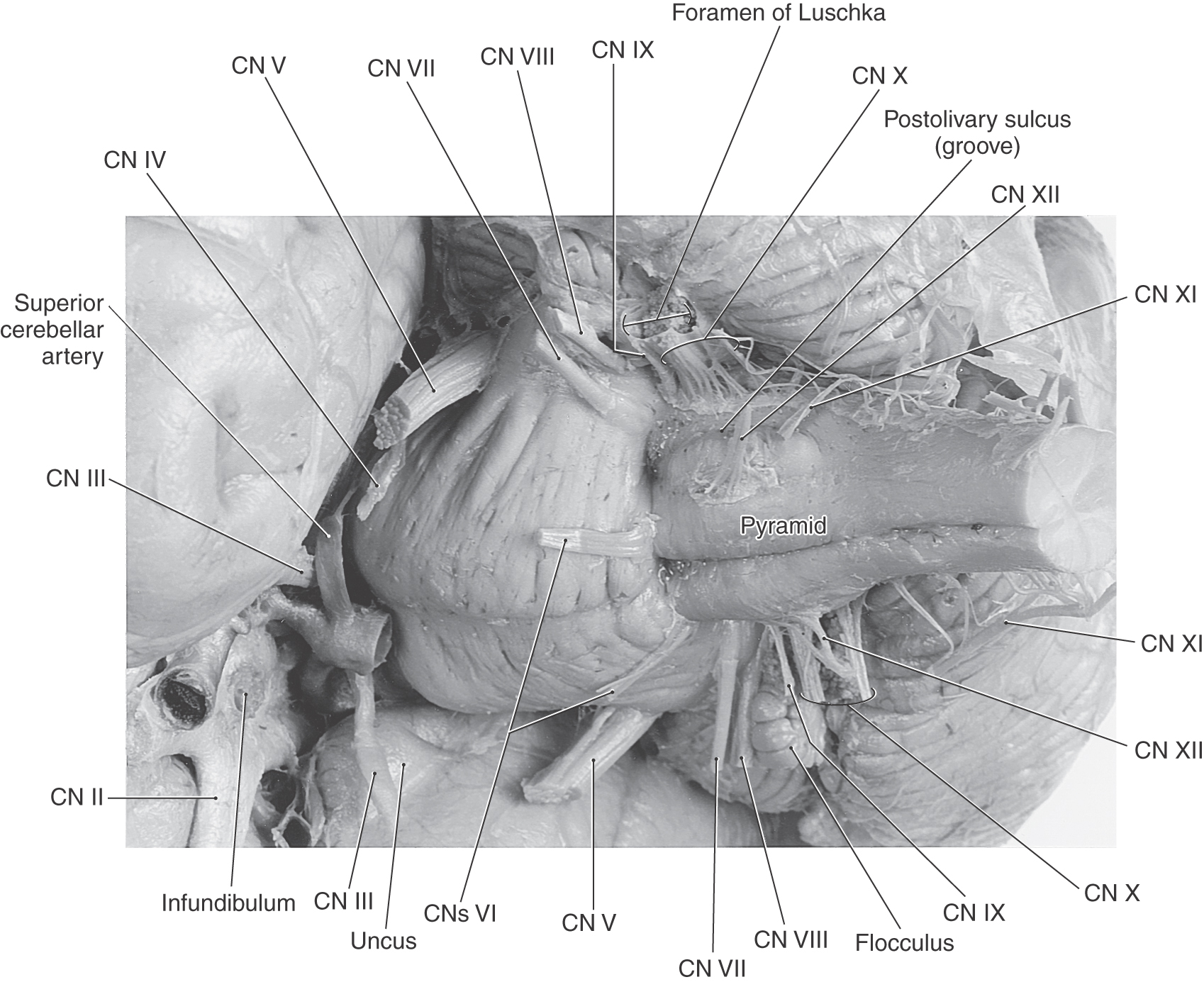
Figure 14-3. An anterior (ventral) view of the brainstem with particular emphasis on cranial nerves (CN).
Hypoglossal Nerve
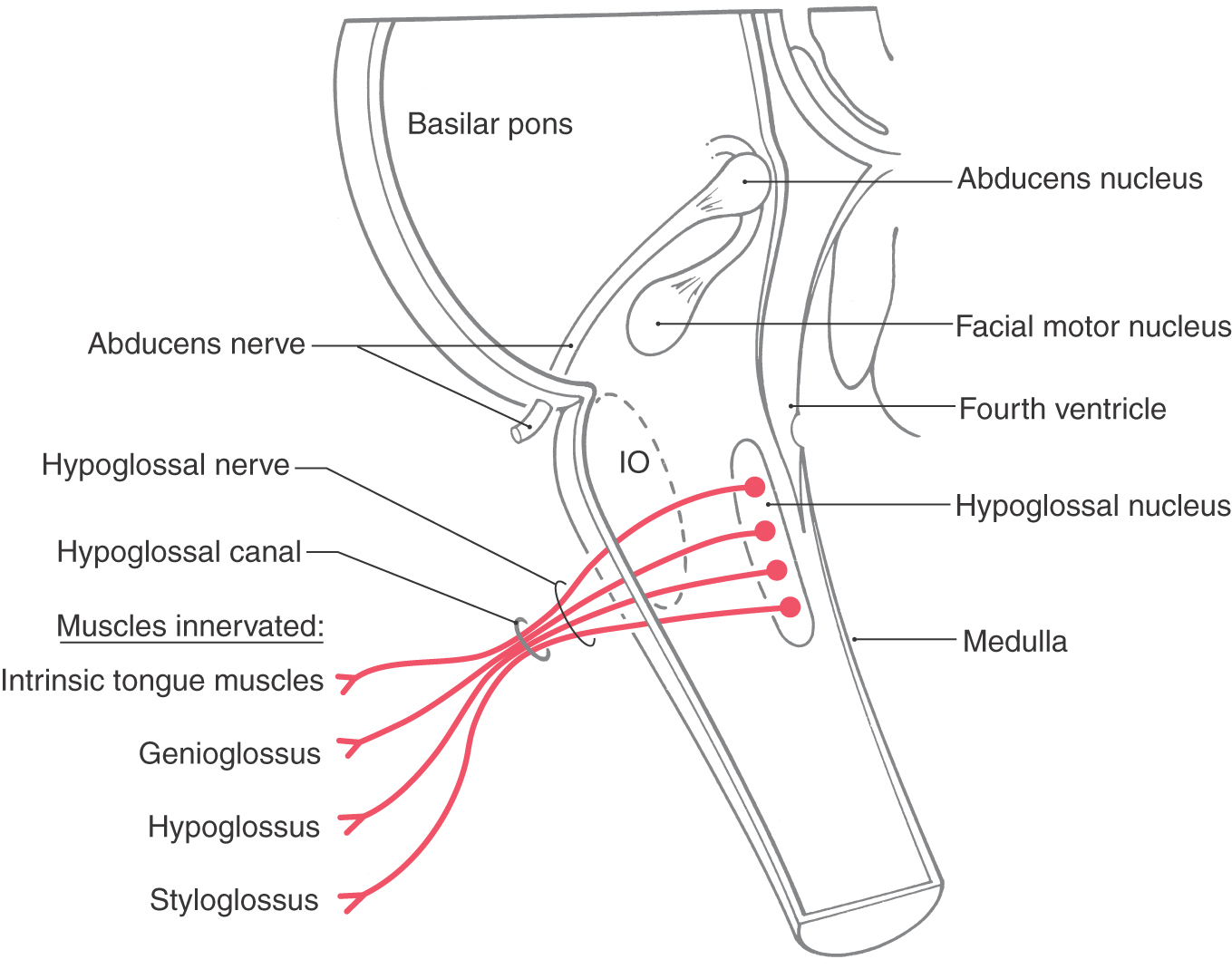
The hypoglossal nucleus is located internal to the hypoglossal trigone. Axons of hypoglossal motor neurons pass anteriorly in the medulla along the lateral aspect of the medial lemniscus and the pyramid (see Fig. 11-11) to exit as a series of rootlets from the preolivary fissure as the hypoglossal nerve (Figs. 14-3 and 14-5). They continue through the hypoglossal canal and distribute to the intrinsic muscles of the tongue plus the hyoglossus, palatoglossus, and genioglossus muscles (Fig. 14-6). In addition to the hypoglossal nerve, the hypoglossal canal may also contain an emissary vein and a small meningeal branch to the dura of the posterior fossa from the ascending pharyngeal artery.
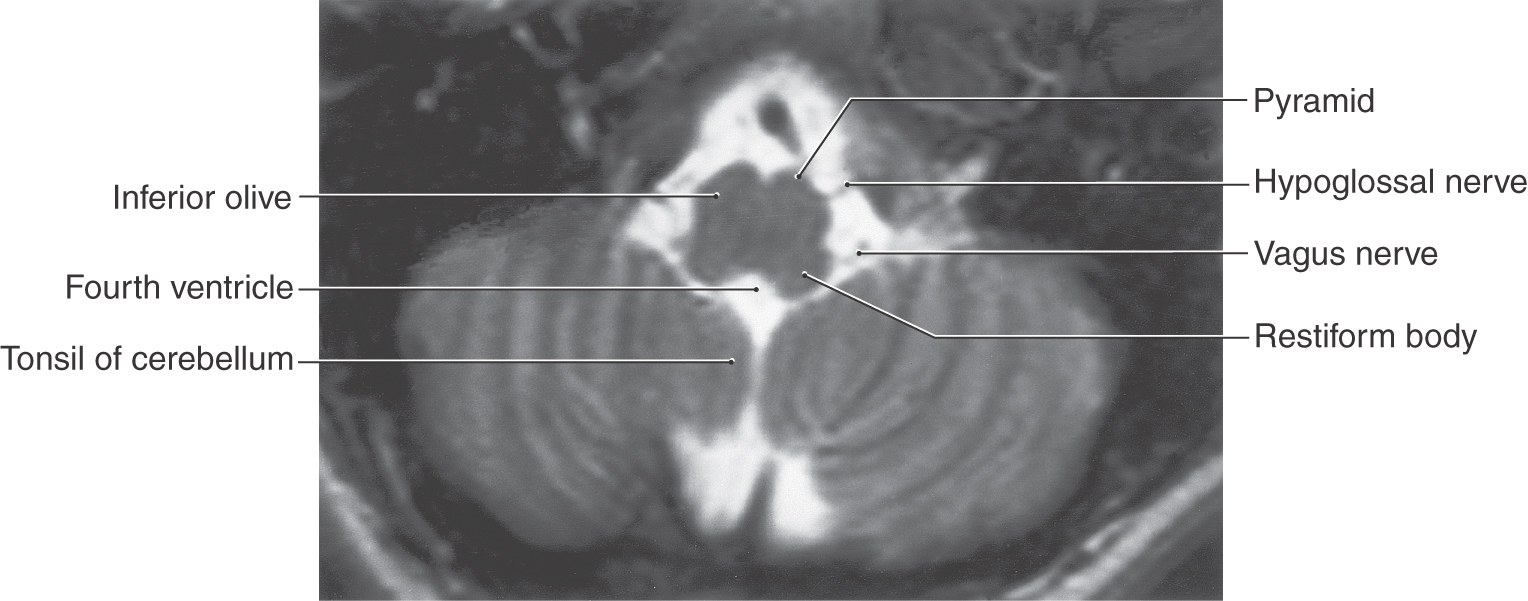
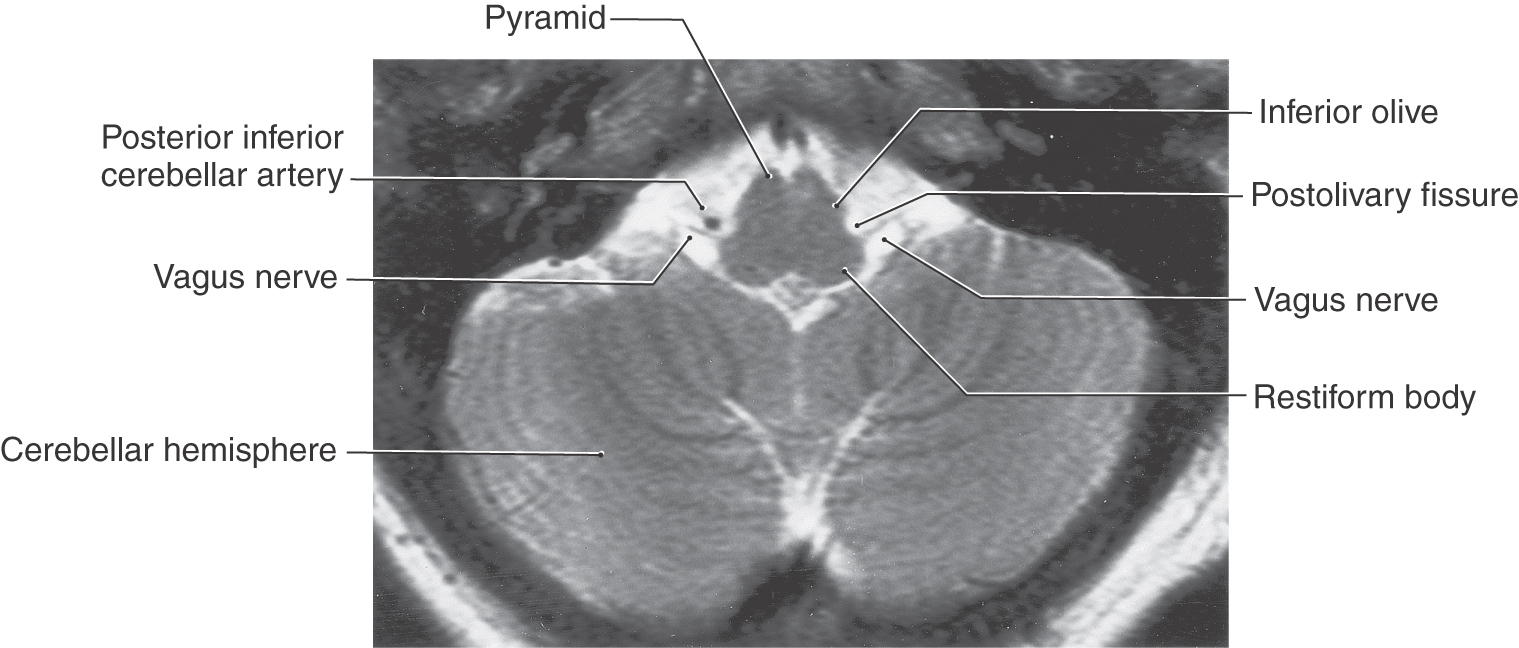
Figure 14-5. Axial T2-weighted magnetic resonance image of the medulla showing the roots of the hypoglossal (from the preolivary fissure) and the vagus (from the postolivary or retroolivary fissure) nerves. Compare the shape of the medulla at this level with Figures 14-3 and 14-4.
Figure 14-6. The central origin and peripheral distribution of the hypoglossal nerve (cranial nerve XII). IO, inferior olive.
The blood supply to the hypoglossal nucleus and its exiting fibers is via penetrating branches of the anterior spinal artery. Occlusion of these branches (as in the medial medullary syndrome) may result in paralysis of the genioglossus muscle with deviation of the tongue toward the side of the lesion (the weak side) on protrusion. In addition, the patient experiences a contralateral hemiparesis (corticospinal tract involvement) and a contralateral loss of position sense, vibratory sense, and two-point discrimination (medial lemniscus involvement) because the anterior spinal artery also serves these structures.
Other lesions that may affect hypoglossal function include a lesion of the root of the nerve only (causing tongue deviation to the side of the lesion with no other deficits) and injury to the internal capsule. In the latter case, corticonuclear fibers to hypoglossal motor neurons innervating the genioglossus muscle are predominantly crossed. Consequently, internal capsule lesions may result in a deviation of the tongue to the contralateral side (side opposite the lesion) on protrusion, in concert with other deficits such as a contralateral hemiplegia and a drooping of the facial muscles in the lower quadrant of the contralateral side of the face. See Chapter 25 for examples of lesions that result in hypoglossal nerve dysfunction.
Accessory Nerve
This so-called cranial nerve was historically described as having a cranial part (from the medulla) and a spinal part (from the cervical spinal cord). However, studies have shown that the neurons that innervate the sternocleidomastoid and trapezius muscles are located in the cervical cord only; these muscles are not innervated by motor neurons located in the medulla. For consistency and in recognition of wide usage, the accessory nerve is considered here as a cranial nerve associated with the medulla.
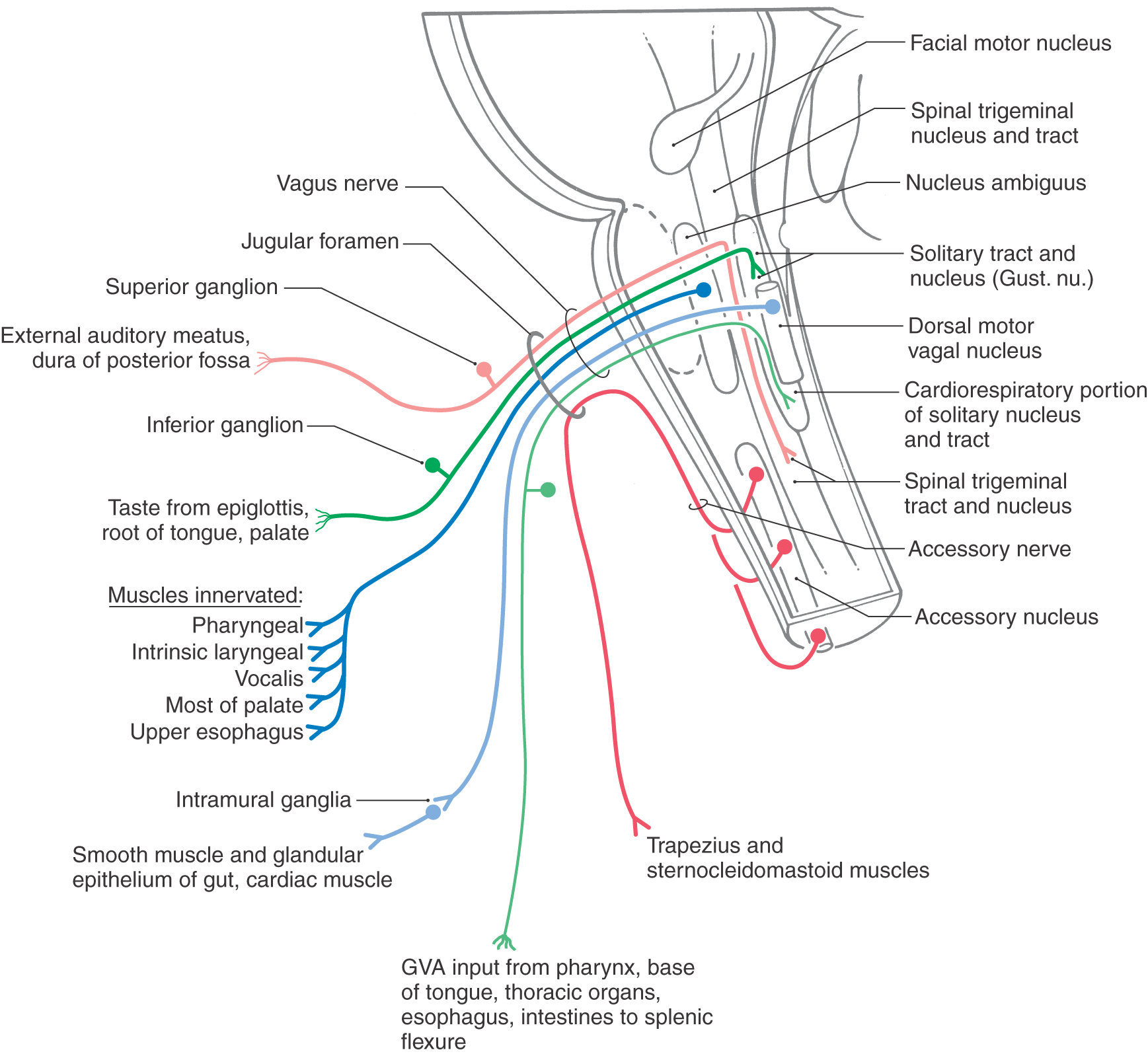
The accessory nerve originates from motor neurons in the cervical spinal cord extending from C1 to C5 (Fig. 14-7). The axons of these neurons exit the lateral aspect of the cord, coalesce to form the nerve (Fig. 14-3), and ascend to enter the cranial cavity via the foramen magnum. As these accessory fibers course through the posterior fossa, they are briefly joined by vagal fibers that originate from the caudal portion of the nucleus ambiguus. These vagal fibers diverge from this temporary association and exit the skull on the tenth cranial nerve. The accessory fibers form the eleventh cranial nerve, receive no contributions from the medulla, and along with cranial nerves IX and X exit the cranial cavity via the jugular foramen (Fig. 14-8).
The relationship of the accessory nerve to the vagus nerve is similar to the relationship of the seventh nerve to the trigeminal nerve via the chorda tympani. In the latter case, the taste fibers from the anterior two thirds of the tongue travel on the trigeminal nerve and then join the seventh nerve via the chorda tympani. However, throughout their extent, these taste fibers are considered part of the seventh nerve, not the fifth. In like manner, fibers of the accessory nerve temporarily join the vagus and then leave it to exit the skull (Fig. 14-7). These accessory nerve fibers do not originate from the medulla, do not distribute peripherally with the vagus, and have their cells of origin in the cervical spinal cord. What has classically been called the cranial part of the accessory nerve is actually a misnomer; these fibers represent the caudal portions of the vagus nerve to which the accessory nerve temporarily relates. Reflecting the fact that the sternocleidomastoid and trapezius muscles in the human originate from paraxial mesoderm caudal to the fourth arch (not in the fourth arch), the functional component associated with these motor neurons is SE.
Lesions of the root of the accessory nerve result in drooping of the shoulder (trapezius paralysis) on the ipsilateral side and difficulty in turning the head to the contralateral side (sternocleidomastoid paralysis) against resistance. Weakness of these muscles is not especially obvious in cervical cord lesions because a hemiplegia (indicating damage to corticospinal fibers) is the overwhelmingly obvious deficit. However, a lesion of the internal capsule may also result in deficits similar to those described previously owing to interruption of the corticonuclear fibers to the accessory nucleus; these corticonuclear fibers are primarily uncrossed.
Vagus Nerve
The vagus nerve is located at an intermediate position between the midline and lateral aspect of the medulla, exits the postolivary sulcus (Figs. 14-3 to 14-5 and 14-9), and contains both motor and sensory components. This cranial nerve exits the cranial cavity via the jugular foramen (Fig. 14-8) and exhibits two ganglia immediately external to the foramen. The superior ganglion contains the cell bodies of SA fibers, whereas the inferior ganglion contains the cell bodies of VA fibers.
The motor cells in the medulla that distribute their axons on the vagus nerve are located in the dorsal motor nucleus of the vagus (VE parasympathetic preganglionic) and in the nucleus ambiguus (SE) (Fig. 14-7). Preganglionic parasympathetic cells of the dorsal motor nucleus send their axons, via branches of the vagus nerve, to end in terminal (intramural) ganglia located adjacent to or within visceral structures of the trachea and bronchi of the lungs, the heart, and the digestive system to a level just proximal to the splenic flexure of the colon (Fig. 14-7). In general, vagal influence causes constriction of the bronchioles, decreases heart rate, and increases blood flow, peristalsis, and secretions in the gut. Axons of motor neurons in the nucleus ambiguus distribute on branches of the vagus nerve to the constrictor muscles of the pharynx, the intrinsic laryngeal muscles (including the vocalis muscle), the palatine muscles (except the tensor veli palatini, which is innervated by the fifth nerve), and the skeletal muscle in about the upper half of the esophagus (Fig. 14-7). These muscles originate from paraxial mesoderm that migrated into the fourth pharyngeal arch.
The sensory fibers conveyed on the vagus nerve relay somatic and visceral sensations. The somatic sensations are represented by SA input (recognized as pain and thermal sensations) from a small area on the ear and part of the external auditory meatus and from the dura of the posterior cranial fossa (Fig. 14-7). These fibers have their cell bodies in the superior ganglion of the vagus nerve and enter the medulla as part of the vagus, but the central processes of these primary afferent fibers enter the spinal trigeminal tract and synapse in the medially adjacent spinal trigeminal nucleus.
The visceral sensations conveyed by the vagus are represented by VA fibers (Fig. 14-7). Visceral sensations from the heart, aortic arch, pharynx and larynx, lungs, and gut to about the level of the splenic flexure are conveyed by these fibers. Their cell bodies are in the inferior ganglion of the vagus nerve, whereas the central processes of these fibers enter the solitary tract and terminate in the surrounding caudal solitary nucleus (the cardiorespiratory nucleus). The same trajectory is also followed by taste fibers on the vagus. These fibers originate from scattered taste buds on the epiglottis and base of the tongue, have their cell bodies in the inferior ganglion, enter the brainstem on the vagus nerve, and centrally distribute to the rostral solitary nucleus (the gustatory nucleus).
Both sensory (SA, VA) and taste (VA) information conveyed on the vagus nerve is eventually relayed to the sensory cortex, where it is interpreted as, for example, pain from the external auditory meatus, a sense of fullness from the gut, or taste. Details of these central pathways are described in later chapters.
A lesion of the root of the vagus nerve will result in dysphagia, owing to a unilateral paralysis of pharyngeal and laryngeal musculature, and dysarthria, owing to a weakness of laryngeal muscles and the vocalis muscle. There are, however, no lasting demonstrable symptoms specifically related to visceromotor (autonomic) dysfunction. Taste loss is not detectable and cannot be tested, and the small somatosensory loss involving the external auditory meatus and canal may be of little consequence.
Unilateral injury inside the medulla, as with tumors, vascular lesions, or syringobulbia, may give rise to similar deficits (as described earlier) that are due to damage to the nucleus ambiguus. Bilateral lesions of the medulla, although rare, result in aphonia, aphagia, dyspnea, or inspiratory stridor. Such lesions may be life-threatening, especially if they involve the dorsal motor nucleus. Dysarthria may also be seen in patients after thyroid surgery if the recurrent laryngeal nerve has been damaged.
Glossopharyngeal Nerve
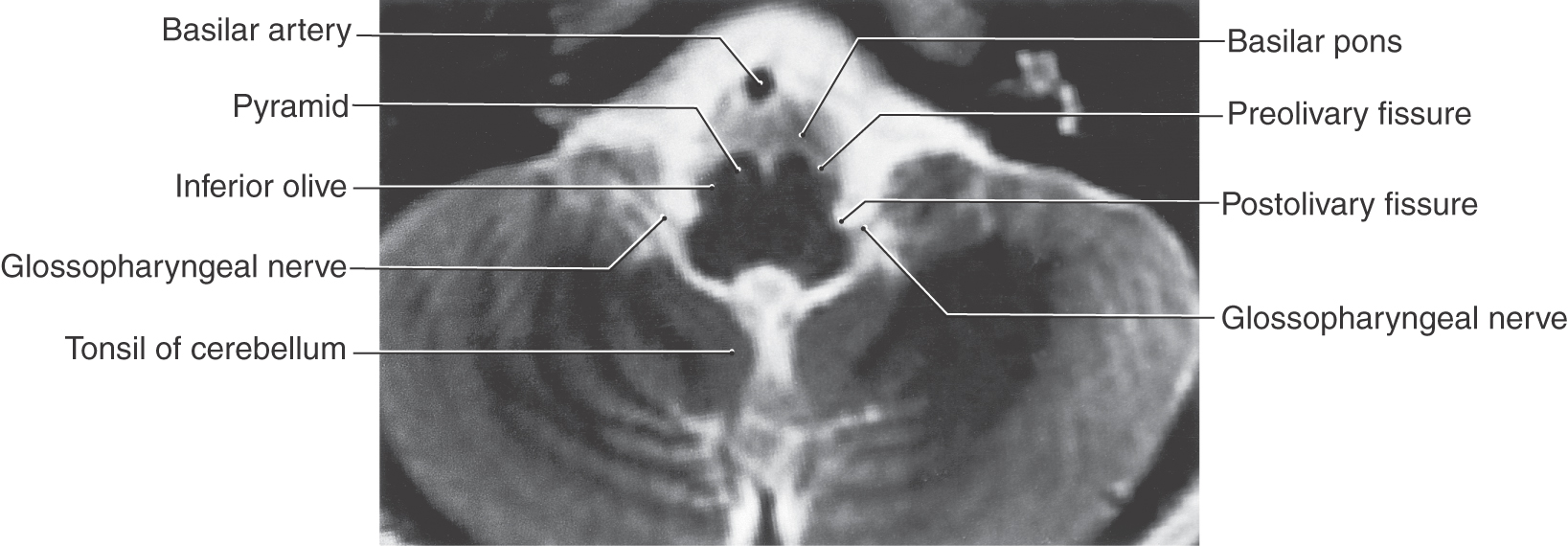
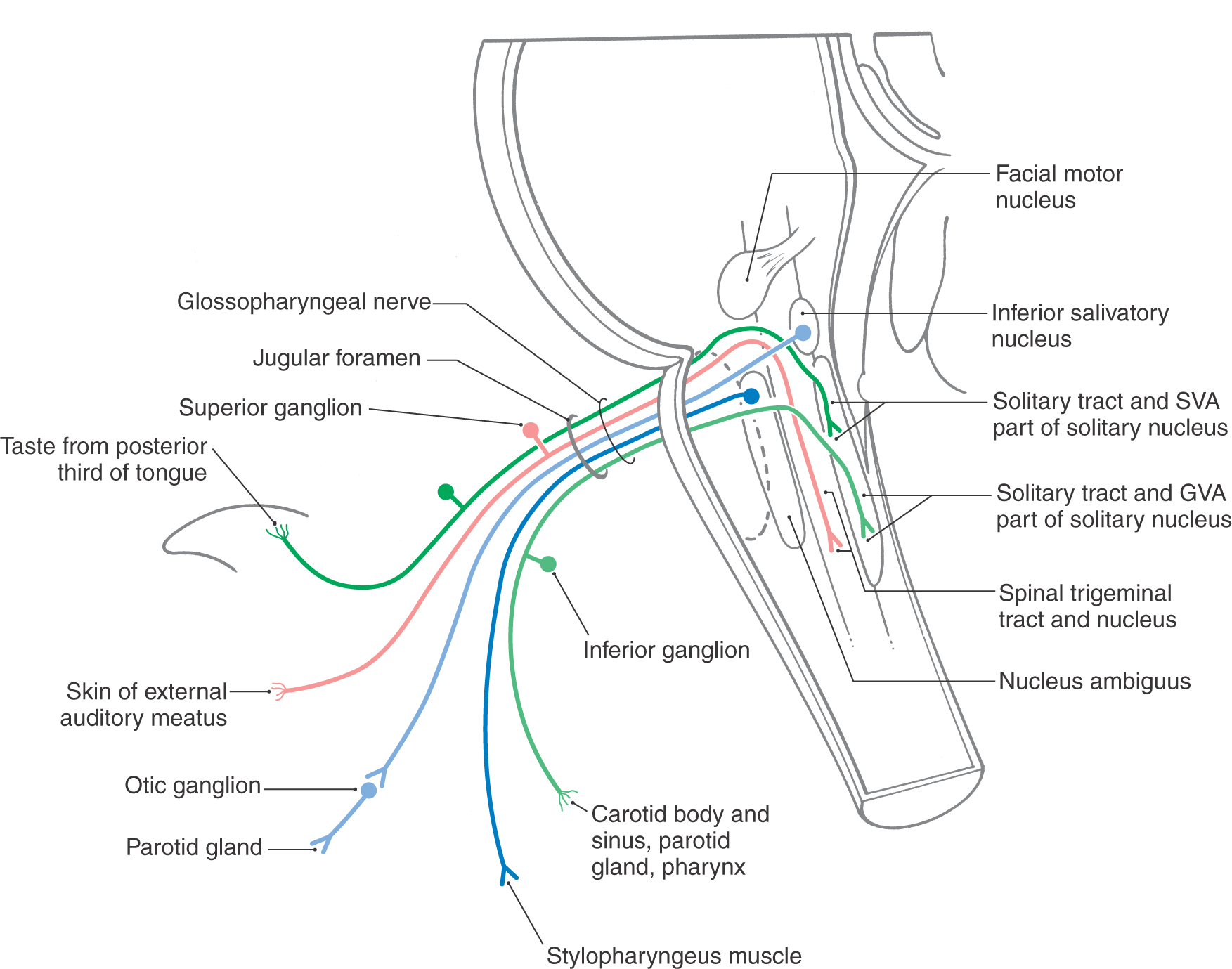
The glossopharyngeal nerve exits the medulla at the postolivary sulcus (Fig. 14-10) immediately rostral to the vagus nerve (Figs. 14-3 and 14-4) and leaves the skull via the jugular foramen (Fig. 14-8) along with the vagus and accessory nerves. Like the vagus, the glossopharyngeal nerve has two ganglia: an inferior ganglion containing VA cell bodies and a superior ganglion containing SA cell bodies.
Motor fibers that distribute on the glossopharyngeal nerve originate from the inferior salivatory nucleus (VE preganglionic parasympathetic fibers) and from the nucleus ambiguus (SE fibers) (Fig. 14-11). Axons of cells of the inferior salivatory nucleus exit on the glossopharyngeal nerve and join the tympanic nerve and then the lesser petrosal nerve to synapse with VE postganglionic neurons in the otic ganglion. These postganglionic parasympathetic cells supply secretomotor input to the parotid gland. The contribution of the nucleus ambiguus to the glossopharyngeal nerve serves to innervate the stylopharyngeus muscle (Fig. 14-11). This muscle assists in swallowing and participates in the efferent part of the gag reflex.
Again, as with the vagus nerve, the sensory fibers of the glossopharyngeal nerve are sensory (SA) and visceral (VA) (Fig. 14-11). The SA fibers originate from cutaneous receptors on a small area of the pinna and external auditory canal and on the posterior third of the tongue. These fibers have their cell bodies in the superior ganglion, and their central processes join the spinal trigeminal tract before terminating in the spinal trigeminal nucleus. The visceral afferent (VA) fibers convey information from the parotid gland and the oropharynx, with an especially important input from the carotid body. The carotid body contains chemoreceptors that are sensitive to changing levels of oxygen and carbon dioxide in the blood. The VA fibers have their cell bodies in the inferior ganglion of the glossopharyngeal nerve, and centrally they enter the solitary tract to terminate in the solitary nucleus. Taste from the posterior third of the tongue (Fig. 14-11) is conveyed centrally by VA fibers that also have their cell bodies of origin in the inferior ganglion of the glossopharyngeal nerve. As is the case for all taste fibers, the central processes of these afferent fibers enter the solitary tract and terminate on cells of the surrounding solitary nucleus. All afferent inputs on the glossopharyngeal nerve are eventually transmitted to relay nuclei of the thalamus and on to the sensory cortex, where the information is fully appreciated and interpreted.
Lesions of the ninth cranial nerve are relatively rare but may occur in combination with the vagal and accessory roots at the jugular foramen (Fig. 14-8). Deficits related only to glossopharyngeal nerve damage are largely restricted to a loss of taste from the posterior third of the tongue and a loss of stylopharyngeus contraction on the side of the lesion; this may affect the gag reflex. The gag reflex depends on the integrity of cranial nerves IX and X. In addition, the ninth nerve is subject to glossopharyngeal neuralgia. This disorder is characterized by attacks of intense idiopathic pain arising from the sensory distribution of the nerve (pharynx, caudal parts of the tongue, tonsil, and possibly areas of the middle ear). The attacks may be spontaneous or may result from artificial stimulation of the back of the oral cavity or from swallowing or even talking. Glossopharyngeal neuralgia may be severe and can be seriously disabling.
Jugular Foramen
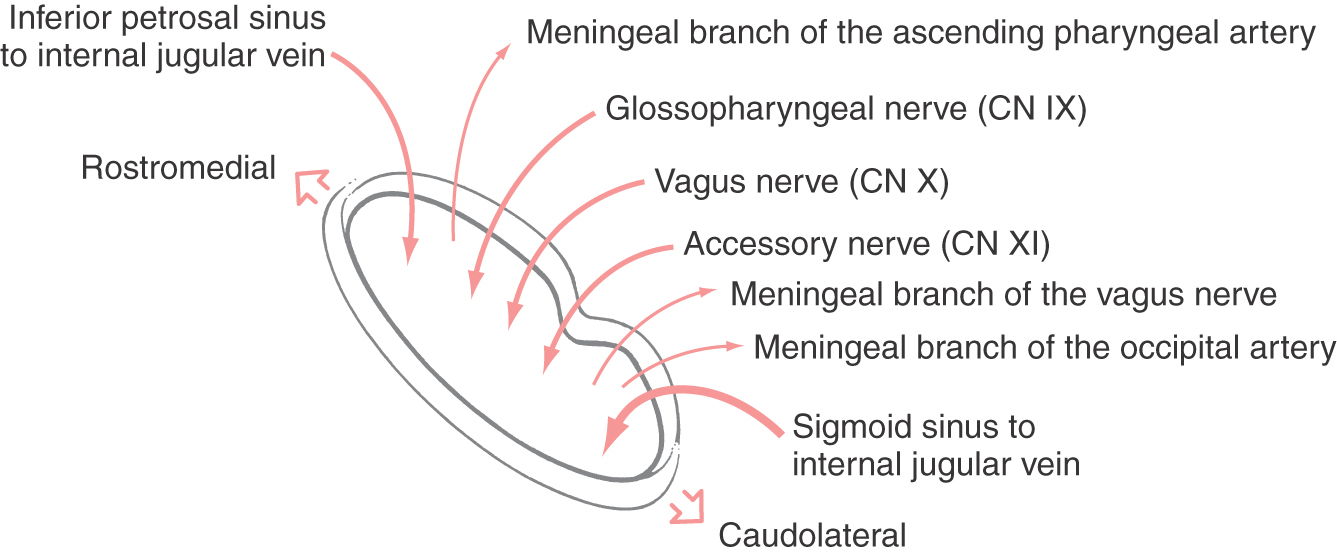
In addition to containing cranial nerves IX, X, and XI, which pass through approximately its middle third, the jugular foramen also serves as a conduit for other important structures (Fig. 14-8). In general, the foramen can be divided into a rostral and slightly medial area, a middle portion (containing cranial nerves IX, X, and XI), and a caudal and somewhat lateral portion.
The rostromedial portion (Fig. 14-8) contains the continuation between the inferior petrosal sinus and the internal jugular vein. The inferior petrosal sinus, although small, represents a communication between the cavernous sinus and the internal jugular vein. In addition the rostromedial portion of the jugular foramen also contains a meningeal branch of the ascending pharyngeal artery. This small vessel is one source of arterial blood to the meninges of the posterior fossa.
The caudolateral portion of the jugular foramen (Fig. 14-8) is the point at which the sigmoid sinus is continuous with the internal jugular vein. The sigmoid sinus is large and represents an important route for venous drainage from the brain. This part of the jugular foramen also contains the meningeal branch of the occipital artery, another source of arterial blood to the meninges of the posterior fossa. The meningeal branch of the vagus nerve enters the cranial cavity through this part of the foramen and serves as one of the nerves for the sensory innervation of the dura of the posterior fossa.
Syndromes of the Jugular Foramen
The deficits seen in jugular foramen syndromes reflect damage to the contents of the foramen or to structures immediately external to the foramen. The syndrome of the jugular foramen (also known as the Vernet syndrome) is usually caused by a lesion at or immediately internal to the foramen. These deficits are a loss of sensation (including taste) in the caudal third of the tongue (cranial nerve IX), loss of sensations in the larynx and pharynx, dysarthria and dysphagia resulting from paralysis of the vocal fold and intrinsic muscles of the larynx on the ipsilateral side (cranial nerve X), and weakness of the ipsilateral sternocleidomastoid and trapezius muscles (cranial nerve XI). Lesions immediately external to the jugular foramen (as in the Collet-Sicard syndrome) result in deficits characteristic of damage to IX, X, and XI plus weakness of the tongue on the side of the lesion; this is because the exit of the hypoglossal nerve (via the hypoglossal canal) is adjacent to the jugular foramen and the root of this nerve is recruited into the lesion. The most inclusive syndrome of the jugular foramen is the Villaret syndrome, also called the retropharyngeal syndrome, which includes deficits that are indicative of damage to cranial nerves IX, X, and XI, the hypoglossal nerve, and sympathetic fibers of the superior cervical ganglion, the last producing a Horner syndrome on the ipsilateral side of the head.
CRANIAL NERVES OF THE PONS-MEDULLA JUNCTION
The cranial nerves of the pons-medulla junction are the vestibulocochlear nerve (the most lateral and, for the purposes of this discussion, exclusively sensory), the facial nerve (intermediate in location and a mixed nerve), and the abducens nerve (the most medial and exclusively motor) (Figs. 14-3 and 14-4).
There are three reasons that cranial nerves VI, VII, and VIII are considered nerves of the pons-medulla junction. First, the abducens motor nucleus and the facial motor nucleus are located at the pons-medulla junction (Fig. 14-1; see also Fig. 12-9 and Fig. 12-10). Second, in humans the exit of all three of these nerves is located at the caudal edge of the pons (Fig. 14-3). Indeed, two of the three (facial and vestibulocochlear) nerves exit the skull via the same foramen. Third, in a broader comparative sense, cranial nerves VI, VII, and VIII do not exit from the medulla. For example, in animals with a more modest cerebral cortex and cerebellum (and consequently a smaller pons), these cranial nerves exit the brain in association with the trapezoid body, which is exposed on the surface of the brainstem. In humans, because of the larger size of the pons, the trapezoid body is located internally but still at the pons-medulla junction. For these reasons, it is appropriate to consider these three cranial nerves in the manner presented here.
Vestibulocochlear Nerve
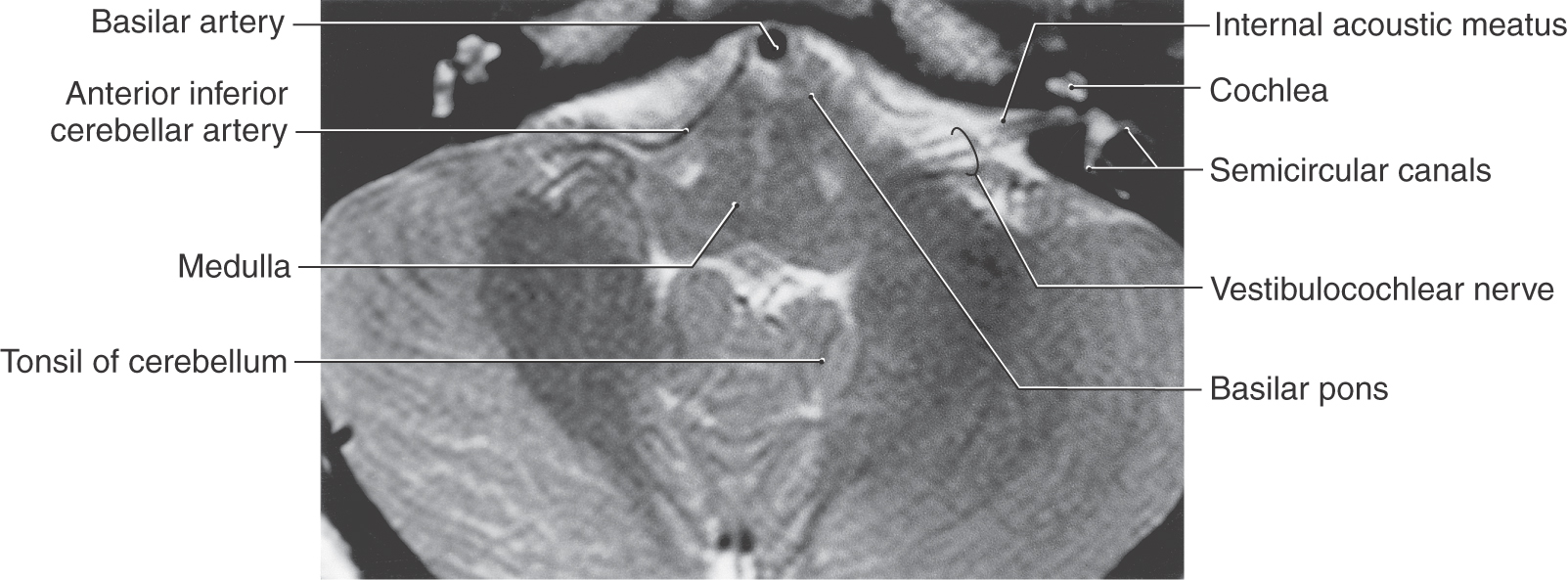
The eighth cranial nerve is the most lateral (Fig. 14-3) of the cranial nerves of the brainstem and is centrally related to the posterior and anterior cochlear nuclei (SA exteroception—hearing) and to the medial, spinal (inferior), superior, and lateral vestibular nuclei (SA proprioception—balance and equilibrium). The two portions of cranial nerve VIII originate from highly specialized receptors that are well protected in the petrous portion of the temporal bone. These receptors are described in Chapter 21 and 22. Although the vestibular and cochlear nerve roots form separate fascicles at their origin, they move to form essentially a combined nerve root as they enter the brainstem. The vestibulocochlear nerve and the facial nerve enter the cranial cavity by passing through the internal acoustic meatus (Figs. 14-12 to 14-14).
The cochlear portion of cranial nerve VIII originates from cells in the spiral ganglion located in the cochlea (Fig. 14-13). This ganglion is made up of bipolar cells, the central processes of which travel on the cochlear division through the internal acoustic meatus to terminate in the posterior and anterior cochlear nuclei. The cochlear nuclei, in turn, project to several brainstem relay nuclei, which ultimately convey auditory information to the medial geniculate nucleus and from here to the auditory cortex and participate in auditory reflexes. These central pathways are described in Chapter 21.
Although the cochlear part of cranial nerve VIII is classically described as exclusively afferent (sensory), cholinergic cells adjacent to the principal and accessory olivary nuclei give rise to axons that exit the brainstem on the cochlear nerve. These small bundles are called the olivocochlear tract (sometimes also called the efferent cochlear bundle or bundle of Rasmussen). These efferent fibers of the cochlear nerve synapse in relation to the inner and outer hair cells and function to inhibit (dampen) the ability of the hair cell to respond to stimuli.
The vestibular division of cranial nerve VIII originates from the bipolar cells of the vestibular ganglion located medial to the semicircular canals (Fig. 14-13; see Chapter 22). The central processes of these cells travel on the root of the nerve, traverse the internal acoustic meatus, enter the brainstem at the pons-medulla junction, and distribute centrally to the vestibular nuclei located in the medulla and caudal pons (Fig. 14-13). The internal acoustic meatus houses not only the vestibulocochlear nerve but also the facial nerve (including its intermediate part) and the labyrinthine artery. After receiving input from the ampullae of the semicircular canals and the utricle and saccule, the vestibular nuclei have important central connections to the cerebellum, to oculomotor nuclei (nuclei of cranial nerves VI, IV, and III), and to other brainstem centers related to balance, position sense, equilibrium, and the coordination of eye movements with head movements. (See also Chapter 22.)
Lesions of the vestibulocochlear nerve may result from a wide range of causes. The clinical manifestations include hearing loss, tinnitus, vertigo (subjective vertigo—the patient senses that he or she is moving; objective vertigo—the patient perceives that objects in the environment are moving), dizziness, and related problems such as ataxia. Injury to the cochlea, spinal ganglion, or cochlear fibers in cranial nerve VIII results in loss of hearing on the side of the lesion; this type of hearing loss is sensorineural hearing loss. Lesions within the brainstem (or at higher levels) may affect the patient’s ability to precisely interpret or to localize a sound in space, but such lesions do not result in deafness in one ear. Conductive hearing loss results from a failure of conduction through the middle ear, usually involving the ossicles. Tinnitus is a ringing, hissing, or roaring sensation perceived by the patient. It is related to the auditory part of cranial nerve VIII and may result from injury to the peripheral part of the nerve or may follow central lesions that damage auditory fibers or structures. Injury to the vestibular fibers of cranial nerve VIII results in vertigo (a perception of movement) and nystagmus that may be accompanied by nausea and vomiting. The vertigo may be subjective (the patient perceives that his or her body is moving) or objective (the patient perceives that objects in the environment are moving). Nystagmus (rhythmic oscillatory movements of the eyes) results from the interruption of vestibular influence over the brainstem motor neurons controlling eye movement, and the nausea and vomiting are a natural consequence of the disconnection between body or eye movements and the environment.
Lesions of the vestibular nuclei and their main central connections, especially the cerebellum, result in a sensation of ataxia and unsteady gait (the patient feels that his or her “balance is off”) and nystagmus. These signs and symptoms may range from mild to severe and may be accompanied by nausea and vomiting. Signs and symptoms of vestibular dysfunction may result from a wide range of causes, such as toxicity from certain medications, trauma, diabetes, cerebellar lesions, and acoustic neuroma. Meniere syndrome is characterized by hearing loss and sound distortion as well as by vertigo and a sensation of dizziness or unsteadiness on walking or standing. The cause is unknown, but there seems to be an increase in endolymphatic pressure with an increase in size of the utricle, saccule, and cochlear duct.
Lesions in the Cerebellopontine Angle
This could rightfully be called tumors of the cerebellopontine angle because most lesions found at this site are vestibular schwannomas (about 85%), meningiomas (5% to 10%), or epidermoid tumors (about 5%). Vestibular schwannomas are sometimes incorrectly called acoustic neuromas; this is incorrect because they are specifically tumors originating from Schwann cells of the vestibular root, not the “acoustic” root. The deficits associated with this specific tumor are initially tinnitus, unsteady gait, and progressive loss of hearing in that ear (as the tumor enlarges and impinges on the acoustic root), with a weakness of ipsilateral facial muscles appearing in later stages. If they are very large (usually more than 3 cm), these tumors may impinge on the root of the trigeminal nerve and also produce sensory deficits or pain sensations similar to tic douloureux.
Meningiomas, in contrast, may arise from the margins of the internal acoustic meatus (frequently the anterior or superior edges) and result in a facial weakness early followed later by hearing loss and pain related to involvement of the trigeminal root. Whereas a meningioma may erode and slightly enlarge the internal acoustic meatus, significant enlargement of this opening is a feature more commonly seen in the computed tomographic scan of patients with a vestibular schwannoma.
Epidermoid tumors (also called epidermoid cysts or cholesteatomas) arise from clusters of epidermis that are entrapped during development and give rise to a benign slow-growing lesion. These tumors are commonly lined with an epithelium and contain cellular debris, proteins, and cholesterol. Spillage of the cyst contents may cause recurrent bouts of aseptic meningitis. Although relatively rare, these lesions may be found anywhere in the central nervous system. When found at the cerebellopontine angle, they cause deficits reflecting damage to cranial nerves V, VII, and VIII.
Facial Nerve
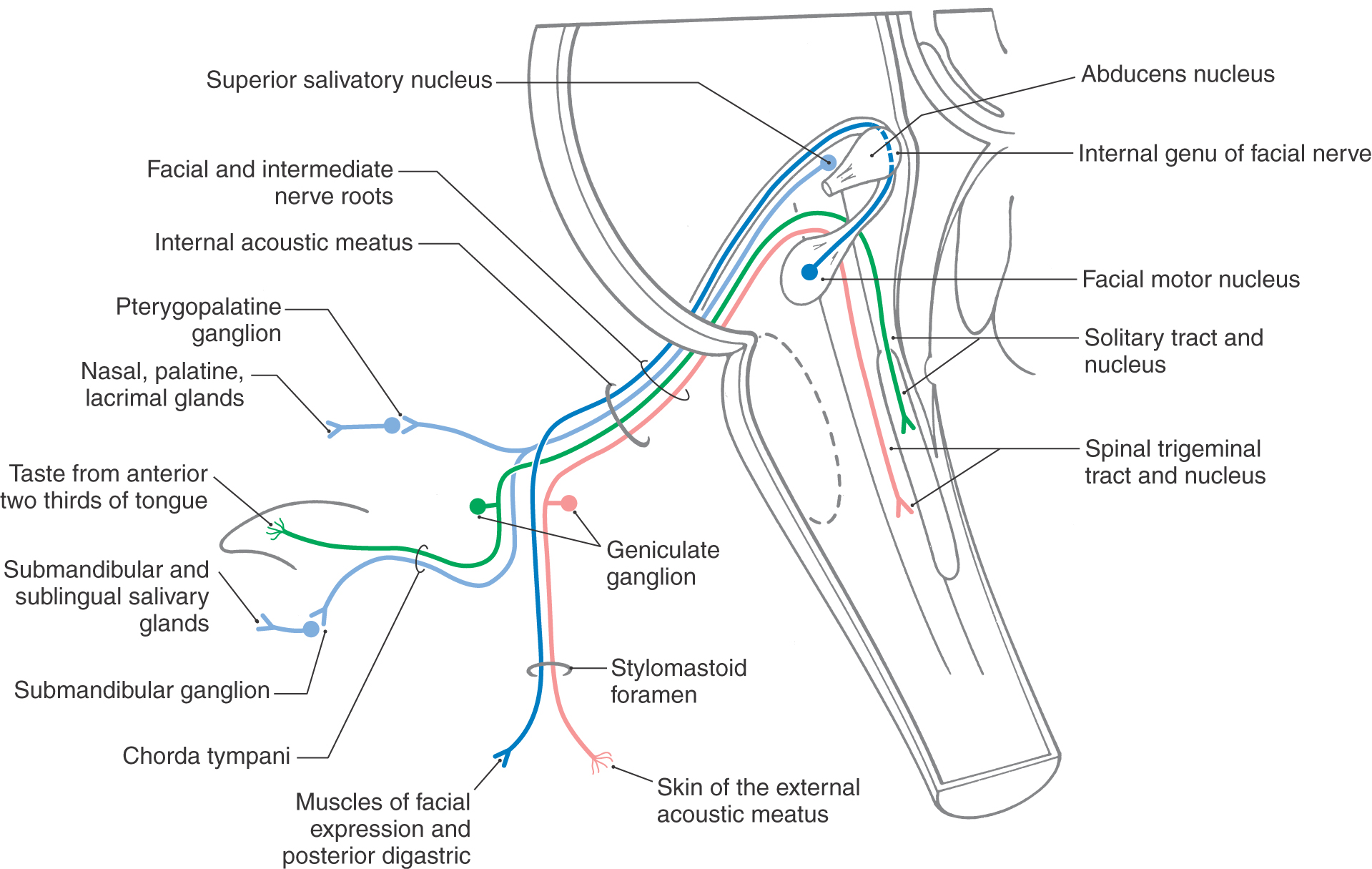
The facial motor nucleus is located in the pons just rostral to the pons-medulla transition (Figs. 14-1 and 14-14). Axons from the SE motor neurons of the facial nucleus course posteromedially to arch around the abducens nucleus from caudal to rostral before turning anterolaterally to exit the brainstem (Fig. 14-14). In their passage through the anterolateral pons, these fibers are joined by VE preganglionic parasympathetic axons from neurons of the superior salivatory nucleus. These VE fibers, along with VA taste fibers from the anterior two thirds of the tongue, SA fibers from the pinna, and a few VA fibers, form the intermediate nerve (Fig. 14-14). The facial nerve fibers and intermediate nerve fibers are intermingled within the pons but emerge from the brainstem as two separate nerve bundles. For all practical purposes, both of these nerve roots form what is commonly called the facial nerve; this nerve enters the internal acoustic meatus along with the vestibulocochlear nerve (Fig. 14-12).
After exiting the brainstem into the cerebellopontine angle, the facial and intermediate nerves merge and are joined by the vestibulocochlear nerve. These nerve trunks enter the petrous portion of the temporal bone through the internal acoustic meatus (Fig. 14-14) accompanied by the labyrinthine artery. Coursing posterolaterally through the facial canal in the petrous temporal bone, the facial nerve approaches the middle ear cavity, where it turns (internal genu) sharply posteriorly and continues superior and posterior to the middle ear cavity to eventually exit the temporal bone via the stylomastoid foramen. The geniculate ganglion (Fig. 14-14) of the facial nerve is located at the internal genu, and it is here that the greater petrosal nerve is formed by VE preganglionic parasympathetic fibers that leave the facial nerve. The greater petrosal nerve courses anteromedially through and on the petrous temporal bone to reach the area just superior to the foramen lacerum, where it joins the deep petrosal nerve to form the nerve of the pterygoid canal. From here, preganglionic parasympathetic axons enter the pterygopalatine ganglion. Postganglionic parasympathetic fibers from this ganglion supply the mucous membrane of the hard and soft palates, nasal cavity, and paranasal sinuses (Fig. 14-14). Other postganglionic parasympathetic fibers join the maxillary division (V2) of the trigeminal nerve and continue on branches that enter the orbit to provide parasympathetic innervation of the lacrimal gland.
En route through the temporal bone, the facial nerve gives off a small branch containing SE motor fibers that supply the stapedius muscle and a larger branch, the chorda tympani (Fig. 14-14). The latter nerve enters the middle ear cavity, passes across the inner surface of the tympanic membrane, and then exits the middle ear through the petrotympanic fissure to reach the infratemporal fossa. Here it joins the lingual branch of V3 to distribute preganglionic parasympathetic fibers to the submandibular ganglion and to collect VA taste afferent fibers from the anterior two thirds of the tongue (Fig. 14-14). Postganglionic fibers from the submandibular ganglion innervate the submandibular and sublingual salivary glands and portions of the mucosal surfaces of the tongue and oral cavity.
On exiting the stylomastoid foramen, the fibers of the facial nerve pass through and around the parotid gland as the nerve divides into its five terminal branches. Most of the fibers that remain in the facial nerve at this point provide motor innervation to the muscles of facial expression (Fig. 14-14), the posterior belly of the digastric muscle, and the stylohyoid muscle. In addition, the facial nerve contains a relatively small number of cutaneous fibers (SA) that supply portions of the external ear and external auditory canal.
The facial nerve includes two varieties of sensory fibers. The first, taste fibers (VA), pass centrally from the anterior two thirds of the tongue first on the lingual branch of V3. These fibers then leave the lingual nerve via the chorda tympani to join the facial nerve to reach their cell bodies in the geniculate ganglion (Fig. 14-14). The central processes of these primary afferent fibers continue with the facial nerve, pass into the cranial cavity through the internal acoustic meatus, and enter the brainstem in the intermediate nerve. These fibers enter the solitary tract and terminate in rostral portions of the solitary nucleus (gustatory nucleus), the central receiving area for all taste sensory signals (Fig. 14-2). In addition, there are a small number of VA fibers conveying sensations from the sublingual and submandibular salivary glands and mucous membranes of the palate and nasopharynx. These cell bodies are also located in the geniculate ganglion, and their fibers enter the more caudal portions of the solitary tract and nucleus (cardiorespiratory nucleus).
The second variety of sensory fibers in the facial nerve is relatively small in number. Cutaneous sensory fibers (SA) from regions of the external ear and external auditory canal course centrally on the facial nerve (Fig. 14-14). Moreover, it appears that the muscles of facial expression contain relatively few muscle spindles; therefore the contingent of muscle afferent fibers normally found within the SA population is small in the facial nerve. The cutaneous fibers reach their cell bodies in the geniculate ganglion and their central processes course into the brainstem with the intermediate nerve. In the brainstem, these SA fibers enter the spinal trigeminal tract and terminate in the spinal trigeminal nucleus (Fig. 14-14); these signals are transmitted rostrally to the thalamus or used in local reflex circuits.
Corticonuclear fibers used in the performance of voluntary movements involving the muscles of facial expression distribute to the facial nuclei in a bilateral manner. The ipsilateral face motor cortex distributes bilaterally to those facial motor neurons that control muscles in the upper face (i.e., frontalis, orbicularis oculi). In contrast, the face motor cortex projects only contralaterally to those facial motor neurons that control the lower facial expression muscles, such as those located near the angle of the mouth that are used to smile voluntarily. Lesions of the efferent fibers from the face motor cortex or of the internal capsule (supranuclear) result in drooping or sagging of the corner of the mouth contralateral to the lesion when the patient is asked to smile voluntarily. Such lesions result in deficits commonly referred to as a central seven lesion. Of interest, although some patients cannot smile when asked to do so by the neurologist, they can sometimes smile “involuntarily” or spontaneously in response to an amusing comment or situation.
Signs and symptoms due to peripheral lesions of the facial nerve (infranuclear; lower motor neuron) depend on the location of the damage. If the injury occurs proximal to the geniculate ganglion (Fig. 14-14) and the origin of the greater petrosal nerve, the patient exhibits a loss of voluntary control of ipsilateral facial expression muscles in the upper and lower portions of the face. This motor (the Bell palsy) deficit is accompanied by decreased mucosal secretion in the nasal and oral cavities and decreased tear fluid production and salivary gland output, all on the ipsilateral side. Cutaneous sensation of the external ear and external auditory canal is also diminished, but innervation of this territory is difficult to assess because cranial nerves IX and X contribute as well. In addition, there is decreased taste sensation (SVA) on the anterior two thirds of the tongue (but general sensation on the face, GSA, is preserved—why?) and hyperacusis on the side ipsilateral to the lesion.
If the lesion occurs distal to the ganglion (Fig. 14-14) but proximal to the origin of the chorda tympani and stapedial nerve, decreased salivation and taste and hyperacusis may be present in conjunction with decreased facial expression throughout the ipsilateral side of the face. However, tear fluid production and the mucosal surfaces of the nasal and oral cavities are unaffected because the greater petrosal nerve is intact. It follows, then, that decreased function of all facial expression muscles on one side of the face in combination with the absence of any deficits involving parasympathetic function or the sense of taste serves to localize the lesion at or distal to the stylomastoid foramen (Fig. 14-14).
The SE fibers originating in the facial nucleus also form the efferent limb of the corneal reflex. The afferent limb of this reflex travels via the ophthalmic division of cranial nerve V. The central processes of these fibers have their cell bodies in the trigeminal ganglion, enter the spinal trigeminal tract, and terminate in the spinal nucleus. Trigeminothalamic fibers originating in the spinal nucleus send collaterals into the facial motor nucleus and then continue rostrally to the thalamus; this collateral connection completes the reflex circuit.
Additional deficits reflecting involvement of the facial nerve are seen in facial diplegia and in hemifacial spasm. Bilateral paralysis of the facial muscles, facial diplegia, may be seen in congenital conditions, such as in myotonic muscular dystrophy or in the Möbius syndrome. In this latter syndrome, there are complex congenital defects that affect movements of the face and eyes (due to partial agenesis of these respective nuclei) and cause defects of the extremities and skeleton. The bilateral facial weakness in the Möbius syndrome frequently affects the upper portions of the face more than the lower portions or the whole face. Facial diplegia is also seen in patients with Lyme disease (characteristic of the disease), in those with the Guillain-Barré syndrome (about 50% of fatal cases will have this feature), or in patients who may have botulism poisoning or infection with Corynebacterium diphtheriae. Hemifacial spasms are irregular and sometimes painful contractions of the facial muscles that may be triggered by voluntary movements of the facial muscles. Whereas the etiology is unknown in some cases, hemifacial spasms may follow an episode of Bell palsy or result from compression of the root of the facial nerve by a lesion or vessel. In this latter case, aberrant branches of the anterior inferior cerebellar artery are the likely candidates.
Abducens Nerve
The abducens nucleus is located internal to the facial colliculus (Fig. 14-1). This structure, in turn, is found in the floor of the rhomboid fossa just lateral to the median sulcus and rostral to the striae medullares of the fourth ventricle. The abducens nucleus contains motor neurons and interneurons.
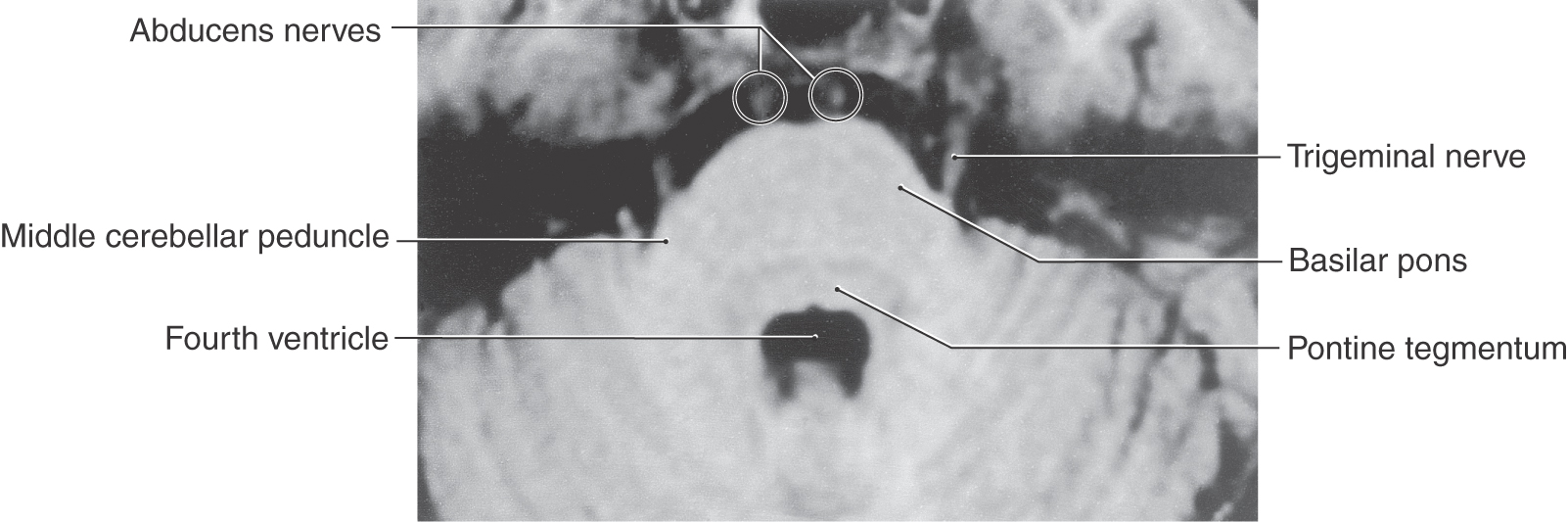
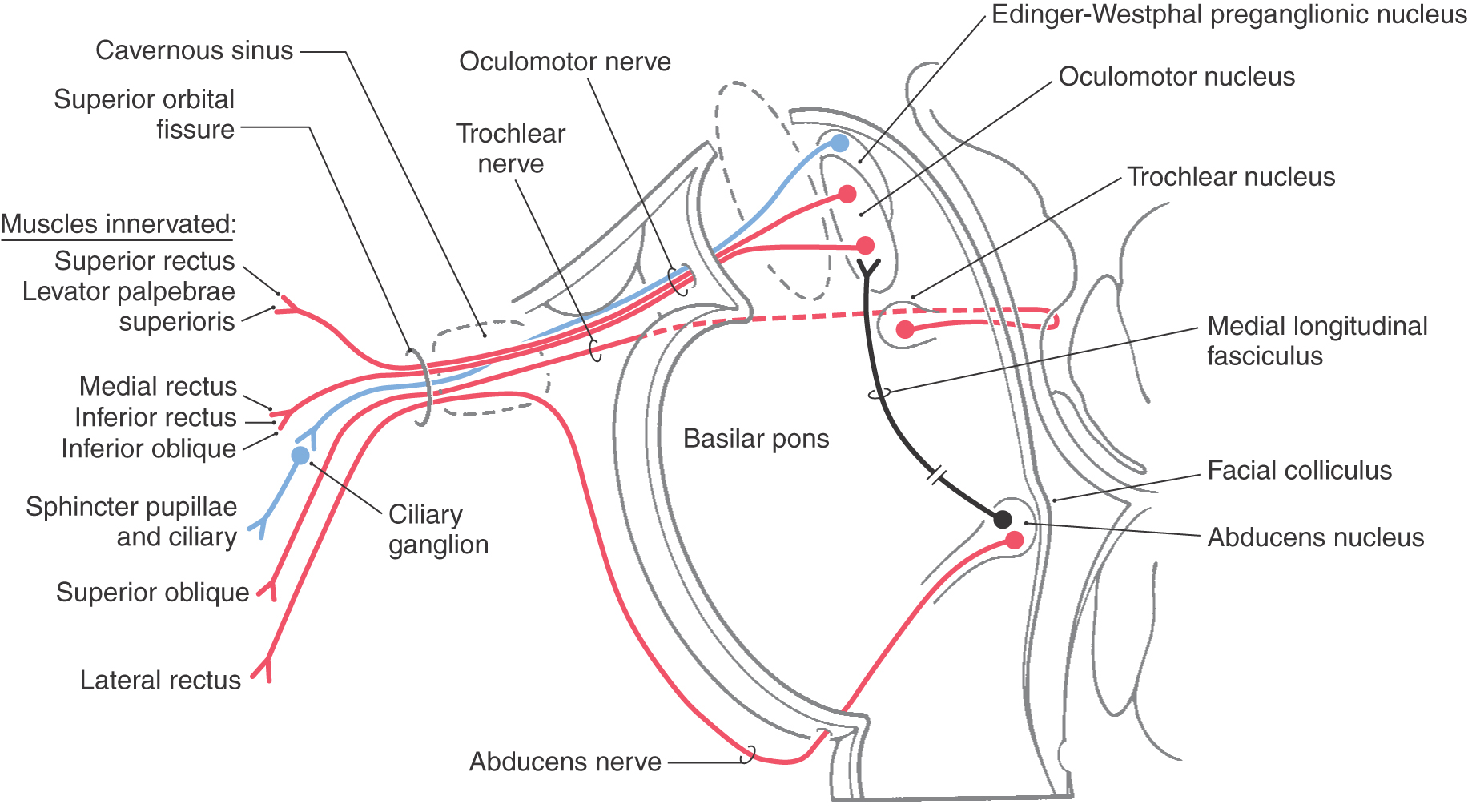
The axons of the SE motor neurons of the abducens nucleus course through the tegmental and basilar pons to exit at the pons-medulla junction generally in line with the preolivary sulcus as the abducens nerve (Figs. 14-3, 14-4, 14-10, 14-15, and 14-16). After the abducens nerve exits the brainstem, it courses rostrad along the surface of the basilar pons closely adjacent to the midline in the prepontine cistern (Figs. 14-15 and 14-16). These axons then enter and pass through the cavernous sinus in close association with the cavernous portion of the internal carotid artery (see Fig. 8-22) and enter the orbit via the superior orbital fissure. The abducens nerve innervates the ipsilateral lateral rectus muscle (Fig. 14-16).
The interneurons in the abducens send their axons into the contralateral medial longitudinal fasciculus, where they ascend to the oculomotor nucleus on that side (Fig. 14-16). These crossed ascending fibers form excitatory synapses on oculomotor neurons that innervate the medial rectus muscle on that side. The connection thus formed is such that when, for example, the patient looks to the right (in the horizontal plane), the right lateral rectus contracts (this eye abducts) and the left medial rectus contracts (this eye adducts). Lesions that involve this pathway lead to the clinical condition known as internuclear ophthalmoplegia.
Lesions of the abducens nerve, the abducens nucleus, or the internuclear axons in the medial longitudinal fasciculus have similar yet individually unique features. Injury to abducens fibers in the pons (as in a medial pontine syndrome) or in its course outside the brain results in a flaccid paralysis of the ipsilateral lateral rectus muscle; the affected eye is slightly introverted and does not abduct on attempted lateral gaze to that side. However, the opposite eye adducts because the internuclear neurons are intact.
A lesion of the abducens nucleus, such as a fourth ventricle tumor invading the facial colliculus, damages both the motor neurons and the internuclear neurons. The result is paralysis of the lateral rectus muscle on the side of the lesion accompanied by failure of the opposite medial rectus muscle to contract on attempted gaze toward the side of the lesion. This particular example combines a lower motor neuron lesion of the lateral rectus on the side of the lesion with an internuclear ophthalmoplegia—a paralysis of the medial rectus on the opposite side seen in attempted gaze toward the side of the lesion.
Damage only to internuclear axons in the left medial longitudinal fasciculus (as in multiple sclerosis) results in an inability to adduct the left eye on attempted gaze to the right. In contrast, with lesions of the left abducens nucleus or nerve, the right eye can abduct because the motor neurons in the right nucleus (and their axons) are intact. An extension of a lesion involving the abducens nucleus (resulting in weakness of the ipsilateral lateral rectus muscle and the contralateral medial rectus muscle) is seen in a lesion that involves the abducens nucleus and fibers of the immediately adjacent medial longitudinal fasciculus, resulting in the one-and-a-half syndrome.
One important source of cerebral cortical influence over abducens motor neurons originates from cells in the frontal eye field. This area of cortex projects bilaterally to the paramedian pontine reticular formation (PPRF), also called the horizontal gaze center, and to the ipsilateral superior colliculus, which in turn projects to the contralateral PPRF. The PPRF, in turn, projects to the ipsilateral abducens nucleus. Sudden cortical damage (such as stroke or trauma) involving the frontal eye field results in an involuntary conjugate deviation of the eyes to the side of the lesion.
CRANIAL NERVE OF THE PONS
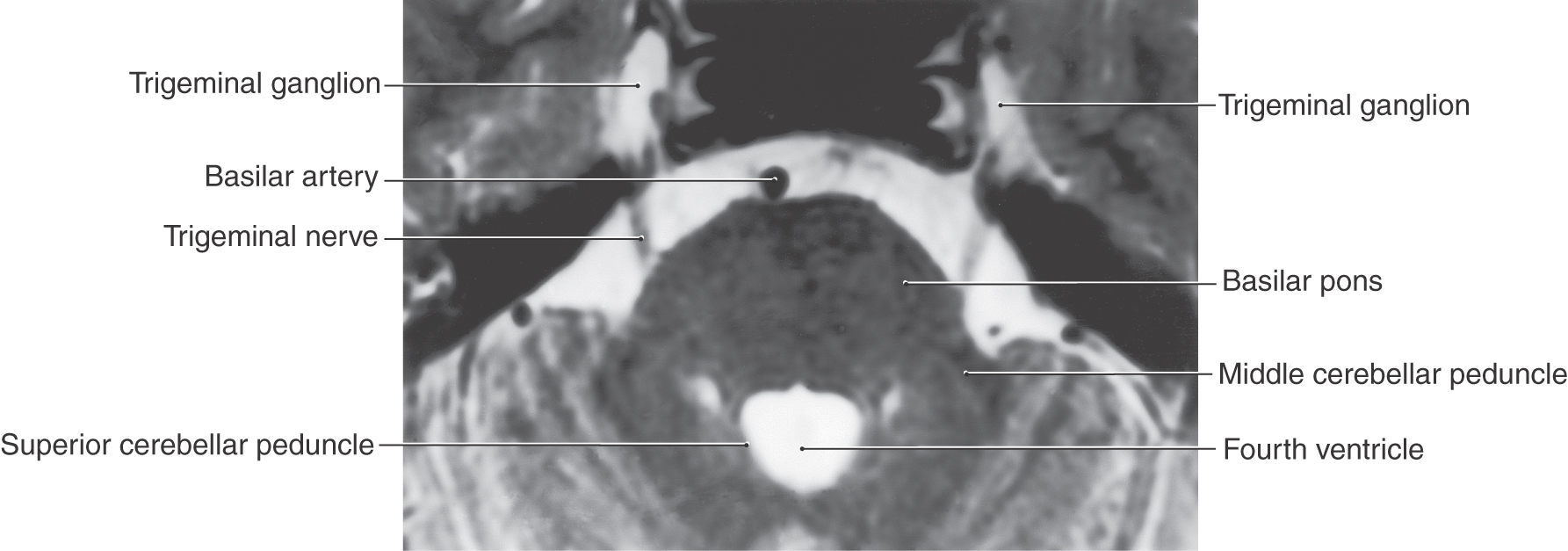
The largest of the cranial nerves of the brainstem, the trigeminal nerve, exits the lateral aspect of the pons (Figs. 14-3, 14-4, and 14-17). It consists of a large sensory root (portio major), which is attached to the trigeminal ganglion, and a small motor root (portio minor), which bypasses the ganglion (Fig. 14-18). The exit point of this nerve is also the border between the basilar pons and the middle cerebellar peduncle, which are located anterior and posterior, respectively, to the nerve roots (Fig. 14-17).
The trigeminal nerve consists of ophthalmic (V1), maxillary (V2), and mandibular (V3) divisions; these are the large sensory branches originating from the trigeminal ganglion (Fig. 14-18). These branches serve the upper eyelid, forehead, and scalp to a point just beyond the vertex of the skull (V1); the lower eyelid, the upper lip and teeth, the maxillary aspect of the face, and a lateral strip in the temporal area (V2); and the lower lip and teeth, much of the oral cavity, and the skin over the mandible and continuing as a band up the lateral side of the head rostral to the ear (V3) (see Chapter 18). The ophthalmic division exits the cranial cavity via the superior orbital fissure (Fig. 14-18) and distributes to the orbit, with some branches exiting the orbit through the supraorbital notch (or foramen). The maxillary division exits the cranial cavity through the foramen rotundum (Fig. 14-18) and then immediately passes through the pterygopalatine fossa and the inferior orbital fissure into the orbit; some of its branches exit the orbit via the infraorbital foramen onto the maxillary region of the face. The mandibular division exits the cranial cavity through the foramen ovale (Fig. 14-18), as does the motor root of the fifth nerve.
Trigeminal Nerve
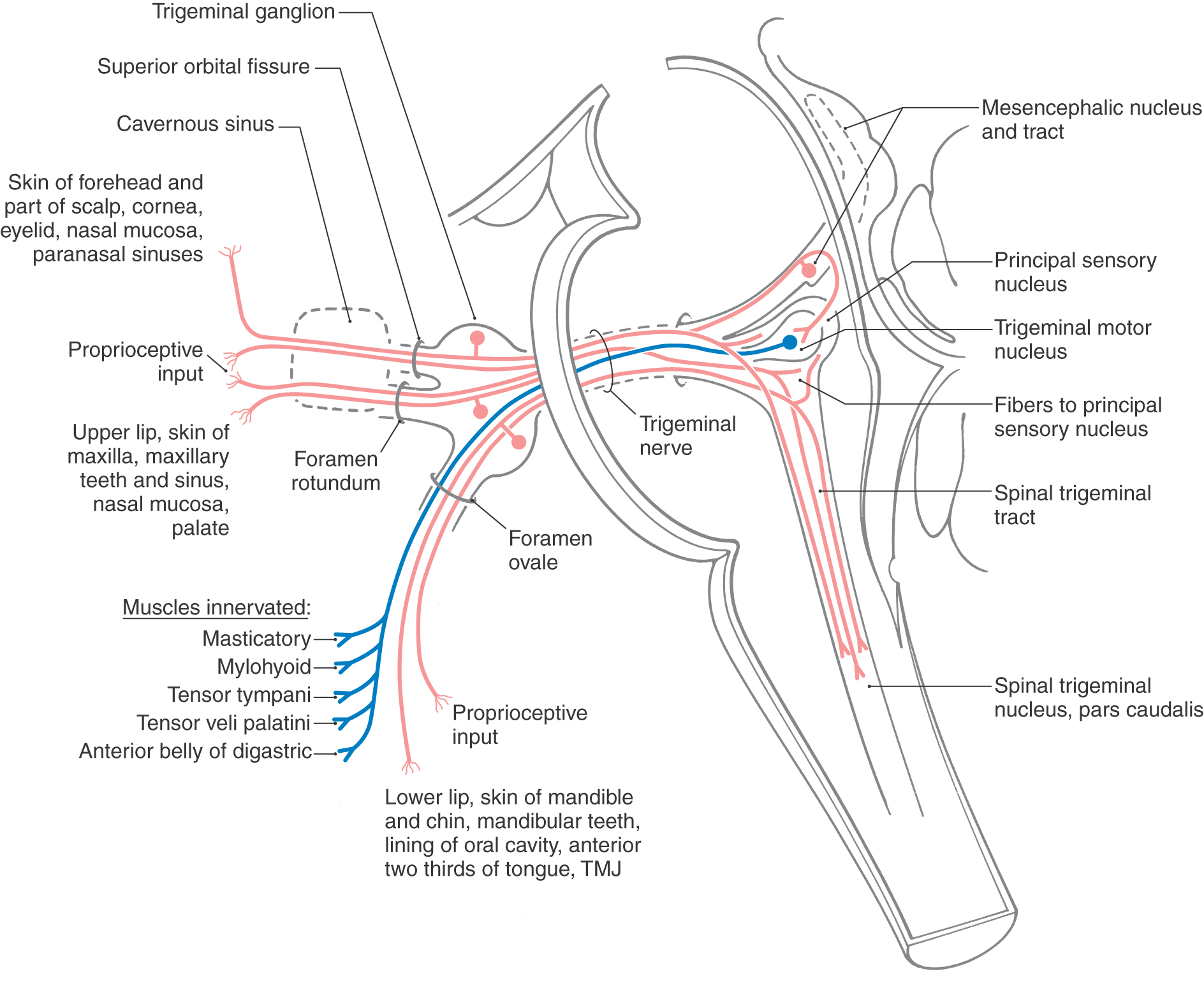
The trigeminal nerve is a mixed nerve, having sensory and motor components. The three sensory nuclei form a continuous cell column that extends from the spinal cord–medulla junction to rostral levels of the mesencephalon. The motor nucleus is located medially adjacent to the principal sensory nucleus at about midpontine levels.
The sensory nuclei of the trigeminal nerve are the spinal trigeminal nucleus, extending from caudal to rostral throughout the lateral medulla and into the caudal pons; the principal sensory nucleus, located in the lateral parts of the pontine tegmentum at about midpontine levels at the rostral end of the spinal nucleus; and the mesencephalic nucleus and mesencephalic tract, which extend rostrally from the principal sensory nucleus into the midbrain along the lateral aspect of the periaqueductal gray (Fig. 14-18). The spinal trigeminal nucleus consists of a pars caudalis (from the spinal cord–medulla junction to the obex), a pars interpolaris (from the obex to the rostral end of the hypoglossal nucleus), and the pars oralis (to the caudal aspect of the principal sensory nucleus).
The trigeminal nerve, along with small contributions from cranial nerves VII, IX, and X, conveys sensory input from the entire face from the posterior border of the mandibular ramus to just caudal to the vertex of the head, the external ear, the cornea and conjunctiva, the mucosa of the nasal cavity and sinuses, the upper and lower teeth, the lining of the oral cavity including the tongue, and the lining of the pharynx and larynx (cranial nerves IX and X) and proprioceptive inputs from the muscles of mastication and extraocular muscles. The sensory information relayed on the fifth nerve is concerned with pain and thermal sensations, discriminative sensations, and proprioception.
Primary afferent fibers conveying pain, thermal sense, and nondiscriminative touch (SA exteroception) from the head have their cell bodies in the trigeminal ganglion and in the geniculate ganglion (cranial nerve VII) and superior ganglia of cranial nerves IX and X (Figs. 14-7, 14-11, 14-14, and 14-18). Their central processes form the spinal trigeminal tract and terminate in the medially adjacent nucleus. Fibers conveying discriminative touch from the face and oral cavity (SA exteroception) follow a similar trajectory, but centrally these fibers terminate in the principal sensory nucleus. Those fibers conveying proprioceptive information from the masticatory muscles, extraocular muscles, and periodontal ligament receptors enter the brainstem via the trigeminal nerve. However, in clear contrast to other primary sensory fibers, these fibers have their cell bodies in the mesencephalic nucleus, not in the trigeminal ganglion. Sensory inputs to the spinal trigeminal nucleus and to the principal sensory nucleus are relayed to the thalamus via the anterior and posterior trigeminothalamic tracts and from the thalamus to the somatosensory cortex, where the sensations are perceived and interpreted.
The motor nucleus of the trigeminal nerve innervates muscles that originate from paraxial mesoderm that migrated into the first pharyngeal arch, hence their SE functional component. The motor root of the trigeminal nerve exits the skull via the foramen ovale, along with V3 (Fig. 14-18). These motor neurons innervate the muscles of mastication (including the medial and lateral pterygoid muscles), the tensor tympani, the tensor veli palatini, the mylohyoid, and the anterior belly of the digastric, all on the ipsilateral side (Fig. 14-18). The motor nucleus receives afferent fibers involved in the jaw jerk reflex.
The most commonly tested reflexes that are associated with the trigeminal nerve are the jaw jerk reflex and the corneal reflex. The afferent limb of the jaw jerk reflex originates at receptors in the muscles of mastication, courses centrally on the mandibular division, and has its cell bodies in the mesencephalic nucleus. Collaterals from these afferent fibers project bilaterally to the trigeminal motor nucleus, the neurons of which give rise to the efferent limb of the reflex. The afferent limb of the corneal reflex originates from pain and touch receptors in the cornea. These fibers travel on the ophthalmic division of cranial nerve V, have their cell bodies in the trigeminal ganglion, and terminate centrally in the ipsilateral spinal trigeminal nucleus. Trigeminothalamic fibers en route to the thalamus send collaterals bilaterally into the facial motor nucleus, which is the origin of the efferent limb of this reflex; in response to a stimulus that touches the cornea, the eyes blink.
The primary deficits in patients with lesions of the trigeminal nerve or its central nuclei are sensory symptoms in the peripheral distribution of the nerve (or its individual division) and paralysis of the masticatory muscles. The sensory deficits are a complete loss of pain, thermal, and tactile sensations on the ipsilateral side of the face and much of the scalp; a loss of the same sensations in the oral cavity (and from the teeth) on the same side; and a loss of the afferent limb of the corneal reflex occurring in response to corneal touch, also on the same side. However, because the efferent limb of the corneal reflex travels via the facial nerve, touching the cornea on the side opposite a trigeminal root lesion results in a blink on that side as well as a blink on the side of the root lesion. An injury to one trigeminal nerve does not necessarily cause a loss of the jaw jerk reflex.
Another sensory disorder associated with the trigeminal nerve is tic douloureux (trigeminal neuralgia). This condition is characterized by severe, unexpected, lancinating pain restricted to one or more of the divisions of the nerve. Paroxysms of intense pain may result from stimulation of a trigger zone frequently located around the lip or nose or on the cheek. A wide variety of stimuli, such as shaving, putting on makeup, talking, chewing, eating, or a breeze on the face, or a sudden facial expression may precipitate an attack. Indeed, some patients may become malnourished because they avoid eating, chewing, and swallowing for fear of an attack. Trigeminal neuralgia is seen more frequently in patients older than 35 years, usually involves the maxillary division more often than the mandibular (second most commonly involved) or ophthalmic (rarely involved) division, and can severely compromise the quality of life. Trigeminal neuralgia may be seen in patients with multiple sclerosis, degenerative changes in the trigeminal ganglion, or vascular malformations. Compression of the trigeminal nerve root by aberrant vessels, usually branches of the superior cerebellar artery, is one known cause of trigeminal neuralgia. Having said this, it is also known that at autopsy some patients have what appears to be compression of the trigeminal root but did not exhibit symptoms of trigeminal neuralgia.
Motor deficits resulting from trigeminal root lesions result in paralysis of the masticatory muscles on that side. The patient has difficulty chewing food, and the jaw deviates toward the side of the lesion (the weak side) on closing. This deviation occurs because of the unopposed action (pull of the mandible toward the midline) of the pterygoid muscles on the opposite (undamaged) side. Although it is relatively rare, some patients with trigeminal neuralgia also experience status trigeminus, which are tic-like and frequently painful contractions of the masticatory muscles.
Sensory or motor deficits related to the trigeminal nerve can also occur from central lesions. Such lesions include but are not limited to tumors or vascular lesions in the medulla or pons, metastatic lesions, and the obstruction of vessels. A well-known example of a lesion due to vascular obstruction is the lateral medullary syndrome (also called the posterior inferior cerebellar artery syndrome or Wallenberg syndrome), in which there is an alternating sensory loss. This lesion (see Chapter 18) results in an ipsilateral loss of pain and thermal sense on the face (spinal trigeminal tract involvement) and a contralateral loss of the same sensations on the body (anterolateral system involvement), in concert with motor deficits most frequently related to damage to the nucleus ambiguus or vestibular nuclei.
CRANIAL NERVES OF THE MIDBRAIN
The cranial nerves of the midbrain are the trochlear nerve (cranial nerve IV) and the oculomotor nerve (cranial nerve III) (Figs. 14-3 and 14-4). The nuclei of both nerves are located adjacent to the midline just ventral to the periaqueductal gray, both nerves are exclusively motor, and, like other exclusively motor nerves, they exit the midbrain just lateral to the midline. These two nerves, along with cranial nerve VI, innervate extraocular muscles.
Trochlear Nerve
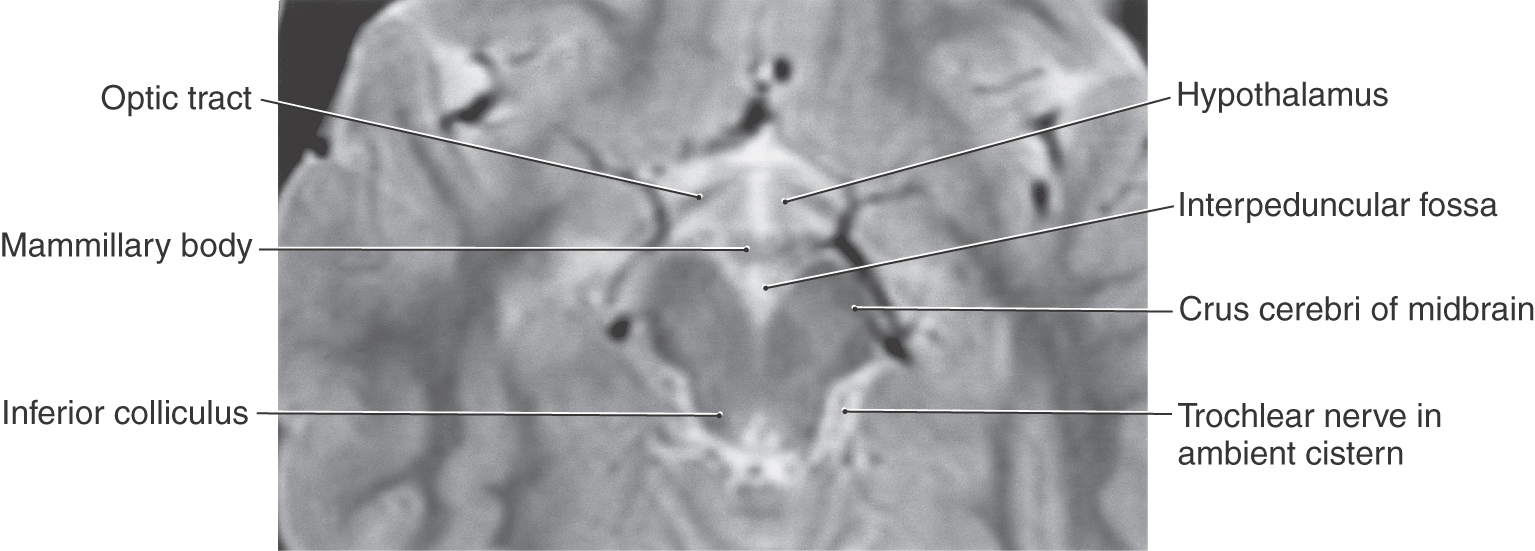
The trochlear nerve is the only motor cranial nerve formed entirely by axons that cross the midline before their exit. For example, axons arising from the left trochlear nucleus arch caudally along the lateral edge of the periaqueductal gray to decussate in the most anterior aspect of the anterior medullary velum and then exit the brainstem immediately caudal to the right inferior colliculus (see Chapter 10 and 28).
The trochlear nucleus is situated posteriorly adjacent to the medial longitudinal fasciculus (MLF) at a cross-sectional level through the inferior colliculus (Fig. 14-16). After arching around the periaqueductal gray, decussating, and exiting from the posterior surface of the midbrain, the axons of these GSE motor neurons have a long intracranial course. They exit into the superior cistern and then course laterally and anteriorly through the ambient cistern on the lateral aspect of the midbrain (Fig. 14-19). From the subarachnoid space, the nerve then enters the overlying dura, courses rostrally within the lateral wall of the cavernous sinus, and enters the orbit via the superior orbital fissure (Fig. 14-16). Cranial nerve IV innervates the superior oblique muscle, which normally functions to direct the eye inferolaterally, that is, downward and outward.
Figure 14-19. Axial T2-weighted magnetic resonance image showing the position of the trochlear nerve in the ambient cistern.
It is important to remember that trochlear motor neurons innervate the contralateral superior oblique muscle as the clinical findings may reflect this innervation. For example, a lesion at the root of the nerve in the ambient cistern or cavernous sinus, or at the superior orbital fissure, results in paralysis of the superior oblique muscle on that side. If the lesion is on the left side, the left eye cannot rotate slightly downward and outward. On the other hand, in a patient with multiple sclerosis involving the MLF, the damage to which has extended to the trochlear nucleus, a variation on this theme may be seen. In this situation, a lesion in the right MLF with trochlear nucleus involvement results in paralysis of the left superior oblique muscle, and the left eye cannot rotate downward and outward to the left. In addition, and in clear contrast to the trochlear nerve root lesion, the patient also has internuclear ophthalmoplegia on the right; on attempted lateral gaze to the left, the right medial rectus muscle does not adduct the eye on that side.
An important source of cerebral cortical input to the trochlear nucleus is from neurons located in the frontal eye field. These projections pass to the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF), also called the vertical gaze center, and to the superior colliculus, which also projects to the riMLF. The riMLF sends a large projection to the ipsilateral trochlear nucleus and a smaller projection to the nucleus on the contralateral side. Sudden cortical damage (such as from stroke or trauma) involving the frontal eye field results in an involuntary conjugate deviation of the eyes to the side of the lesion.
Oculomotor Nerve
The oculomotor nucleus is located within the ventral portion of the periaqueductal gray just posterior to the medial longitudinal fasciculus and is present in about the rostral half of the midbrain (Fig. 14-16). It is divided into several smaller subnuclei that contain SE motor neurons that innervate all the extraocular muscles except the superior oblique and lateral rectus. This innervation involves ipsilateral muscles except for the superior rectus motor neurons, whose axons decussate within the nucleus to enter the contralateral oculomotor nerve. Although seemingly significant, this crossed pathway is typically ignored in the clinical setting because the effect of losing the innervation to the (contralateral) superior rectus muscle is usually masked by the actions of the functionally intact muscles in that orbit.
Also located in the midbrain periaqueductal gray matter posterior to the oculomotor complex are the Edinger-Westphal cell groups (Fig. 14-16). These consist of the Edinger-Westphal centrally projecting nucleus (EWcp), which projects to the brainstem and spinal centers involved in behavioral aspects of stress, eating, and drinking, and the Edinger–Westphal preganglionic nucleus (EWpg), whose axons provide preganglionic parasympathetic fibers that reach the ciliary ganglion via the oculomotor nerve.
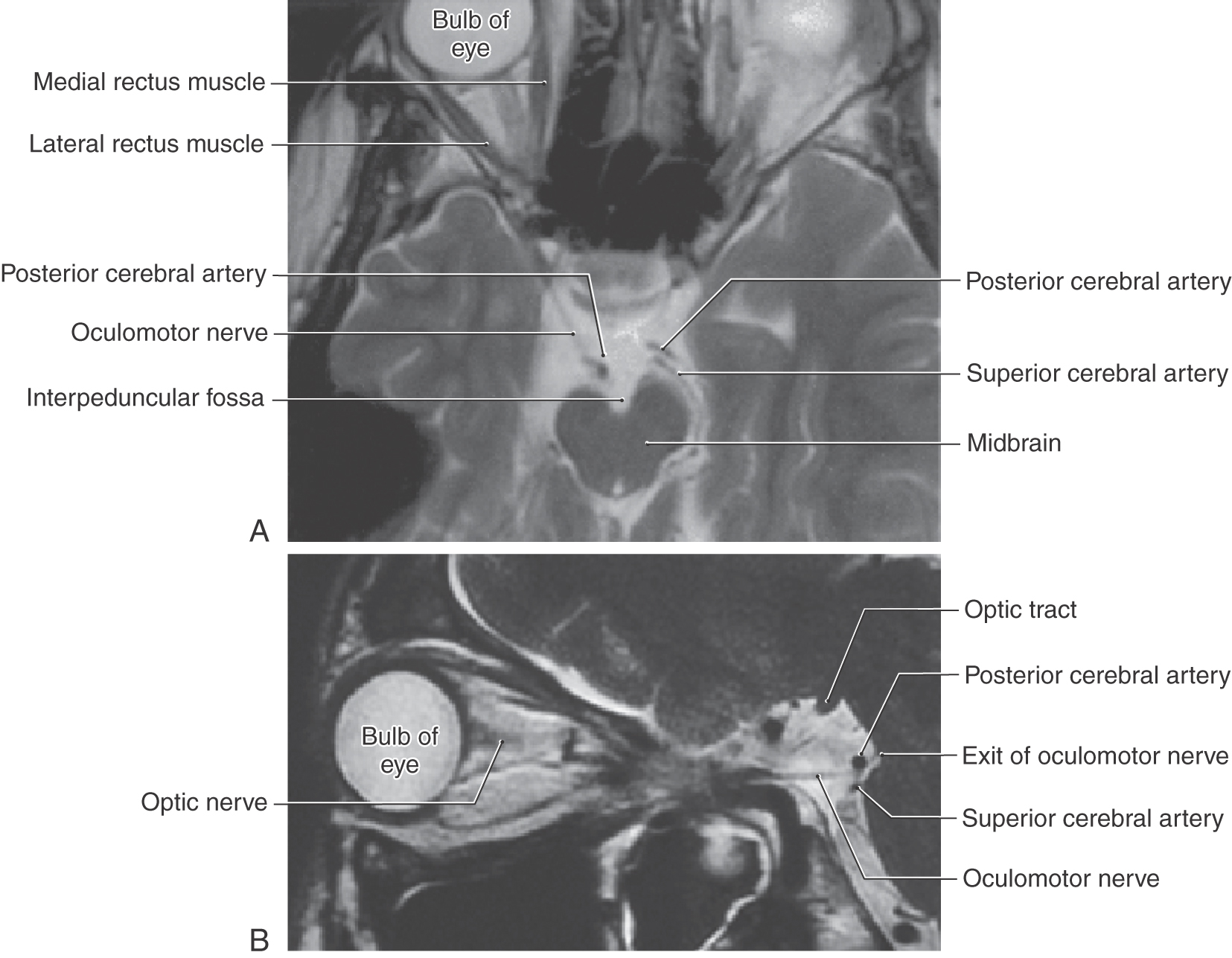
Oculomotor nerve fibers pass anteriorly through and around the red nucleus to eventually exit the midbrain in the interpeduncular fossa (Fig. 14-20). Emerging from the midbrain, the nerve passes between the posterior cerebral and superior cerebellar arteries, enters the interpeduncular cistern, and then penetrates the dura lateral to the sella turcica to course within the lateral wall of the cavernous sinus (see Chapter 8). The nerve then exits the dura and passes through the superior orbital fissure, along with cranial nerves IV, VI, and V1, to enter the orbit (Figs. 14-16 and 14-18). In the orbit, the oculomotor nerve divides into superior and inferior divisions, each division forming a few small communicating branches to the ciliary ganglion in addition to their muscle branches. The ciliary ganglion then gives off several small, short ciliary nerves, which reach the posterior aspect of the globe of the eye, where they penetrate the sclera and course anteriorly to reach the sphincter pupillae and ciliary muscles (Fig. 14-16).
There are four smooth muscles related to each orbit that require visceromotor innervation. The VE fibers in the oculomotor nerve enter the ciliary ganglion, the postganglionic parasympathetic fibers of which innervate the sphincter pupillae and ciliary muscles. In contrast, the dilator pupillae muscle and the superior tarsal muscle are activated by sympathetic innervation. Postganglionic sympathetic fibers exit the superior cervical ganglion and course, via the internal carotid plexus, to join the ophthalmic artery. Coursing into the orbit with the latter artery via the optic canal, sympathetic postganglionic fibers may join the ciliary ganglion directly, may join the nasociliary nerve (a branch of V2 from which the long ciliary branches originate), or may join the oculomotor nerve and then enter the ciliary ganglion. Once in the ciliary ganglion, the sympathetic fibers continue, without synapsing, into the short ciliary nerves to reach the dilator pupillae muscle. Some of the sympathetic postganglionic fibers that travel via the oculomotor nerve continue beyond the ciliary ganglion with the levator palpebrae superioris muscle to reach the superior tarsal muscle.
Although it is known that the extraocular muscles contain muscle spindles, the oculomotor, trochlear, and abducens nerves are regarded as purely motor nerves. The spindle afferent fibers appear to join sensory nerves in the orbit, such as the frontal and nasociliary nerves, and eventually pass through V1 to reach their cell bodies in the trigeminal ganglion. From here, sensory information enters the brainstem via the sensory root of the trigeminal nerve.
The oculomotor nucleus does not receive direct cortical projections via the corticonuclear system. The cortex of the frontal eye field exerts its control over oculomotor neurons via projections to the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF, the vertical gaze center) and the superior colliculus. This part of the tectum also projects to the riMLF, which in turn sends numerous fibers to the ipsilateral oculomotor nucleus and a smaller contingent to the contralateral side. Consequently, cortical and capsular lesions have an effect on the actions of muscles innervated by the oculomotor nerve, but this effect is indirect and results from the loss of cortical input to the brainstem gaze control centers. Cortical damage (such as from stroke or trauma) involving the frontal eye fields produces an involuntary conjugate deviation of the eyes toward the side of the injury. One easy way to remember this correlate is that the patient “involuntarily looks to the lesioned side.” If the cortical lesion is large enough to involve corticospinal fibers, the deviation of the eyes is toward the side of the cortical damage but away from the side of the resulting hemiparesis.
Lesions involving the oculomotor nucleus, the oculomotor nerve in the interpeduncular cistern, or the nerve in the lateral wall of the cavernous sinus all generally have the same result. Loss of the SE motor fibers paralyzes all of the extraocular muscles in the ipsilateral orbit except the superior oblique and lateral rectus muscles. As a result, the ipsilateral eye assumes an abducted and depressed position (down and out) owing to the unopposed action of the lateral rectus and superior oblique muscles. The patient also experiences diplopia (double vision) because the image seen by each eye cannot be directed to corresponding portions of each retina because most of the extraocular muscles in one eye are not functional. Furthermore, interruption of the preganglionic parasympathetic fibers in the oculomotor nerve results in characteristic signs and symptoms in the ipsilateral eye. First, the pupil is dilated (mydriasis) and nonreactive to light because the sphincter pupillae muscle is denervated (the dilator pupillae muscle, innervated by sympathetic fibers, is intact). Second, the lens in the ipsilateral eye cannot accommodate because the ciliary muscle is also denervated. Third, although the innervation to the superior tarsal muscle is intact because the course of the sympathetic fibers does not involve the oculomotor nerve outside the orbit, the upper eyelid exhibits ptosis (droop) because the levator palpebrae has been denervated by the oculomotor nerve lesion.
Because the parasympathetic fibers are located near the periphery (outer surface) of the oculomotor nerve, visceromotor signs and symptoms, such as a subtle ptosis or mildly diminished pupil reactivity, can appear before the onset of or in the absence of any extraocular muscle dysfunction with external compressive injury to the oculomotor nerve. The external compression affects the superficially located, smaller diameter visceromotor fibers first. In contrast, in diabetic patients, the onset of an eye movement disorder may not be accompanied by visceromotor signs or symptoms. This is because diabetes is a vascular problem and the larger vessels inside the nerve are compromised first, thereby affecting the internally located, larger diameter GSE motor axons but sparing the smaller and more superficially located visceromotor fibers. Isolated lesions of the oculomotor nerve distal to its passage through the superior orbital fissure are relatively rare and produce variable symptoms, depending on the location of the lesion.
Sources and Additional Reading
Carpenter MB, Sutin J. Human Neuroanatomy. ed 8 Baltimore: Williams & Wilkins; 1983.
Greenberg MS. Handbook of Neurosurgery. ed 5 New York: Thieme; 2001.
Haerer AF. DeJong’s The Neurologic Examination. Philadelphia: JB Lippincott; 1993.
LeBlanc A. The Cranial Nerves: Anatomy, Imaging, Vascularisation. New York: Springer-Verlag; 1995.
Parent A. Carpenter’s Human Neuroanatomy. ed 9 Baltimore: Williams & Wilkins; 1996.
Pryse-Phillips W. Companion to Clinical Neurology. ed 2 New York: Oxford University Press; 2003.
Rowland LP. Merritt’s Neurology. ed 10 Philadelphia: Lippincott Williams & Wilkins; 2000.
Victor M, Ropper AH. Adams and Victor’s Principles of Neurology. ed 7 New York: McGraw-Hill; 2001.