Chapter 8
A Survey of the Cerebrovascular System
Cerebral Arteries and Watershed Infarcts
Veins and Venous Sinuses of the Brain
Internal Veins of the Hemisphere
About 50% of the problems that occur inside the cranial cavity and result in neurologic deficits are vascular in origin. Consequently, a detailed understanding of cerebrovascular patterns is absolutely essential to establish an accurate diagnosis of the neurologically compromised patient. The brain is a voracious consumer of oxygen and therefore requires a great deal of oxygenated blood. Although it makes up only about 2% of total body weight in adults, the brain receives 15% to 17% of the total cardiac output and consumes about 20% of the oxygen used by the entire body.
An ongoing flow of oxygenated blood is essential for continued brain function. The average person will lose consciousness if the brain is deprived of blood for 10 to 12 seconds; after 3 to 5 minutes, irreparable brain damage or death may result. There are exceptions, however. Individuals who become hypothermic with a subsequent decrease in arterial blood flow to the brain, as in a winter near-drowning, may be revived after 10, 15, or even 20 minutes with little or no permanent damage. In these cases, the reduction in body temperature protects the brain against the consequences of reduced blood flow.
OVERVIEW
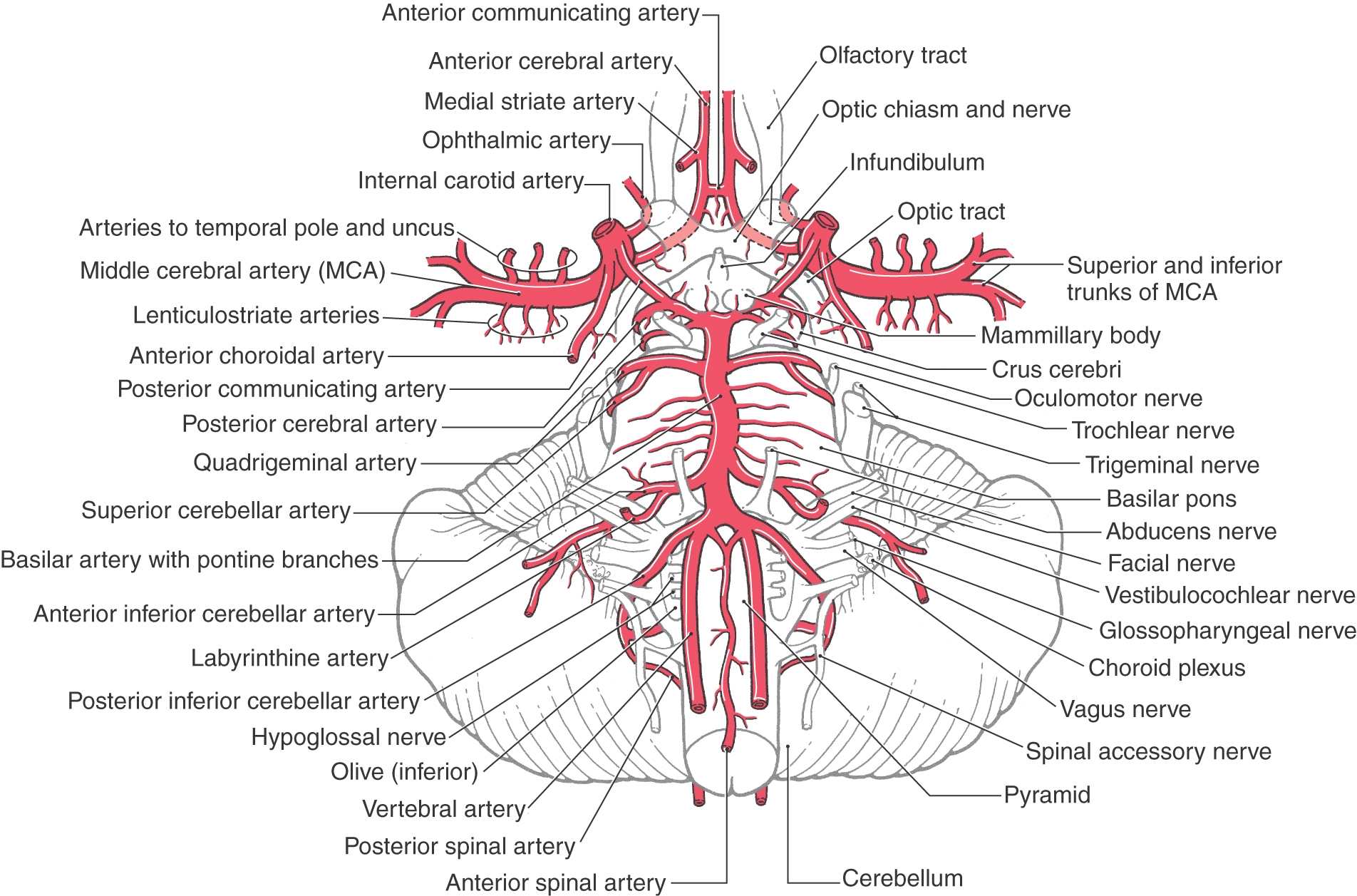
Blood is supplied to the brain by the internal carotid and vertebral arteries. The internal carotid arteries enter the skull and then divide into the anterior and middle cerebral arteries. The vertebral arteries pass through the foramen magnum and join to form the basilar artery; hence, the term vertebrobasilar is frequently applied to this part of the cerebral circulation. The basilar artery branches into right and left posterior cerebral arteries.
Venous outflow from the brain travels through superficial and deep veins, which drain into the dural venous sinuses. Blood in the sinuses, in turn, enters the internal jugular vein. Superficial veins in the scalp and veins in the orbit also may communicate with the dural sinuses, but these are not major conduits for venous drainage from the brain.
This chapter presents an overview of the cerebrovascular system, with particular emphasis on the distribution pattern of vessels on the surface of the brain and spinal cord. Details of the distribution of blood vessels to internal structures are covered in later chapters.
CAUSES OF VASCULAR COMPROMISE
Intracranial hemorrhage originates from arteries or veins and may result from diseases, trauma, developmental defects, or infections. Such bleeding is classified according to its location. Meningeal hemorrhages are found in relation to the coverings of the brain, whereas bleeding into the subarachnoid space is called subarachnoid hemorrhage. Hemorrhage may occur into the ventricular spaces (intraventricular) or into the substance of the brain (parenchymatous). Although many events can lead to cerebrovascular problems with resultant dysfunction, only three examples—aneurysm, cerebral embolism, and arteriovenous malformation—are considered here.
Aneurysm
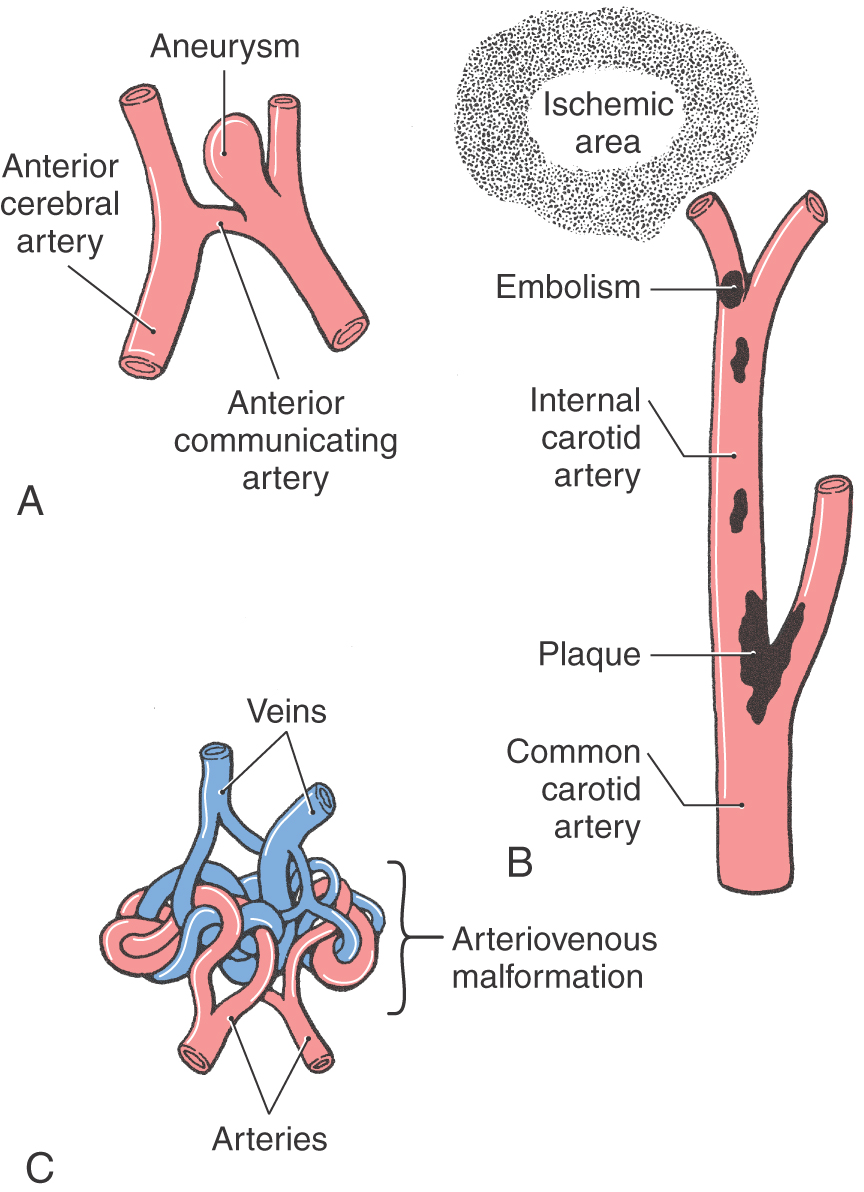
An aneurysm is the dilation of a vessel wall (Fig. 8-1A), usually an artery, that extends from the lumen to the vessel surface. Aneurysms located inside the skull, cerebral aneurysms, may range from small (berry or saccular aneurysms) to very large (giant aneurysms are more than 2 cm in diameter), or they may involve an elongated portion of the vessel (fusiform aneurysm). Larger aneurysms may cause signs or symptoms by compression of adjacent structures such as cranial nerve roots. The second most common cause of blood in the subarachnoid space (subarachnoid hemorrhage) is rupture of an aneurysm, the first most common cause being trauma.
Figure 8-1. Representation of an aneurysm (A), an embolism (B), and an arteriovenous malformation (C).
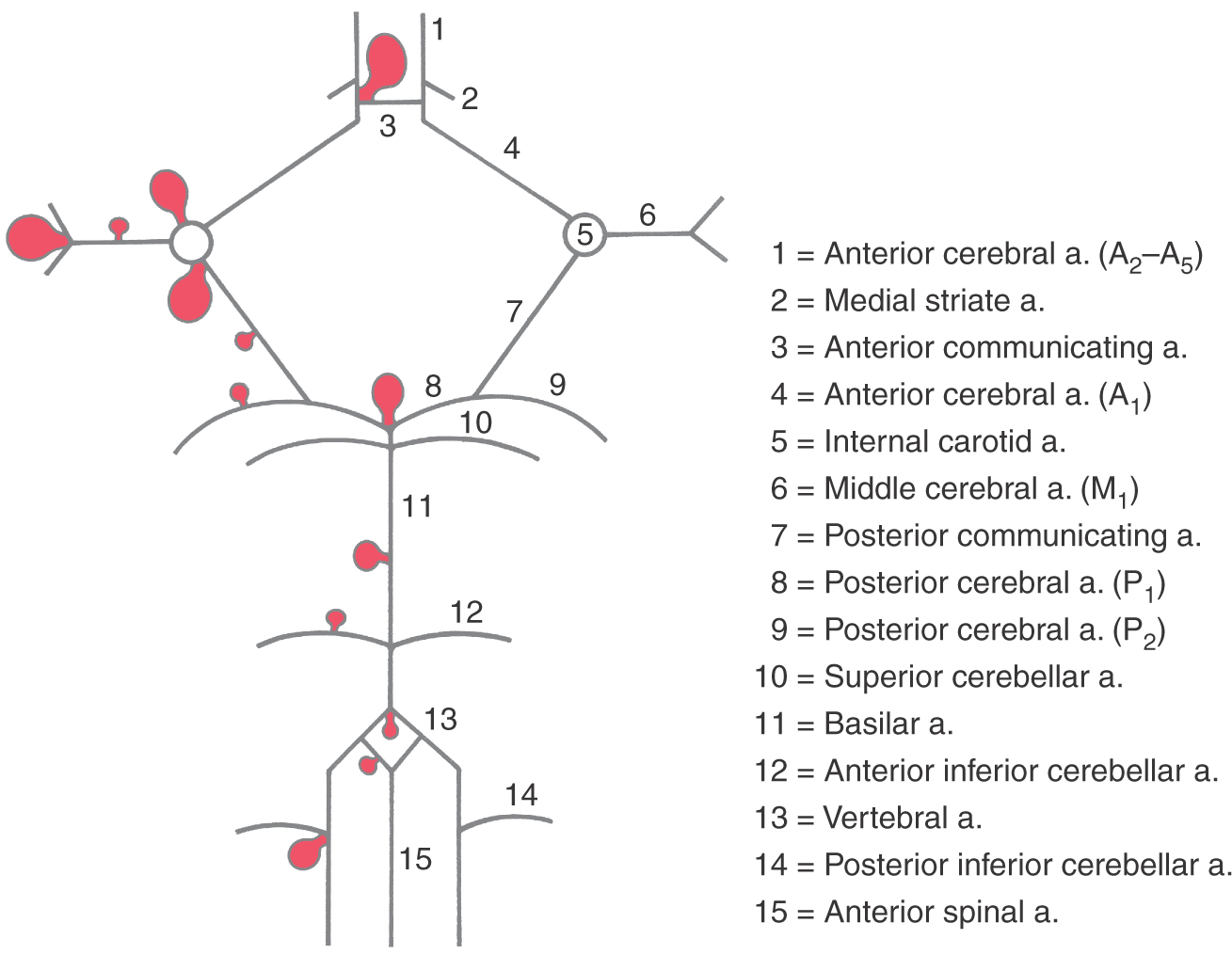
Most intracranial (or cerebral) aneurysms (about 85%) are found on the branches of the internal carotid artery system (Fig. 8-2). On this portion of the vascular tree, aneurysms are most frequent on the anterior communicating artery or at its junction with the anterior cerebral artery (30% to 35%), on the internal carotid artery or at its junction with the posterior communicating artery (25% to 30%), or at the bifurcation of the M1 segment of the middle cerebral artery (20%) (Fig. 8-2). About 10% to 15% of intracranial aneurysms are located on branches of the vertebrobasilar system (Fig. 8-2). When aneurysms are present on these vessels, they are more likely to be located at the bifurcation of the basilar artery (5% to 10%), on the basilar artery, or on the posterior inferior cerebellar artery or at its junction with the vertebral artery. Regardless of where they may occur, intracranial aneurysms are frequently located at branch points of vessels or at points where the vessels may make a sharp abrupt turn in their course. The treatment of choice is to clip the stalk of the aneurysm to separate its friable sac from the cerebral circulation.
Cerebral Embolism
A cerebral embolism is the occlusion of a cerebral vessel by some extraneous material (such as a clot, tumor cells, a clump of bacteria, air, or plaque fragments). This occlusion leads to ischemia (a localized anemia) and, if prolonged, ultimately to infarction (a localized vascular insufficiency resulting in necrosis) of the area served by the vessel (Fig. 8-1B). In many cases the deficits seen in the patient reflect the loss of function of the damaged area of the brain or spinal cord. An embolus made up exclusively of blood products is called a thrombus.
The size of the embolus determines where it lodges. Very small emboli may temporarily occlude small cerebral vessels and give rise to a transient ischemic attack, a sudden loss of neurologic function that usually resolves within a few minutes (about 70% of cases), a few hours (about 20% of cases), or in a minority of cases up to 24 hours. On the other hand, large emboli that suddenly occlude major vessels may cause sudden and catastrophic neurologic problems that result in permanent deficits or death.
One well-known cause of cerebral embolism is seen in patients with atherosclerotic disease. Plaques form at many locations, but those at the bifurcation of the common carotid into the external and internal carotid arteries (Fig. 8-1B) are especially problematic. Pieces of plaque may dislodge, pass into the cerebral circulation, and block distal branches of the internal carotid system; the deficits reflect the brain territory damaged. Septic emboli are composed of bacteria usually originating from an extracranial location. This type of embolus may cause an interruption of blood supply, with a consequent infarction, or result in an infection within the central nervous system (CNS) once the bacteria become lodged in a vessel. Septic emboli may also infect and weaken the vessel wall itself, resulting in a mycotic aneurysm. Air embolism may occur in surgical procedures in which a dural sinus is opened. Air may enter the sinus, and movement of blood through the sinus is compromised; if the air gets into the general vascular system and to the heart, other and equally serious problems may arise.
Penumbra
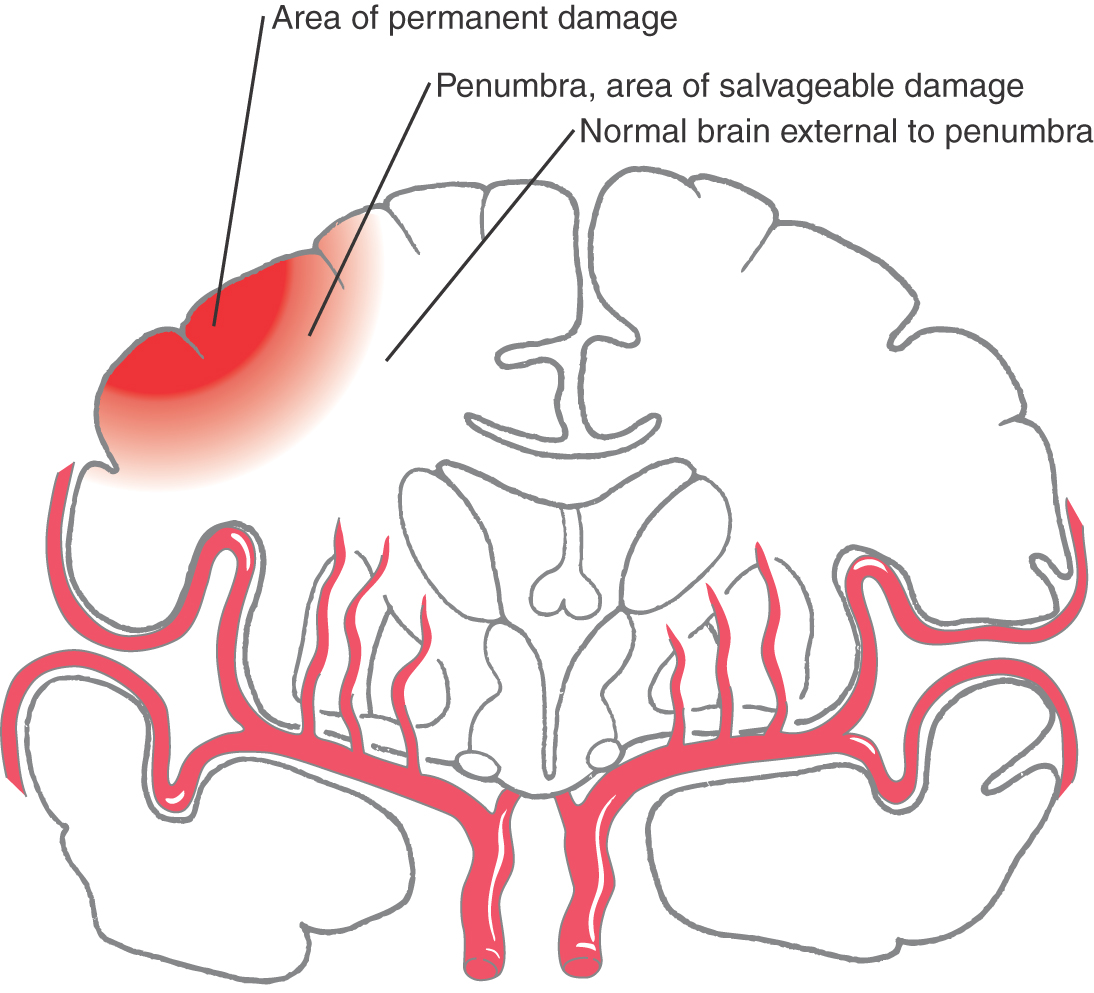
“Time is brain” is a phrase well known to physicians dealing with stroke. In the case of a stroke resulting from vascular occlusion (Fig. 8-1B), it may be described as an occlusive stroke or ischemic stroke; there are three brain areas of particular concern. If a central portion of the ischemic area is permanently lost, an immediately surrounding area, the penumbra, may be salvaged with appropriate and rapid treatment, and an area outside the penumbra will most likely survive (Fig. 8-3). For this type of stroke, treatment may be by the use of tPA (tissue plasminogen activator, infused within 3 to 4.5 hours after the onset of symptoms) or endovascular removal of the clot (within 8 hours). Successful treatment, if it is initiated early, may save the area of the penumbra, decrease brain loss, and result in fewer or less severe neurologic deficits. If treatment is not initiated quickly, the penumbra may be recruited into the area of permanent brain tissue loss, and the neurologic deficits will be accordingly greater. Hemorrhagic strokes cannot be treated with tPA.
Arteriovenous Malformation
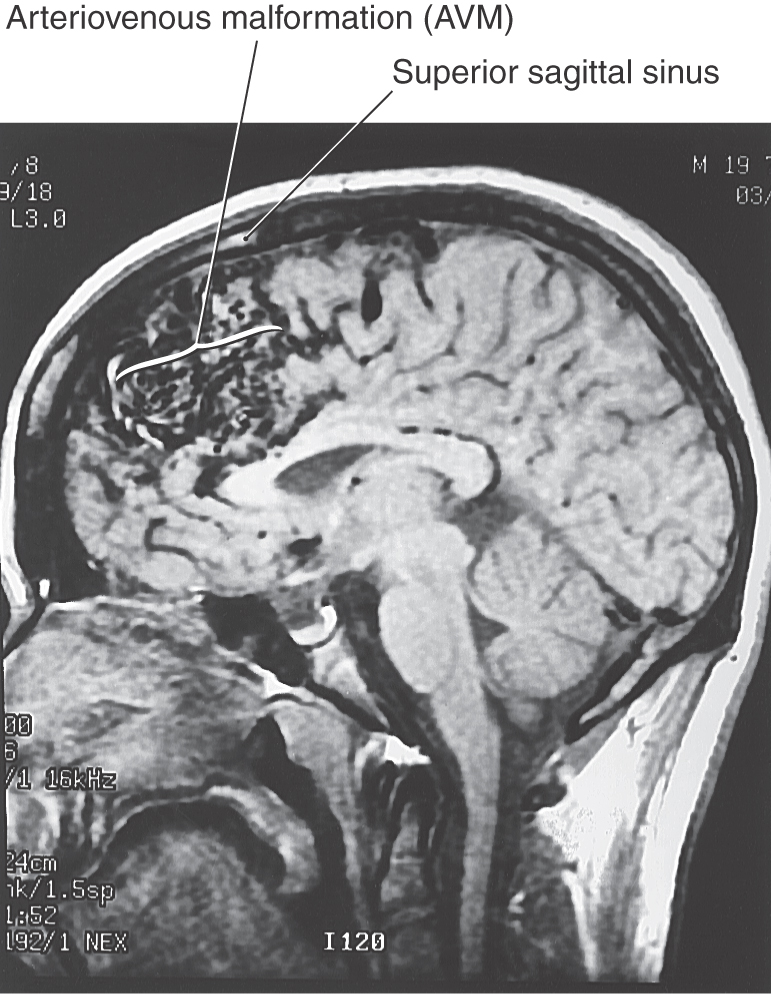
An arteriovenous malformation (AVM) results when the communications between major arteries and veins do not develop normally (Fig. 8-1C). These lesions consist of masses of tortuous, interconnecting channels composed of large arteries connecting with large veins. The intervening capillary bed is missing, and there is little or no normal brain tissue in this vascular mass. An AVM may be located on the surface or within the substance of the brain (Fig. 8-4).
Whereas in the strict sense an AVM is not a neoplasm, an AVM shares important features with this type of lesion. Like neoplasms, AVMs are dynamic lesions that will grow, change in their configuration, and cause additional deficits by damaging adjacent brain areas in the process. Degenerative changes in the abnormal vessels within the AVM may lead to hemorrhage. Such hemorrhage may be into the substance of the brain, subarachnoid space, ventricles, or brainstem, depending on the location of the AVM.
The treatment of choice for AVMs is surgical removal. Superficially located lesions, with few feeding arteries and draining veins, are more easily removed, whereas those located deep within the hemisphere or within the brainstem are much more difficult to treat with surgery. However, newer interventional methods (endovascular embolization) now make it possible to pass a small cannula into an AVM to inject substances that occlude the larger channels. Embolization alone is usually inadequate to definitively treat the lesion. In some cases this method is used as a preparatory step to eventual surgical removal.
AVMs (Fig. 8-4) are commonly identified in the second or third decade of life, although signs and symptoms (hemorrhage [into the brain, subarachnoid, ventricular], seizures, mass effect [cranial nerve signs], evidence of increased intracranial pressure, hydrocephalus) may be noted earlier. Bleeding from AVMs is common and may be “silent” or may result in obvious neurologic deficits. AVMs are a part of a larger category of vascular lesions found in the brain that fall under the general classification of vascular hamartomas. These include capillary telangiectases, cavernous angiomas, and venous malformations commonly called venous angiomas.
INTERNAL CAROTID SYSTEM
The internal carotid artery system consists of the internal carotid artery (ICA), as it enters the base of the skull, and its branches. There are several important branches of the ICA; its terminal branches are the anterior cerebral and the middle cerebral arteries.
Internal Carotid Artery
The ICA consists of a cervical part that ascends in the neck, a petrous part, a cavernous part, and a cerebral part (Fig. 8-5). The petrous part is located in the carotid canal and has no branches of consequence. The cavernous part passes through the cavernous sinus and gives rise to the inferior hypophysial and meningeal arteries.
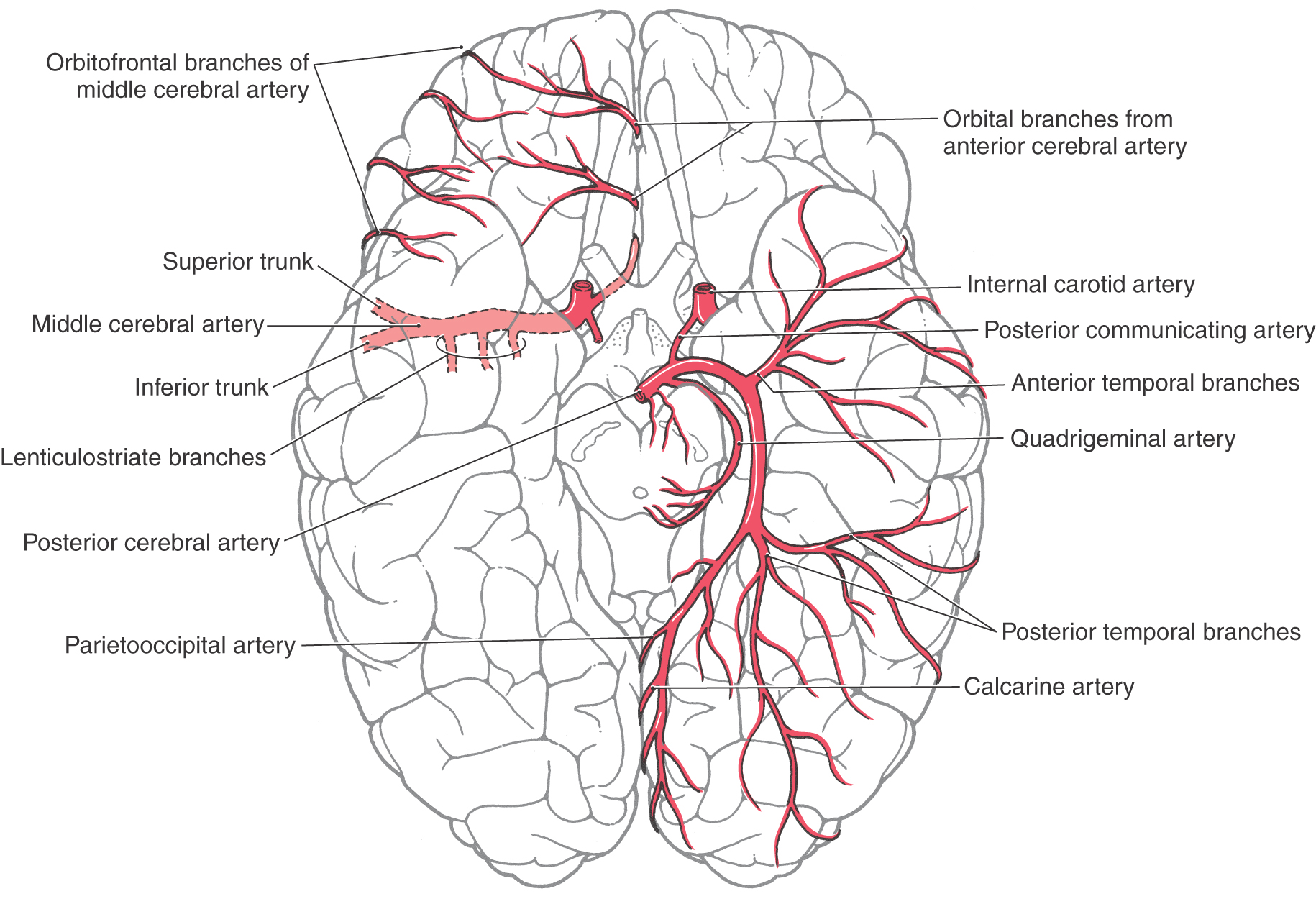
The cerebral part of the internal carotid begins where this vessel penetrates the dura just anterior (ventral) to the optic nerve. Its branches are the ophthalmic, posterior communicating, anterior choroidal, and superior hypophysial arteries (Fig. 8-6).
Figure 8-6. Arteries on the base of the brain showing the relationship of vessels to structures and the arrangement of the circle of Willis (see Fig. 8-11).
The ophthalmic artery, after entering the orbit via the optic foramen, gives rises to the central artery of the retina just distal to the foramen. This latter vessel then passes along the ventral aspect of the optic nerve (it may be inside or outside the dura) to eventually enter the nerve about 10 to 15 mm behind the bulb of the eye to serve the retina. Occlusion of the ophthalmic artery may result in significant visual loss in the ipsilateral eye. Also, aneurysms at the ophthalmic-carotid intersection may cause visual loss because of direct pressure on the optic nerve.
The posterior communicating artery joins the posterior cerebral artery, and the anterior choroidal artery follows caudolaterally along the optic tract (Fig. 8-6). Occlusion of the anterior choroidal artery will result in a combination of visual deficits and weakness of the opposite upper and lower extremities; this is called the anterior choroidal artery syndrome. The ICA ends by dividing into the anterior and middle cerebral arteries.
Anterior Cerebral Artery
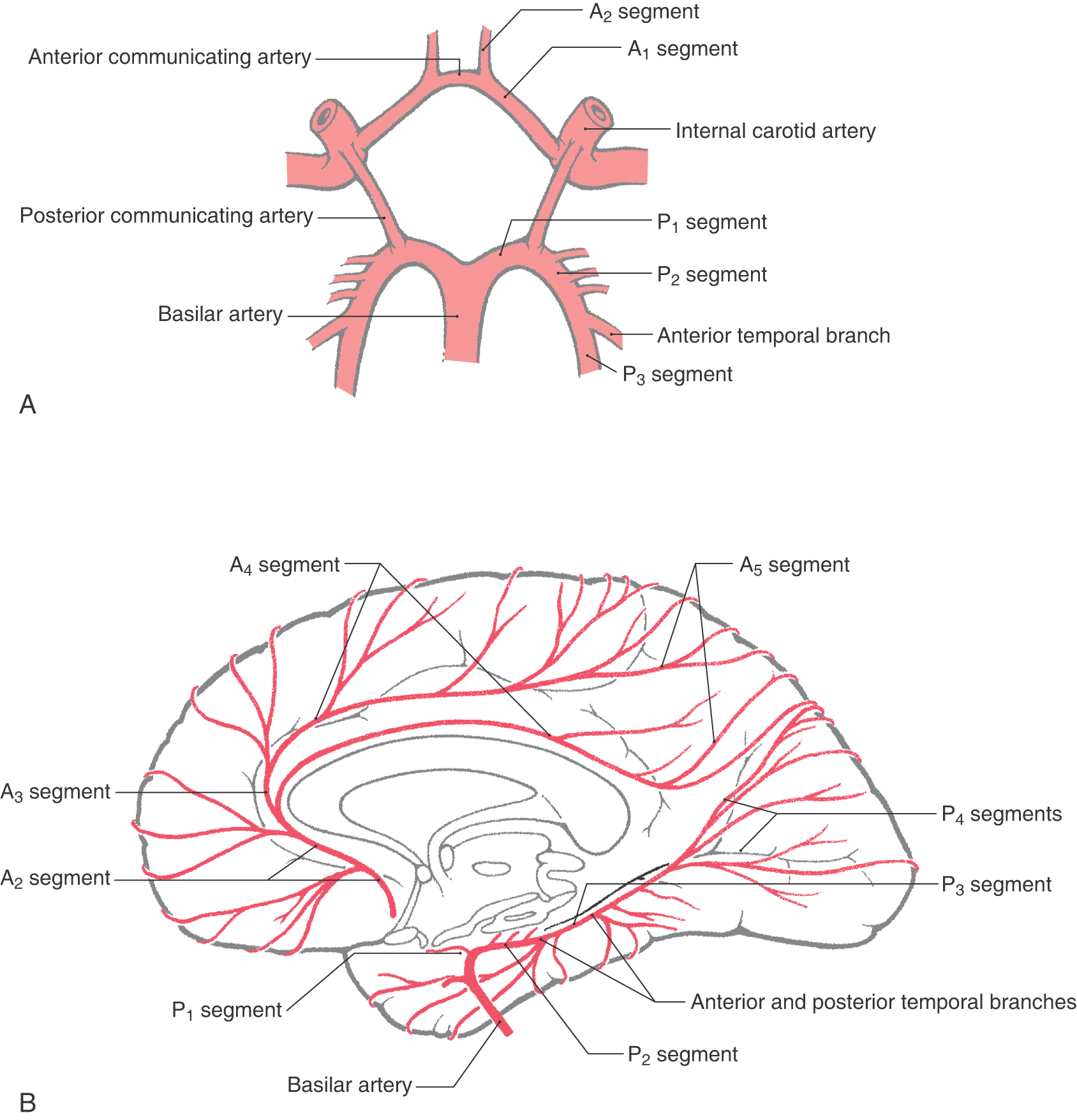
Taking its entire extent into consideration, from its origin at the internal carotid to its termination at about the parietooccipital sulcus, the anterior cerebral artery (ACA) is divided into five segments designated A1 to A5 (Fig. 8-7). The precommunicating segment, A1, extends from the internal carotid artery to the anterior communicating artery. A2, the infracallosal segment, extends from the anterior communicating artery to about where the rostrum and genu of the corpus callosum meet. The precallosal segment, A3, arches around the genu of the corpus callosum; this segment ends as the vessels turn sharply caudal. Segments A4, supracallosal, and A5, postcallosal, are located superior and caudal to the corpus callosum; A5 ends at about the level of the parietooccipital sulcus (Figs. 8-7 and 8-8).
Figure 8-8. Arteries on the medial surface of the cerebral hemisphere and on the inferior surface of the temporal lobe.
The A1 segment of the ACA passes superiorly over the optic chiasm and is joined to its counterpart by the anterior communicating artery (Fig. 8-6). The anterior communicating artery and the distal parts of the A1 segments are located in the cistern of the lamina terminalis and give rise to small branches that serve structures in the immediate area, such as parts of the anterior hypothalamus and the optic chiasm.
About 30% to 35% of all intracranial aneurysms are found either on the anterior communicating artery or where this vessel joins the ACA (Figs. 8-1A and 8-2). Patients with aneurysms at these locations may have visual deficits because of the proximity to the optic chiasm. Rupture of aneurysms in this area always results in blood in the interhemispheric fissure between the frontal lobes, and these patients frequently have hematomas in the brain itself or blood in the ventricles.
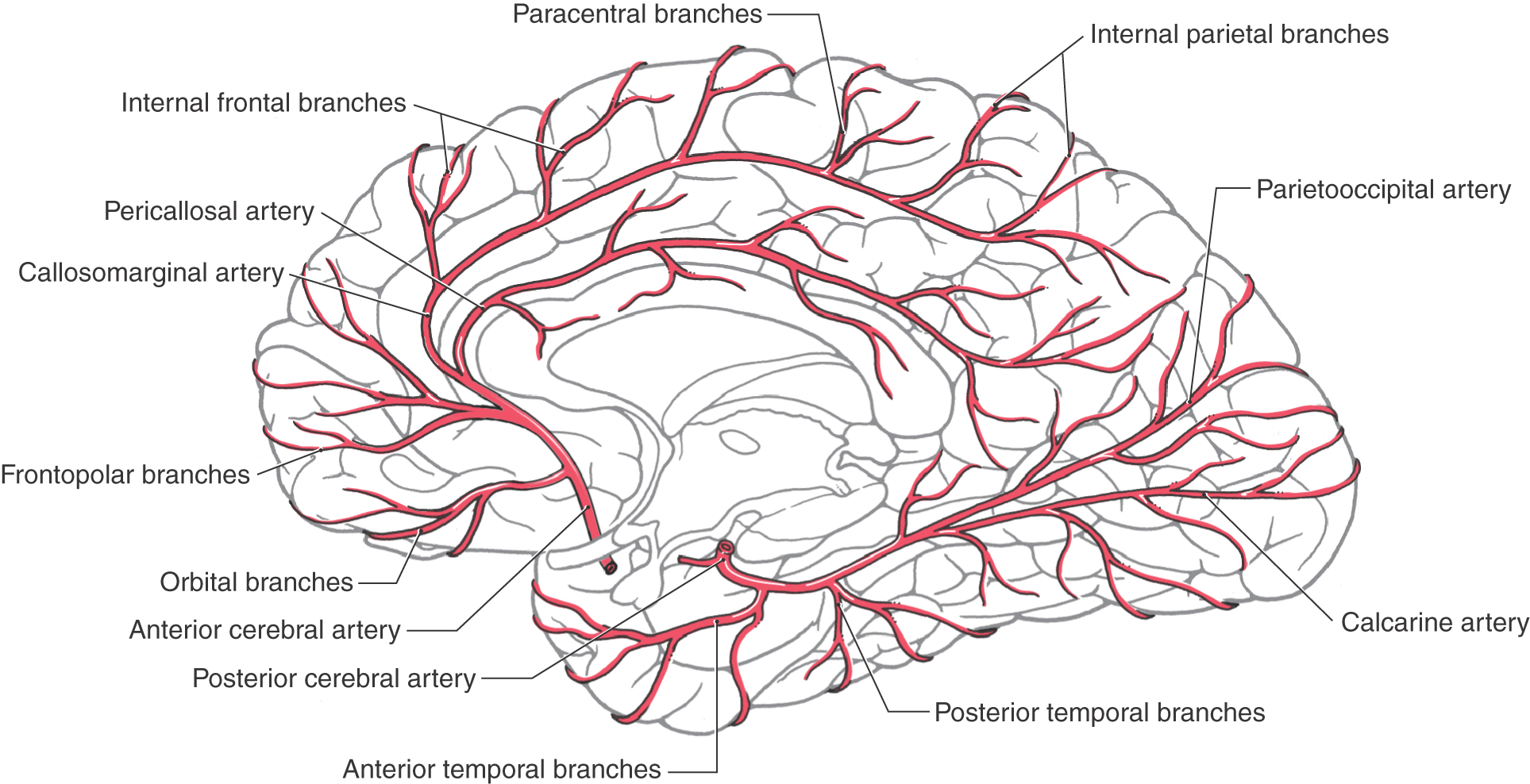
Distal to the anterior communicating artery, the A2 segment passes through the cistern of the lamina terminalis rostrally toward the genu of the corpus callosum and usually gives rise to frontopolar and orbitofrontal branches (Figs. 8-7 and 8-8). The A3 segment arches around the genu and usually gives rise to the callosomarginal artery and to the more anterior and middle of the internal frontal branches. A4 and A5 segments of the ACA are composed of the pericallosal and callosomarginal arteries and their branches on the medial aspect of the cerebral hemisphere (Figs. 8-7 and 8-8). These distal portions of the ACA serve the lower extremity areas of the somatomotor and somatosensory cortex; damage to this vascular territory may result in hemimotor and hemisensory deficits of the lower extremity.
The main branches of A3 to A5 lie within the callosal cistern. When aneurysms occur on the more distal parts of the ACA, they are usually located at the branch points of the frontopolar and callosomarginal arteries.
Middle Cerebral Artery
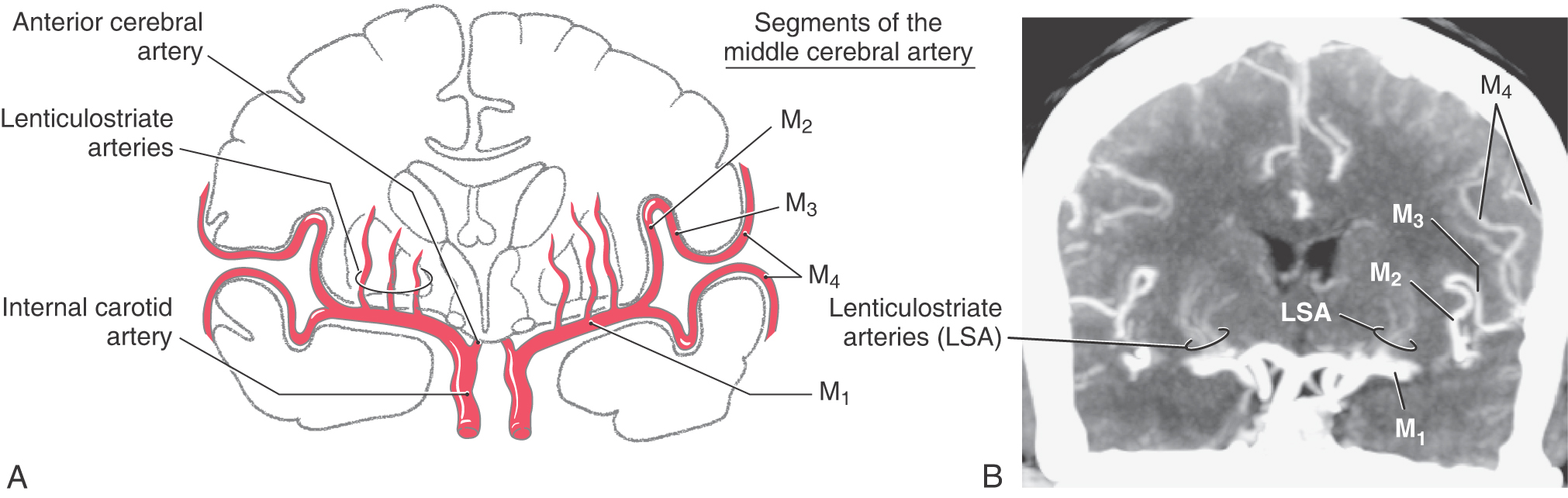
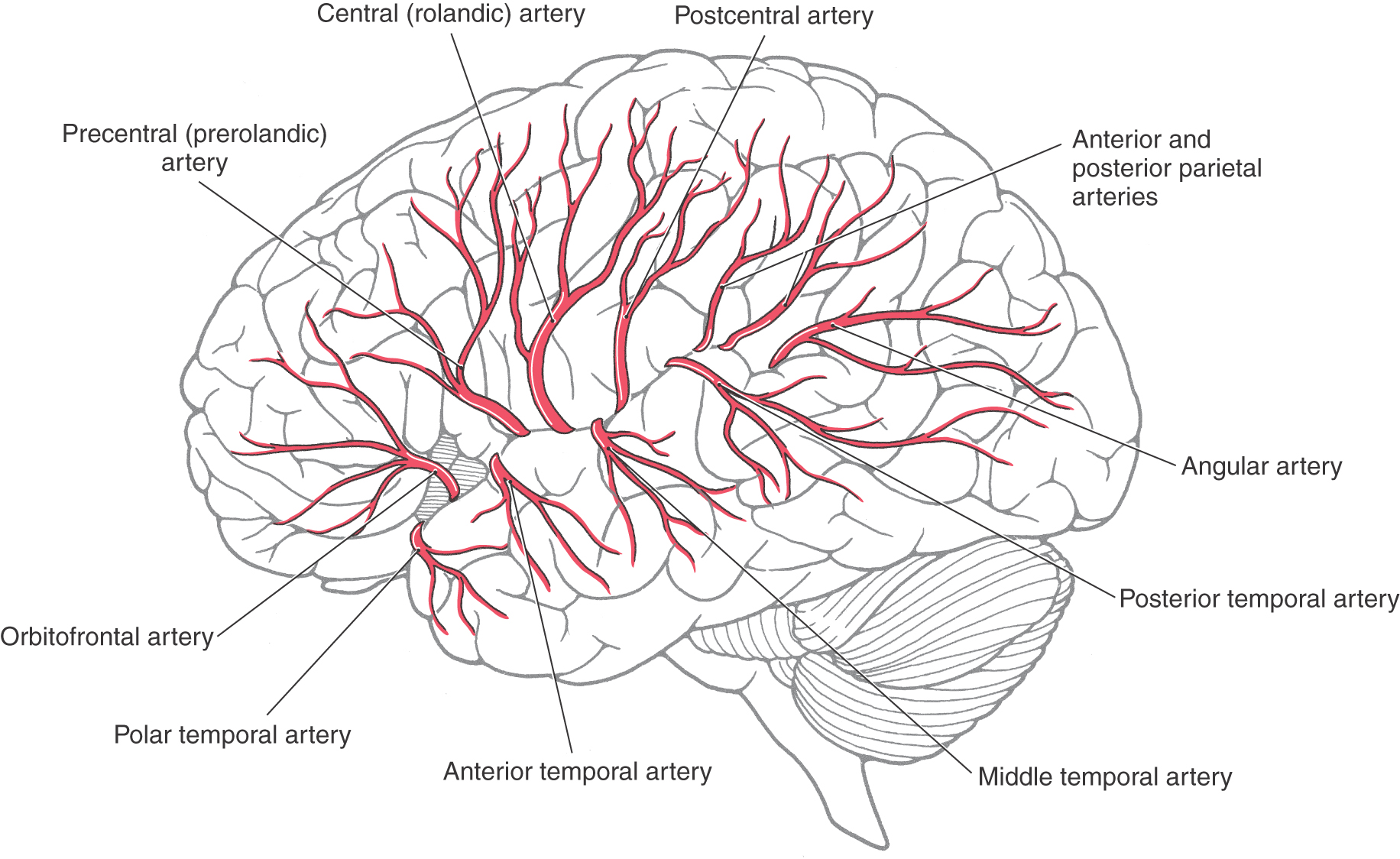
The middle cerebral artery (MCA) is usually (70% of the time) the larger of the two terminal branches of the internal carotid artery (Figs. 8-5 and 8-6). The part of this vessel located between its origin from the internal carotid and the point where it branches at the ventromedial aspect of the insula (the limen insulae) is the M1 segment (Fig. 8-9A). Branches from M1 serve adjacent medial and rostral aspects of the temporal lobe and, via lenticulostriate arteries, structures located inside the hemisphere. Whereas it is common to refer to the lenticulostriate arteries (plural), in about 40% of patients, this vessel originates as a single trunk that immediately divides into a number of branches (the arteries) that penetrate the hemisphere. The other patterns are two main trunks (30%) that immediately divide and 10 to 15 small arteries (30%) arising directly from M1.
The M1 segment is located in medial portions of the sylvian cistern. On the ventromedial aspect of the insular cortex, the M1 segment usually bifurcates into superior and inferior trunks (Figs. 8-5, 8-6, and 8-9A). Aneurysms on the MCA frequently arise at the bifurcation of M1 into the superior and inferior trunks; the aneurysm represents the true position of the bifurcation in cases in which there may appear to be multiple bifurcations of M1. These trunks and their distal branches collectively serve the insular cortex, the inner aspects of the opercula, and the lateral surface of the cerebral hemisphere. Those branches on the insular cortex (insular part of the MCA) are the M2 segment (Figs. 8-5 and 8-9A, B). The larger vessels continue on to the inner aspects of the opercula (frontal, parietal, temporal), where they are designated M3, the opercular part of the MCA (Fig. 8-9A, B). Distal branches of the superior and inferior trunks exit the lateral fissure and serve, respectively, cortical areas located above and below this fissure. These cortical branches (cortical part of the MCA) collectively form the M4 segment (Figs. 8-5 and 8-9A, B). Most of these distal branches are named according to the general area or structure they serve; their distribution patterns are shown in Figure 8-10. M4 branches serve trunk, upper extremity, and face areas of the somatomotor and somatosensory cortex; occlusion of these vessels may produce deficits affecting these body regions.
VERTEBROBASILAR SYSTEM
The vertebrobasilar system is so named because it is formed by the distal segments of the vertebral arteries as they join to form the basilar artery (Figs. 8-6 and 8-11A, B). This portion of the cerebrovascular system is the primary source of blood supply to the brainstem.
Vertebral Artery
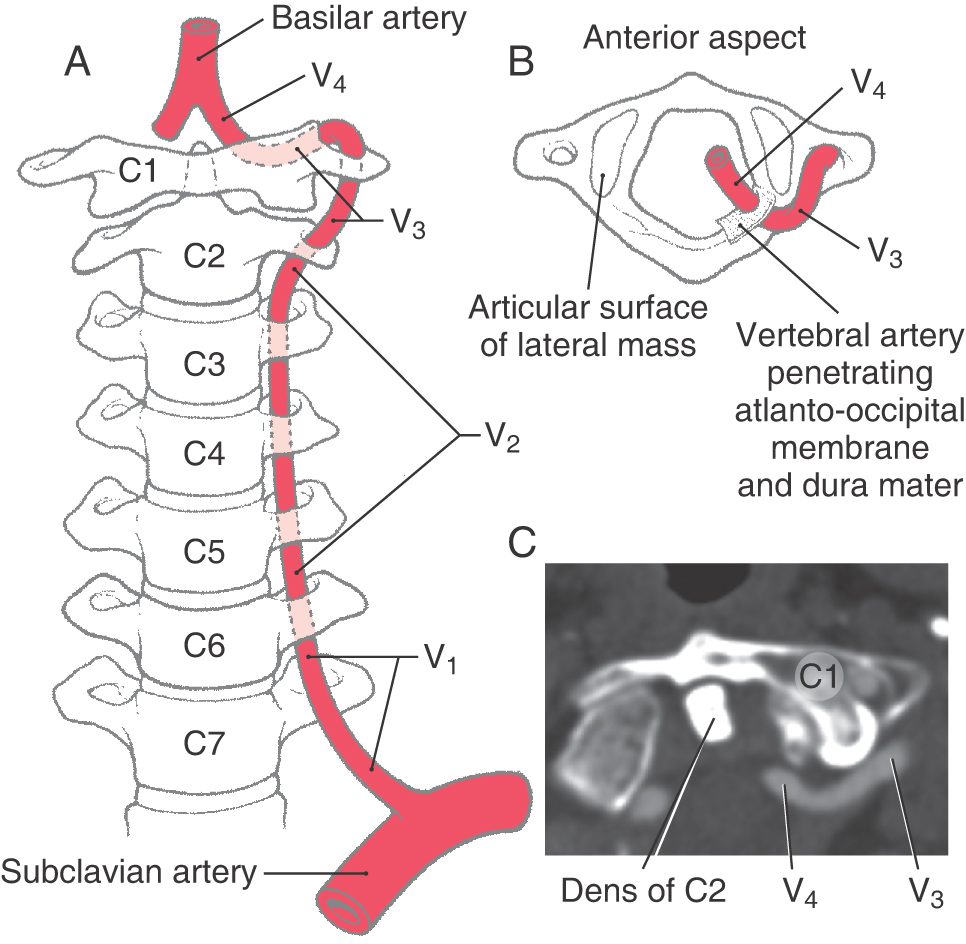
The vertebral artery is divided into four segments (V1 to V4, Fig. 8-12A-C) on the basis of its anatomic relationships. Segment V1 is that portion located between its origin from the subclavian artery and the point where this artery enters the transverse foramen of the sixth cervical vertebra. The second segment, V2, is that portion of the vertebral artery that ascends through the transverse foramina of C6 to C2. The third segment, V3, is located between the exit of the artery from the C2 foramen and the point where it penetrates the atlantooccipital membrane; this includes the loop that passes through the transverse foramen of C1 and arches caudally and medially around the lateral mass of the atlas (Fig. 8-12B, C). The V4 segment passes through the dura, is located within the cranial cavity internal to the atlantooccipital membrane and dura, and will join its counterpart to form the basilar artery (Figs. 8-11 and 8-12A).
This circuitous segment of V4 is vulnerable to injury. For example, hyperextension of the head may compress the vertebral artery between the occipital bone and the posterior arch of the atlas, and extreme rotation of the head may put torsion on this artery and restrict blood flow. The deficits seen in these patients fall under a general classification referred to as vertebrobasilar insufficiency. Once it is inside the subarachnoid space, the vertebral artery is located in the lateral cerebellomedullary cistern.
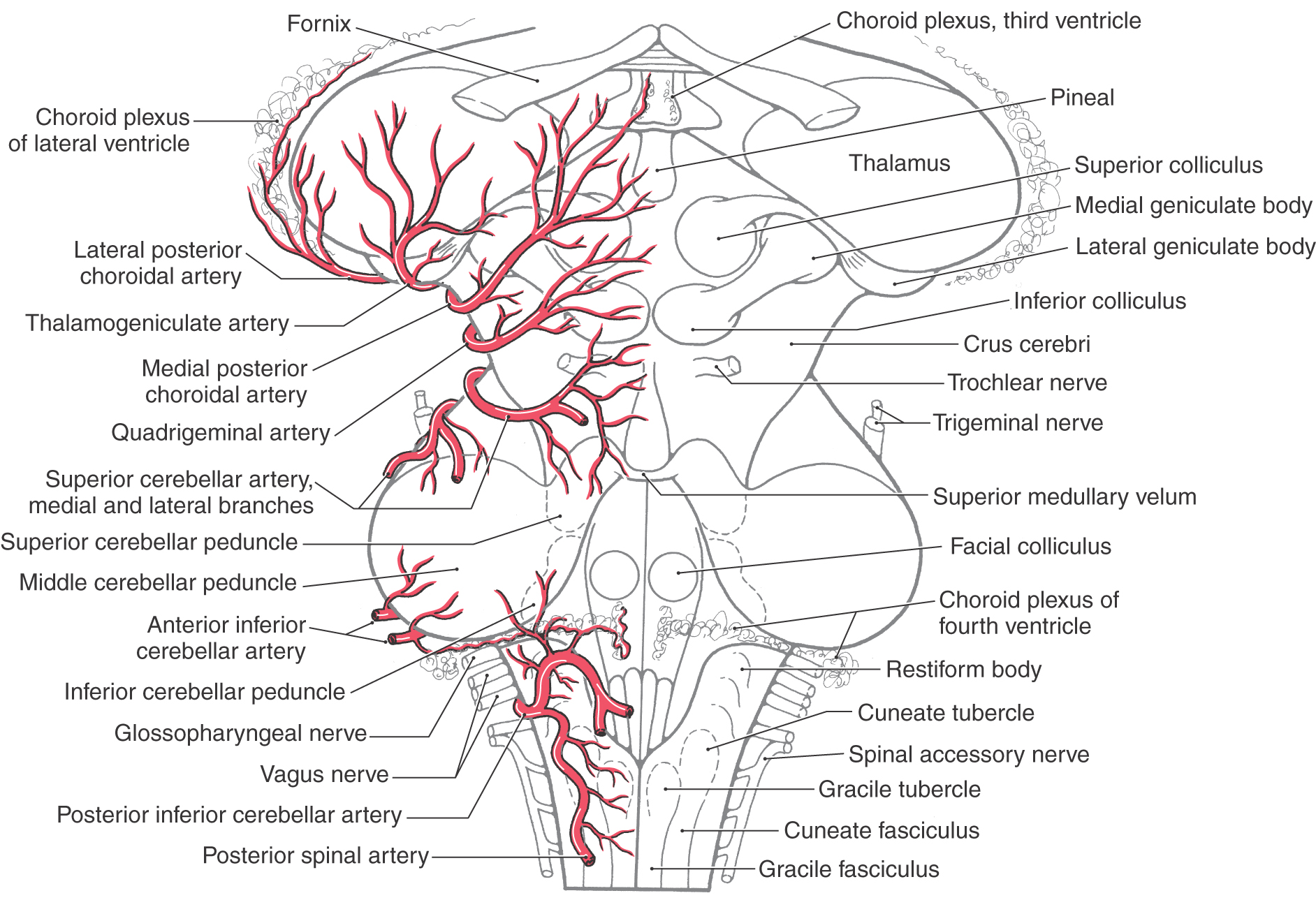
Branches of the vertebral artery supply the medulla, parts of the cerebellum, and the dura of the posterior fossa (Figs. 8-5 and 8-13). Its first major branch, the posterior inferior cerebellar artery (PICA), arches around the posterolateral medulla and sends branches to this part of the brainstem (Fig. 8-11A). Posteriorly the PICA is located in the cisterna magna (dorsal cerebellomedullary cistern). It serves the choroid plexus of the fourth ventricle and then branches over medial parts of the inferior cerebellar surface (Fig. 8-13). In about 75% of brains, the posterior spinal artery is a branch of the PICA; in the other 25%, it arises from the vertebral artery. The posterior spinal artery serves dorsolateral regions of the medulla caudal to the area served by the PICA (Fig. 8-13). The vertebral artery supplies the anterolateral medulla and, just before joining its counterpart on the opposite side, gives rise to the anterior spinal artery. This vessel usually (85% of cases) originates as two small trunks, which join to form a single artery that courses caudally in the ventral median fissure of the medulla and continues into the spinal cord (Fig. 8-6; see also Fig. 8-26). The anterior spinal artery is found in the premedullary cistern.
Aneurysms of the vertebral artery or its major branches are not common. When present, they are usually found where the PICA branches from the vertebral artery.
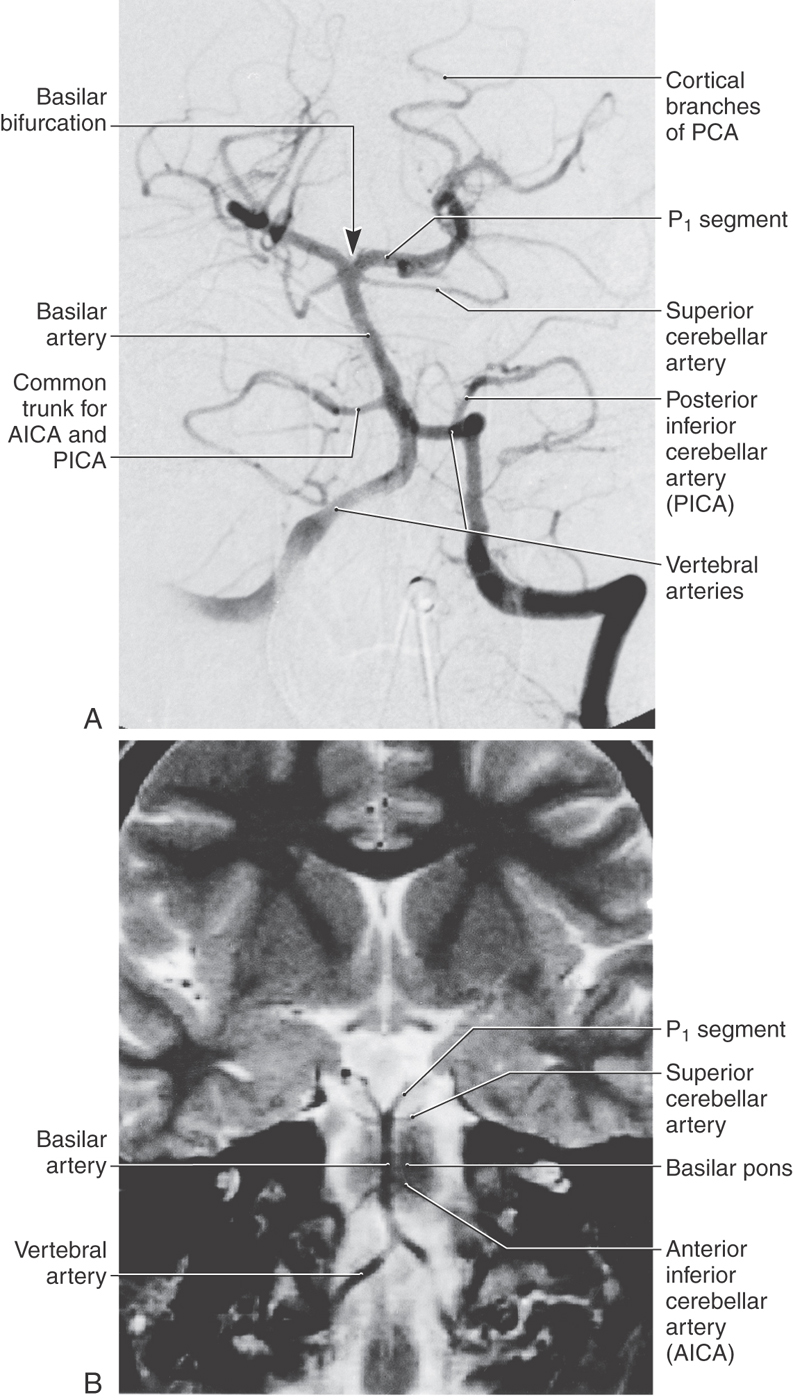
Basilar Artery
The basilar artery lies in a shallow depression, the basilar sulcus, on the anterior surface of the pons in the prepontine cistern (Figs. 8-6 and 8-11B). Its first large branch, the anterior inferior cerebellar artery (AICA), generally arises from the lower third of the basilar artery and passes through the cerebellopontine cistern as it wraps around the caudal aspect of the middle cerebellar peduncle. The AICA serves ventral and lateral surfaces of the cerebellum, parts of the pons, and the portion of choroid plexus that extends out of the foramen of Luschka into the cerebellopontine angle (Fig. 8-13). The labyrinthine artery is usually a branch of the AICA. It arises close to the origins of the facial and vestibulocochlear nerves (Fig. 8-6) and enters the internal acoustic meatus along with these nerves.
The basilar artery gives rise to numerous pontine arteries (Fig. 8-6). These arteries may penetrate the pons immediately as paramedian branches, travel for a short distance around the pons as short circumferential branches, or pass for longer distances as long circumferential branches.
The last major branches of the basilar artery are the superior cerebellar arteries. Just distal to their origin, each superior cerebellar artery divides into medial and lateral branches, which serve their respective regions of the superior surface of the cerebellum (Figs. 8-6, 8-11, and 8-13) and most of the cerebellar nuclei. These vessels pass laterally just caudal to the root of the oculomotor nerve and wrap around the brainstem in the ambient cistern to ultimately serve caudal parts of the midbrain and the entire superior surface of the cerebellum (Figs. 8-5 and 8-12).
Intracranial aneurysms on the vertebrobasilar system are frequently found in relation to the basilar bifurcation and may therefore involve the oculomotor nerve (Fig. 8-2). In similar fashion, an aneurysm of the AICA may produce symptoms of facial or vestibulocochlear nerve involvement because this vessel travels adjacent to these nerves.
Posterior Cerebral Artery
At the pons-midbrain junction, the basilar artery bifurcates in the interpeduncular cistern and gives rise to the posterior cerebral arteries (PCAs) (Fig. 8-11). Each PCA passes laterally just rostral to the root of the oculomotor nerve (Fig. 8-6), wraps around the midbrain in the ambient cistern, and then joins the anterior and medial surfaces of the temporal lobes (Figs. 8-8 and 8-14). The PCA sends branches to the midbrain and thalamus and to the ventral and medial surfaces of the temporal and occipital lobes as far as the level of the parietooccipital sulcus (Figs. 8-7 and 8-13).
Figure 8-14. Distribution pattern of the major branches of the posterior cerebral artery.
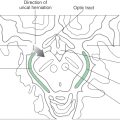
The PCA is divided into segments P1 to P4. The P1 segment is located between the basilar bifurcation and the posterior communicating artery (Fig. 8-7A). It gives rise to small perforating vessels and to quadrigeminal and thalamoperforating arteries (Fig. 8-6). The portion of the posterior cerebral artery between the posterior communicator and the inferior temporal branches is the P2 segment (Fig. 8-7A). Medial and lateral posterior choroidal and thalamogeniculate arteries as well as small perforating branches to the midbrain originate from P2 (Fig. 8-13). The P3 segment is the portion of the artery that gives rise to its temporal branches, and the parietooccipital and calcarine arteries form the P4 segment (Figs. 8-8 and 8-14). P4 branches, particularly the calcarine artery, serve the primary visual cortex; occlusion of these vessels will likely result in a homonymous hemianopia of the opposite visual fields. In fact, this deficit may also be seen in occlusion of more proximal segments of the PCA.
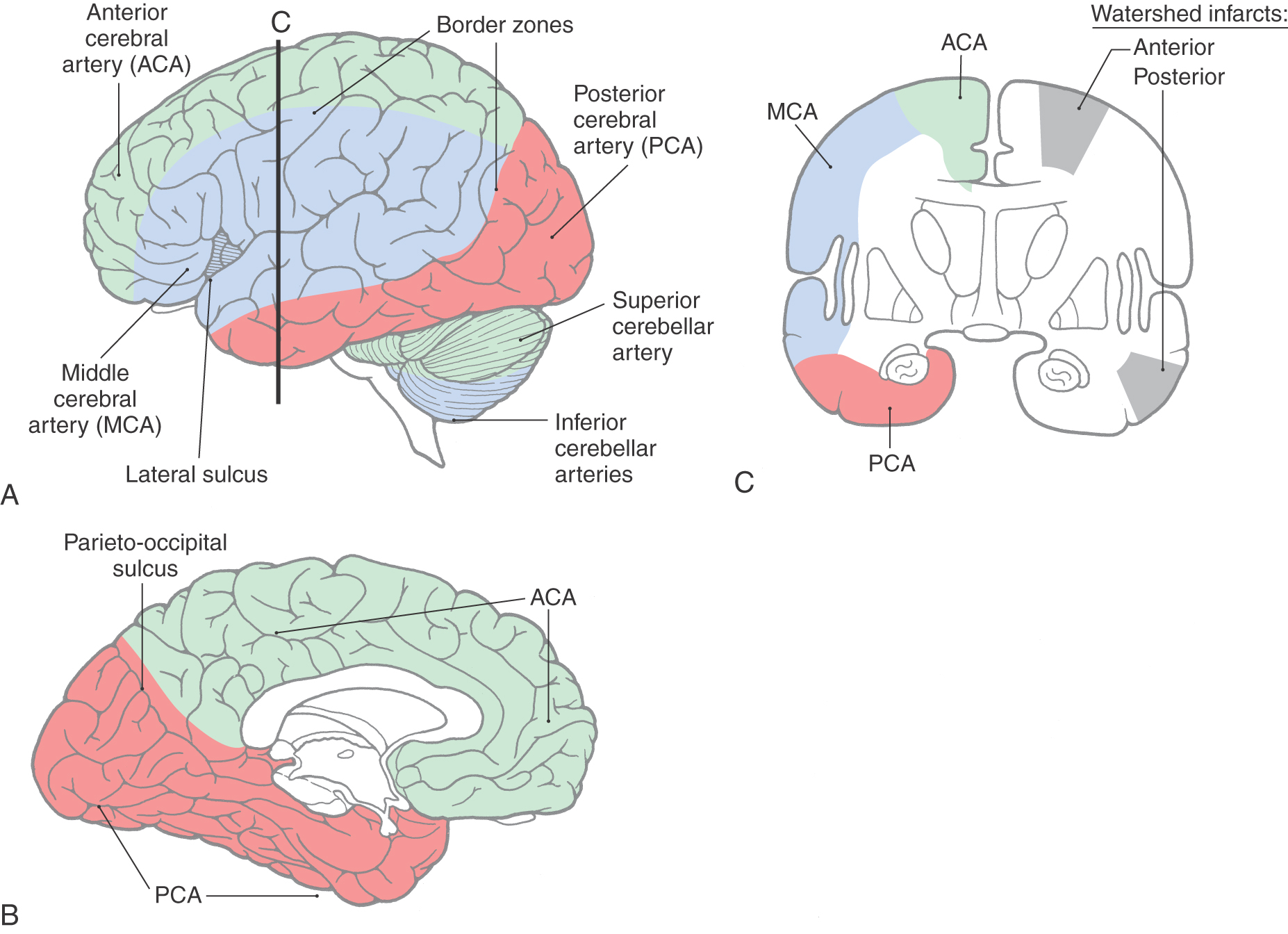
CEREBRAL ARTERIES AND WATERSHED INFARCTS
On the lateral surface of the hemisphere, the terminal branches of anterior and middle and middle and posterior cerebral arteries overlap, forming border zones between the areas served by these arteries (Fig. 8-15). Smaller border zones are also located between the territories of the anterior and posterior cerebral arteries at the parietooccipital sulcus and between the territories of the cerebellar arteries (Fig. 8-15). The brain tissue located in these border zones is particularly susceptible to damage under conditions of sudden systemic hypotension or when there is hypoperfusion of the distal vascular bed of a major cerebral artery.
In the case of the cerebral arteries, inadequate perfusion of the border zones may result in watershed infarcts (Fig. 8-15). Such lesions represent about 10% of all brain infarcts and may be caused by, for example, hypotension or embolic showers. Damage to the anterior cerebral–middle cerebral border zone in one hemisphere (anterior watershed infarcts; see Fig. 8-15) causes a contralateral hemiparesis of the lower extremity and expressive language deficits or behavioral changes. A posterior watershed infarct (damage at the middle cerebral–posterior cerebral border zone) commonly produces a partial visual loss accompanied by a variety of language problems.
CIRCLE OF WILLIS
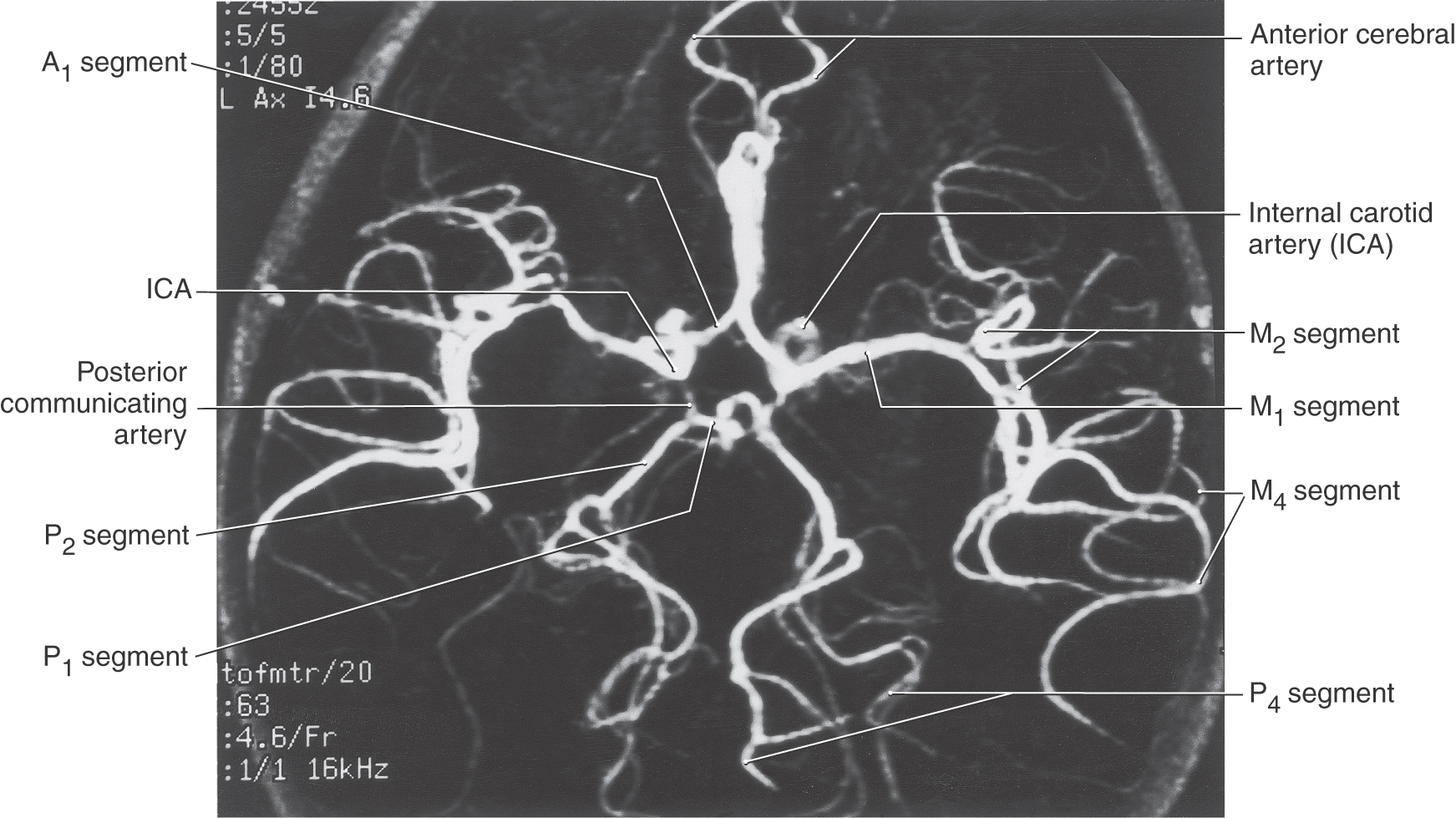
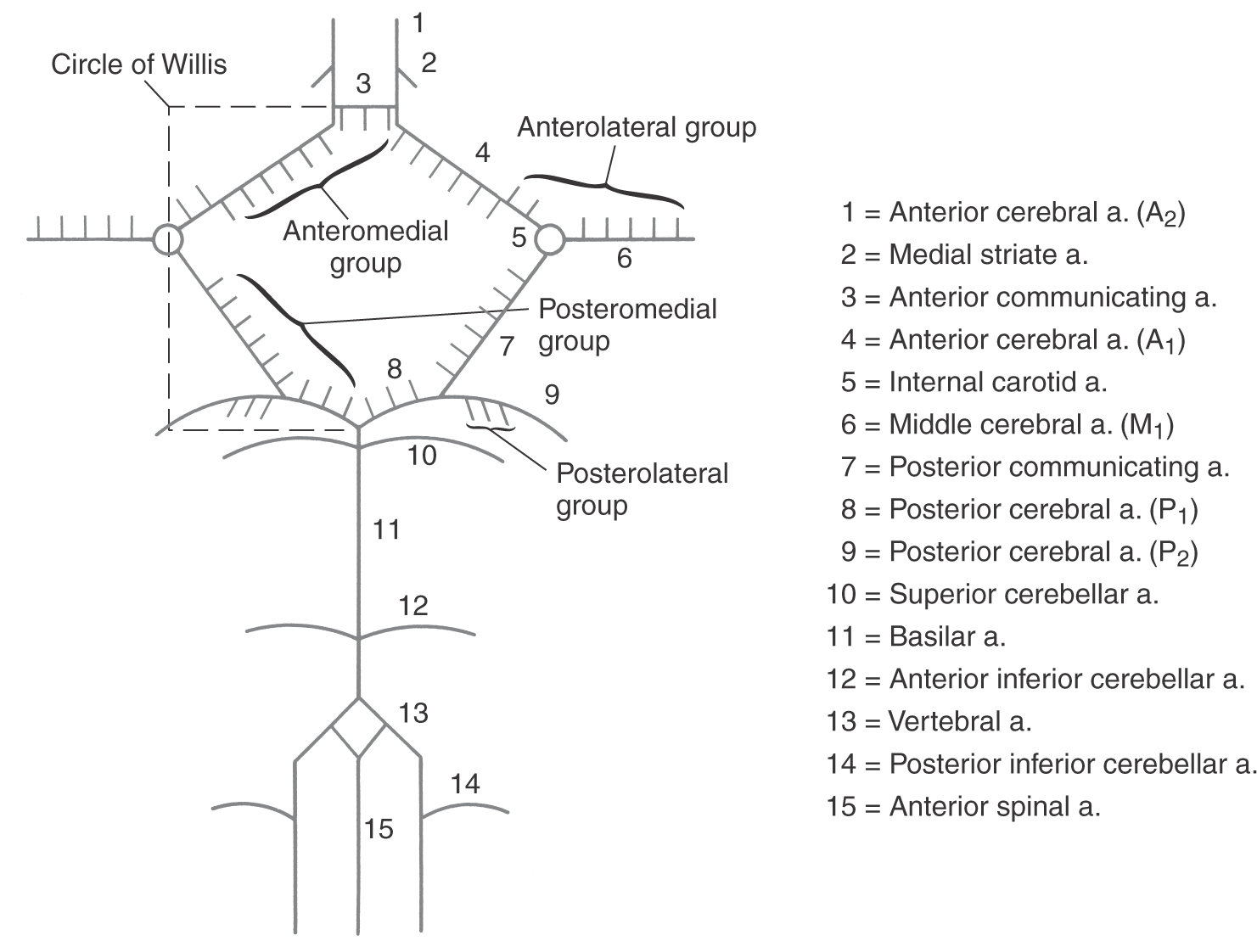
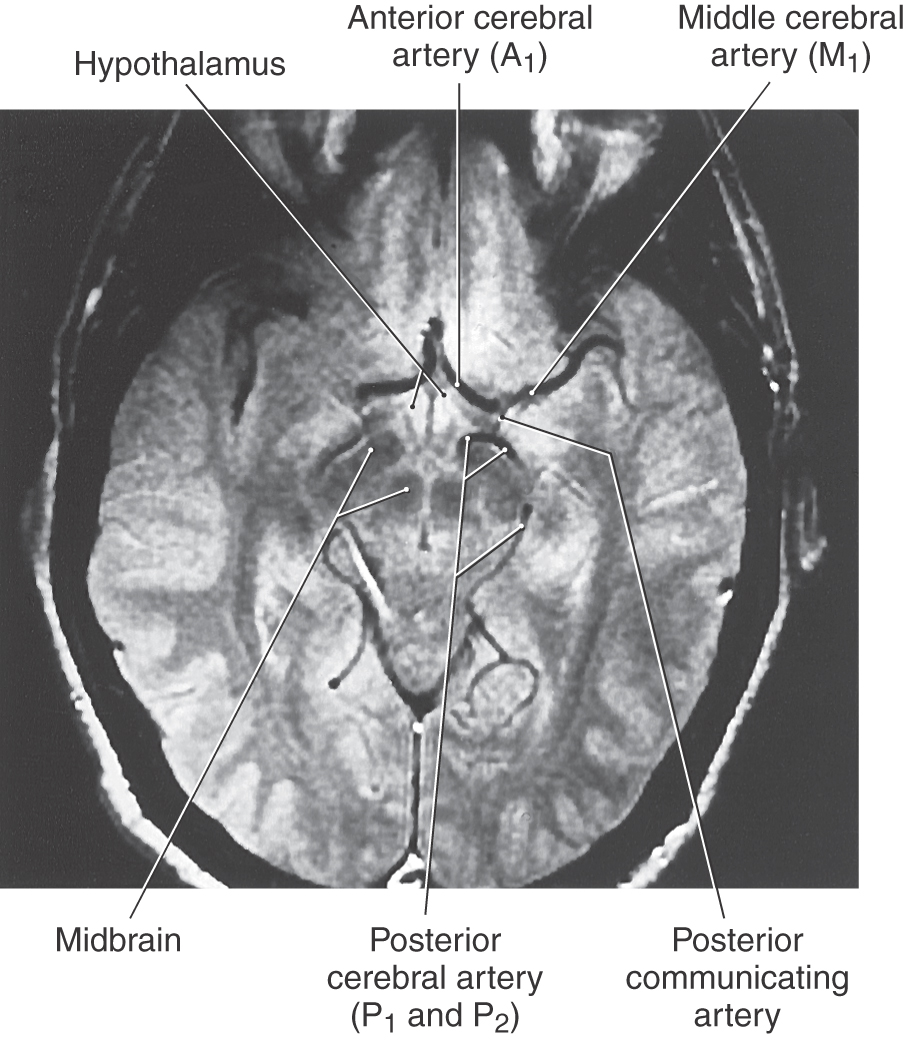
The circle of Willis (cerebral arterial circle) is actually a roughly shaped heptagon of arteries located on the inferior surface of the brain (Figs. 8-16 to 8-18). This loop of vessels passes around the optic chiasm and the optic tract, crosses the crus cerebri of the midbrain, and joins at the pons-midbrain junction. Important structures located inside this circle include the optic chiasm and tracts, the infundibulum and tuber cinereum, the mammillary bodies, the hypothalamus, and structures of the interpeduncular fossa (Figs. 8-6 and 8-18). Arteries forming the circle of Willis give rise to numerous perforating (central or ganglionic) branches, which serve structures located deep to their origin (Figs. 8-6 and 8-17), and to the large cortical branches (anterior, middle, and posterior cerebral arteries) discussed earlier (Fig. 8-18).
Figure 8-16. Magnetic resonance angiography showing the arrangement of the cerebral arterial circle (circle of Willis) and its main branches. Compare with Figure 8-6.
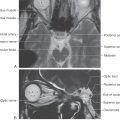
Figure 8-18. An axial magnetic resonance image obtained through the anterior (ventral) aspect of the hemisphere showing most of the vessels forming the circle of Willis (compare with Figs. 8-4 and 8-11).
Central Branches
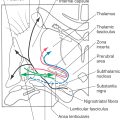
The perforating branches of the circle of Willis are divided into four groups (Fig. 8-17). The anteromedial group originates from A1 and from the anterior communicating artery. These vessels serve structures in the area of the optic chiasm and anterior parts of the hypothalamus. The anterolateral group arises from M1 and A1. Included in this group are the lenticulostriate arteries, which serve the interior of the hemisphere. Vessels of the anterolateral group enter the hemisphere via the anterior perforated substance. The posteromedial group originates from P1 and from the posterior communicating artery (Fig. 8-17). These vessels supply the crus cerebri and the middle and caudal portions of the hypothalamus and enter the interpeduncular fossa via the posterior perforated substance. The thalamoperforating arteries are part of the posteromedial group, and as their name implies, they serve the thalamus. The posterolateral group arises from P2 and is composed of the thalamogeniculate and posterior choroidal arteries (medial and lateral) and some small penetrating branches that enter the midbrain (Fig. 8-17). These vessels serve parts of the thalamus and the choroid plexus.
VEINS AND VENOUS SINUSES OF THE BRAIN
In contrast to the arterial supply of the brain, which comes from two major sources, the venous drainage of the brain exits the skull through one major vessel. Venous blood from superficial and deep veins enters the dural sinuses, which in turn drain into the internal jugular vein. The major venous sinuses are endothelium-lined channels in the meningeal reflections. The superior and inferior sagittal sinuses are located in the attached and free edges of the falx cerebri, respectively. The straight sinus is found where the falx cerebri attaches to the tentorium cerebelli. The other venous sinuses are located adjacent to the inner surface of the skull at specific locations.
Cerebral Hemispheres
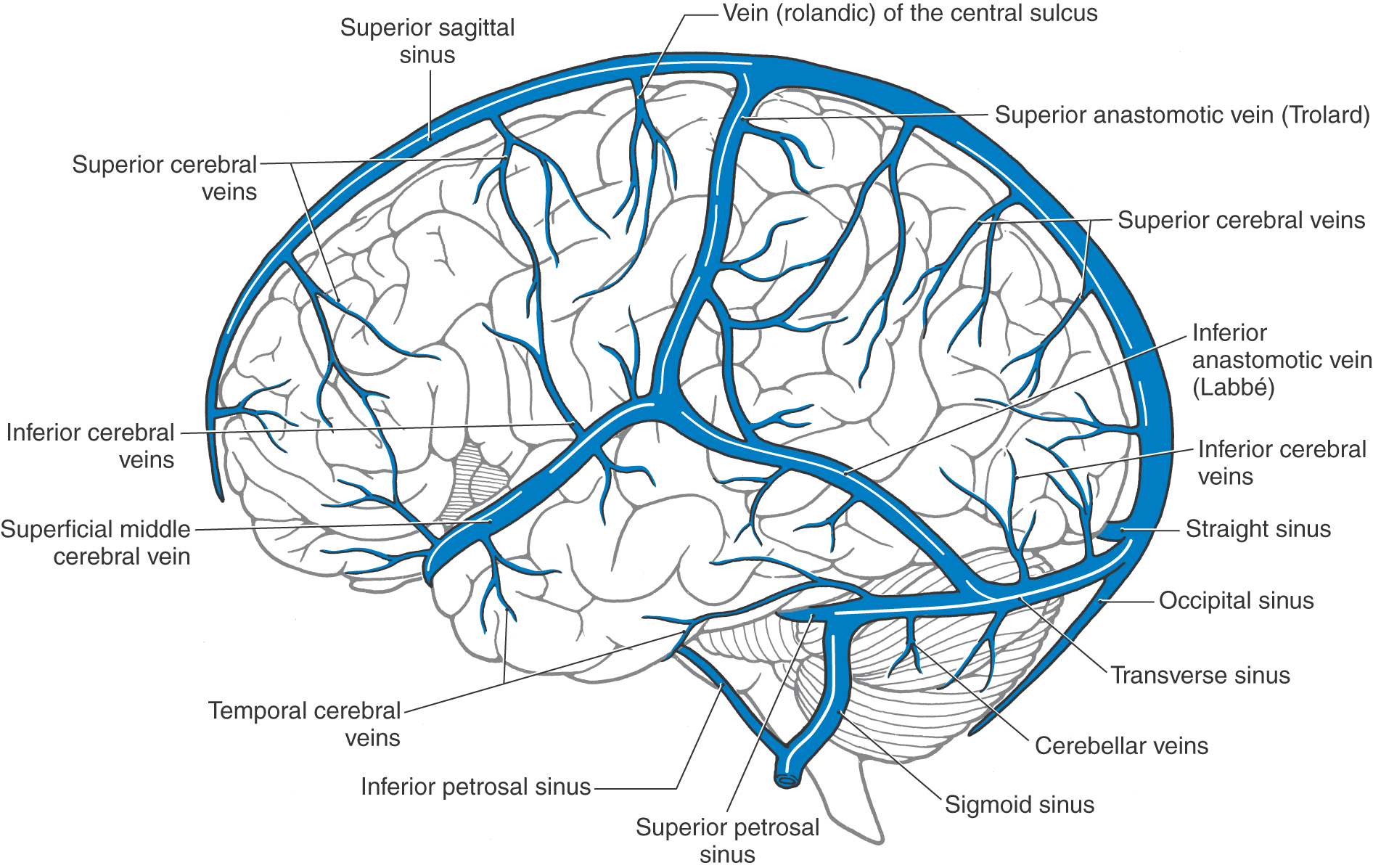
The cerebral veins on the lateral surface of the hemisphere drain into the superior sagittal and transverse sinuses and into the superior anastomotic vein (of Trolard) and the inferior anastomotic vein (of Labbé) (Fig. 8-19). These large anastomotic veins form channels between the superior sagittal and transverse sinuses and the superficial middle cerebral vein. The latter vessel courses medially around the temporal pole to end in the cavernous sinus (Fig. 8-20).
Figure 8-19. Veins and sinuses of the brain from the lateral aspect.
Small vessels on the midsagittal surface of the hemisphere drain into the sagittal sinus and, from the medial region of the temporal lobe, into the basal vein (of Rosenthal) (Fig. 8-21). The venous blood in these channels and from the corpus callosum and the interior of the hemisphere (internal cerebral veins) drains into the great cerebral vein (of Galen) and then into the straight sinus. The confluence of sinuses (confluens sinuum) is formed by the junction of the straight sinus, the superior sagittal sinus, and both transverse sinuses (Figs. 8-20 and 8-21). Rather than a true confluence, the superior sagittal sinus usually drains into the right transverse sinus and the straight sinus into the left.
Figure 8-21. Veins and sinuses on the medial surface of the hemisphere and anterior (ventral) part of the temporal lobe.
Basal Aspect of the Brain
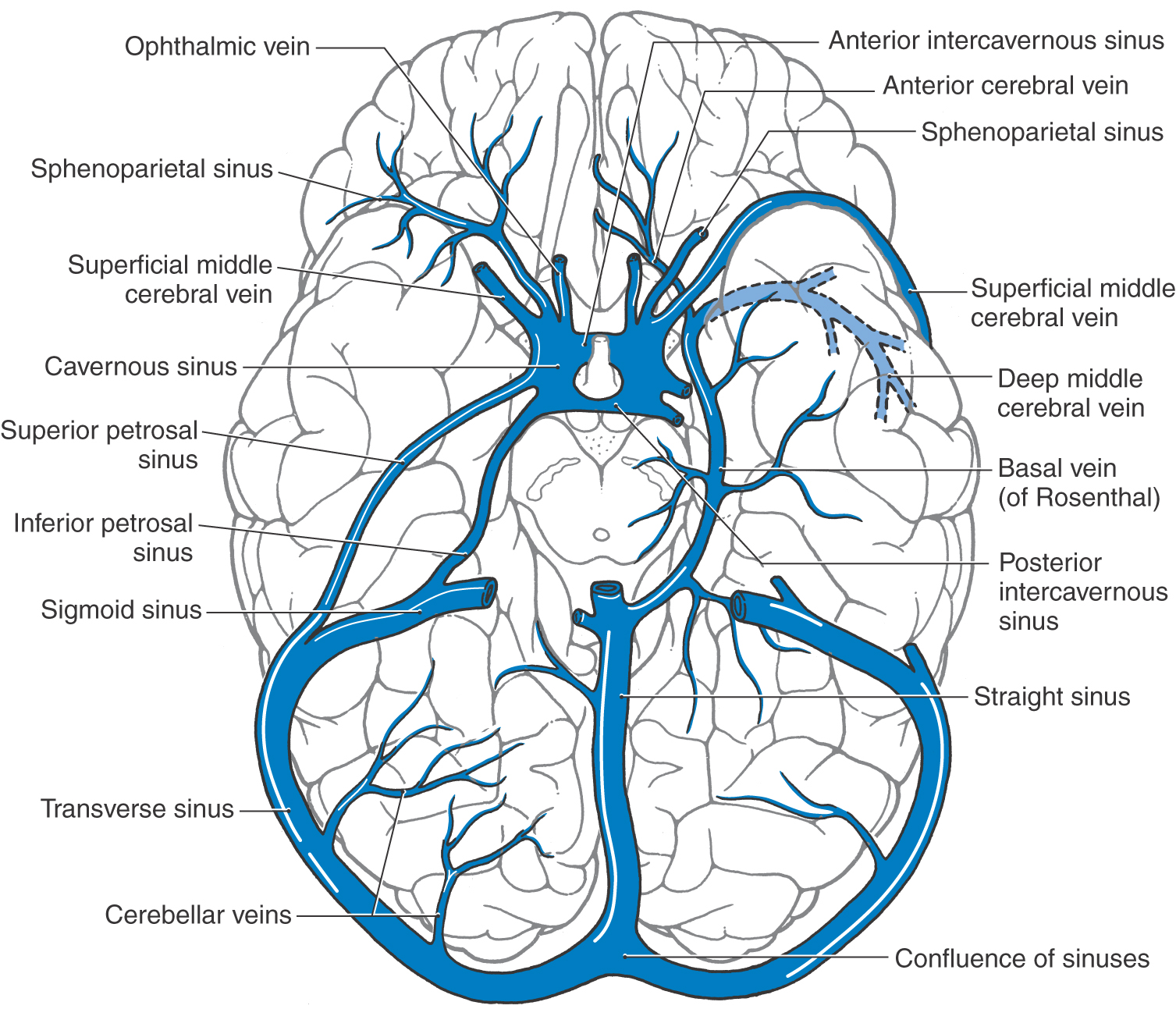
The basal vein (of Rosenthal) begins on the orbital cortex as the anterior cerebral vein and in the sylvian fissure as the deep middle cerebral vein and proceeds around the medial edge of the temporal lobe to join the straight sinus. It receives venous blood from the midbrain and medial areas of the temporal lobe (Figs. 8-20 and 8-21). The transverse and sigmoid sinuses form a shallow groove on the internal surface of the occipital and temporal bones, respectively, and receive several tributaries. Venous blood travels from the transverse and petrosal sinuses into the sigmoid sinus and then into the internal jugular vein at the jugular foramen (Figs. 8-19 and 8-20).
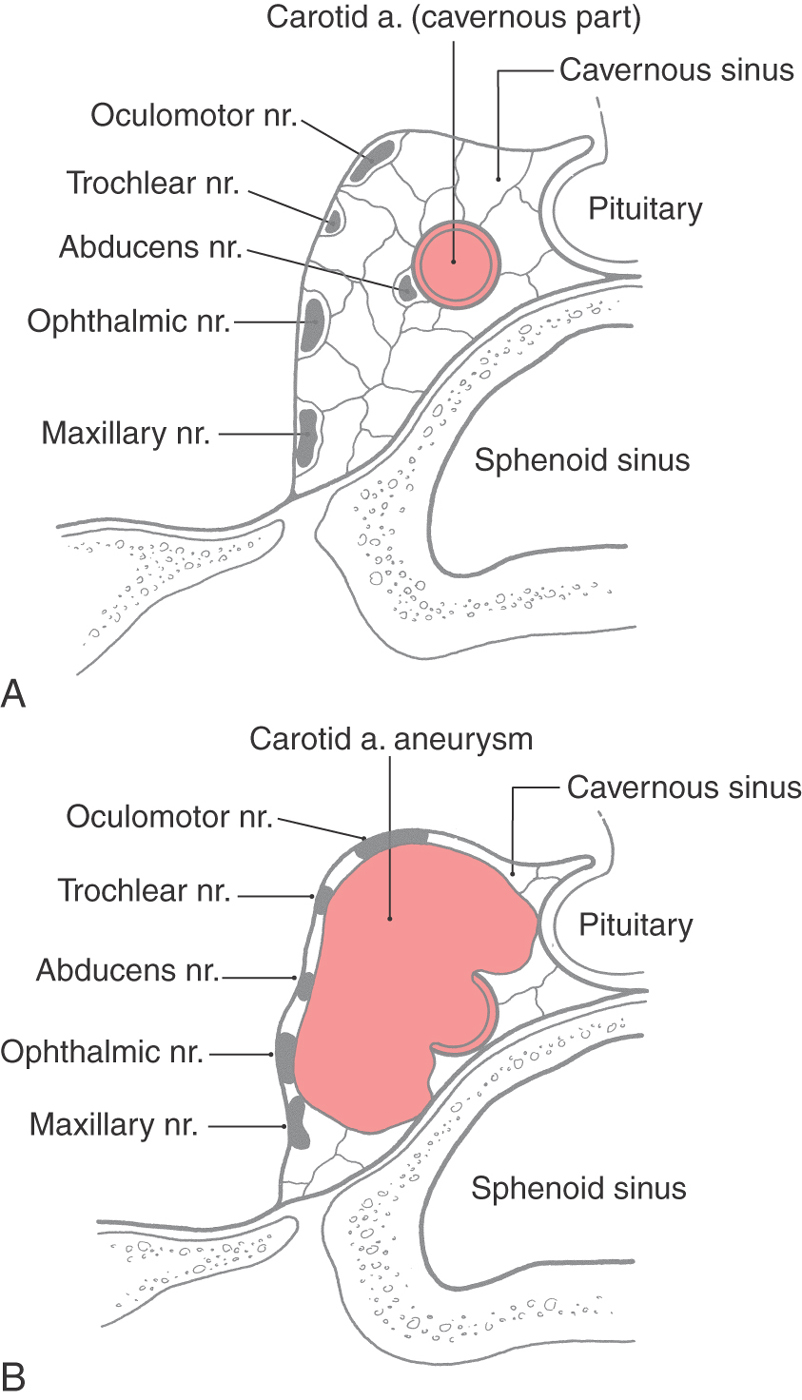
The cavernous sinus is located on either side of the body of the sphenoid bone. It contains the cavernous part of the internal carotid artery; the abducens, oculomotor, and trochlear nerves; and the ophthalmic and maxillary branches of the trigeminal nerve (Fig. 8-22). These nerves are found internal to the dura surrounding the sinus but are external to its endothelial lining.
Each cavernous sinus communicates with its counterpart through the intercavernous sinuses (Fig. 8-20). Caudally the cavernous sinus drains into the superior and inferior petrosal sinuses and the basilar plexus on the ventral aspect of the brainstem.
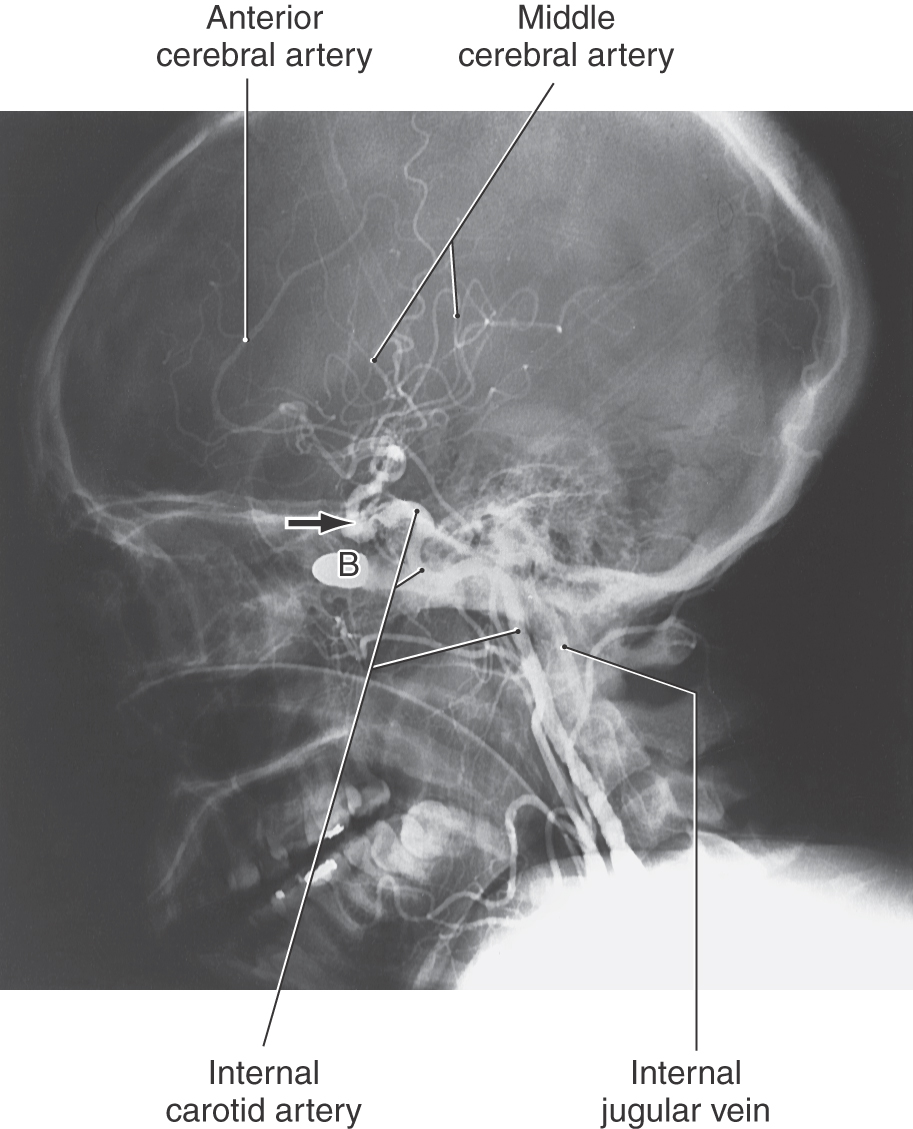
The neurologic deficits seen in patients with an aneurysm of the cavernous part of the carotid artery are related to the compact nature of the sinus and the close apposition of cranial nerves III, IV, and VI and V1 and V2 to the carotid artery (Fig. 8-22). An expanding aneurysm will affect the adjacent nerves, resulting in a partial or complete paralysis of eye movement, loss of the corneal reflex, and paresthesias or pain within the distribution of the ophthalmic and maxillary nerves. The direct shunting of blood from the internal carotid artery into the cavernous sinus, a carotid-cavernous fistula, is rarely the result of a ruptured aneurysm but may occur secondary to trauma (Fig. 8-23).
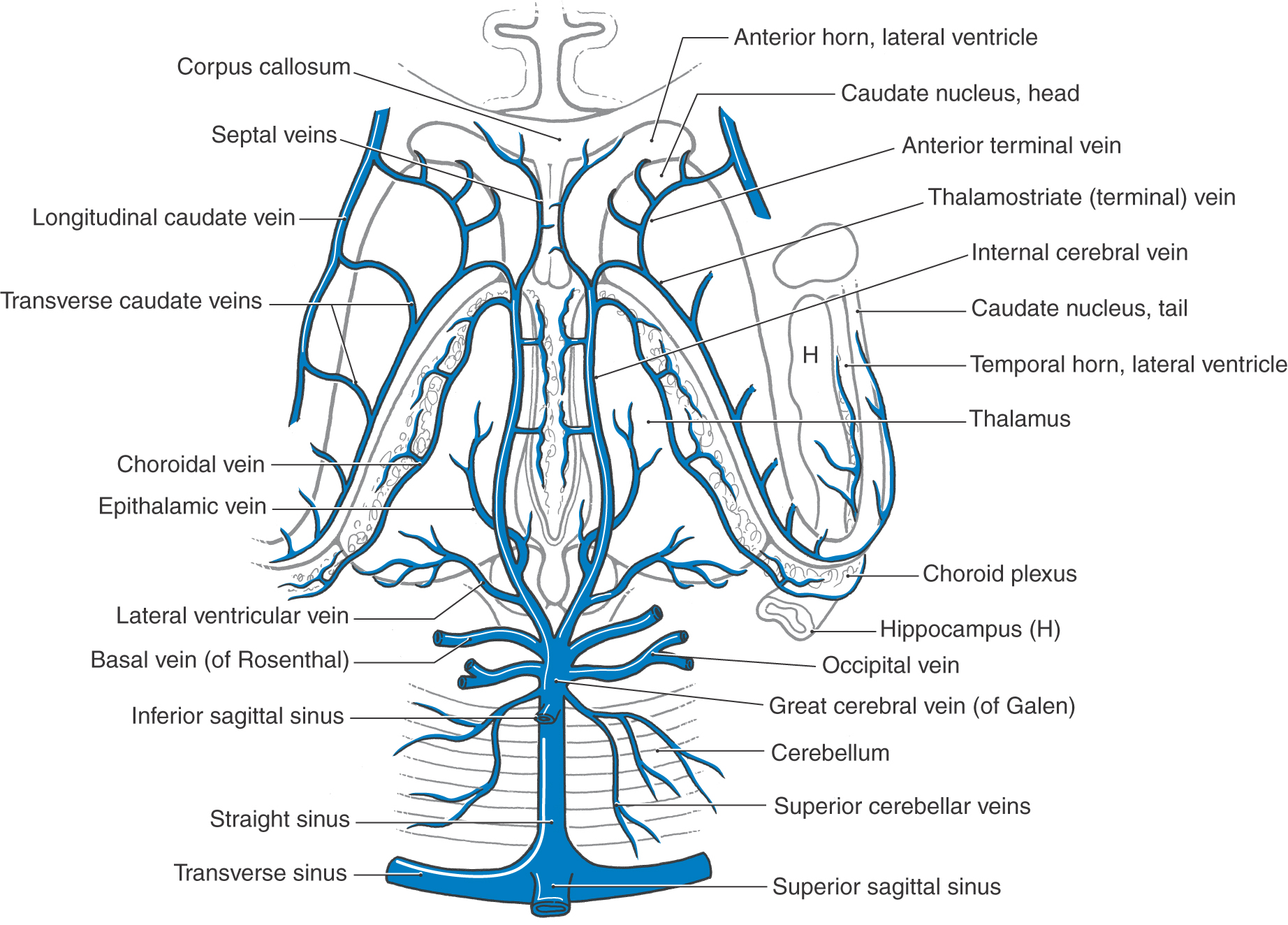
Internal Veins of the Hemisphere
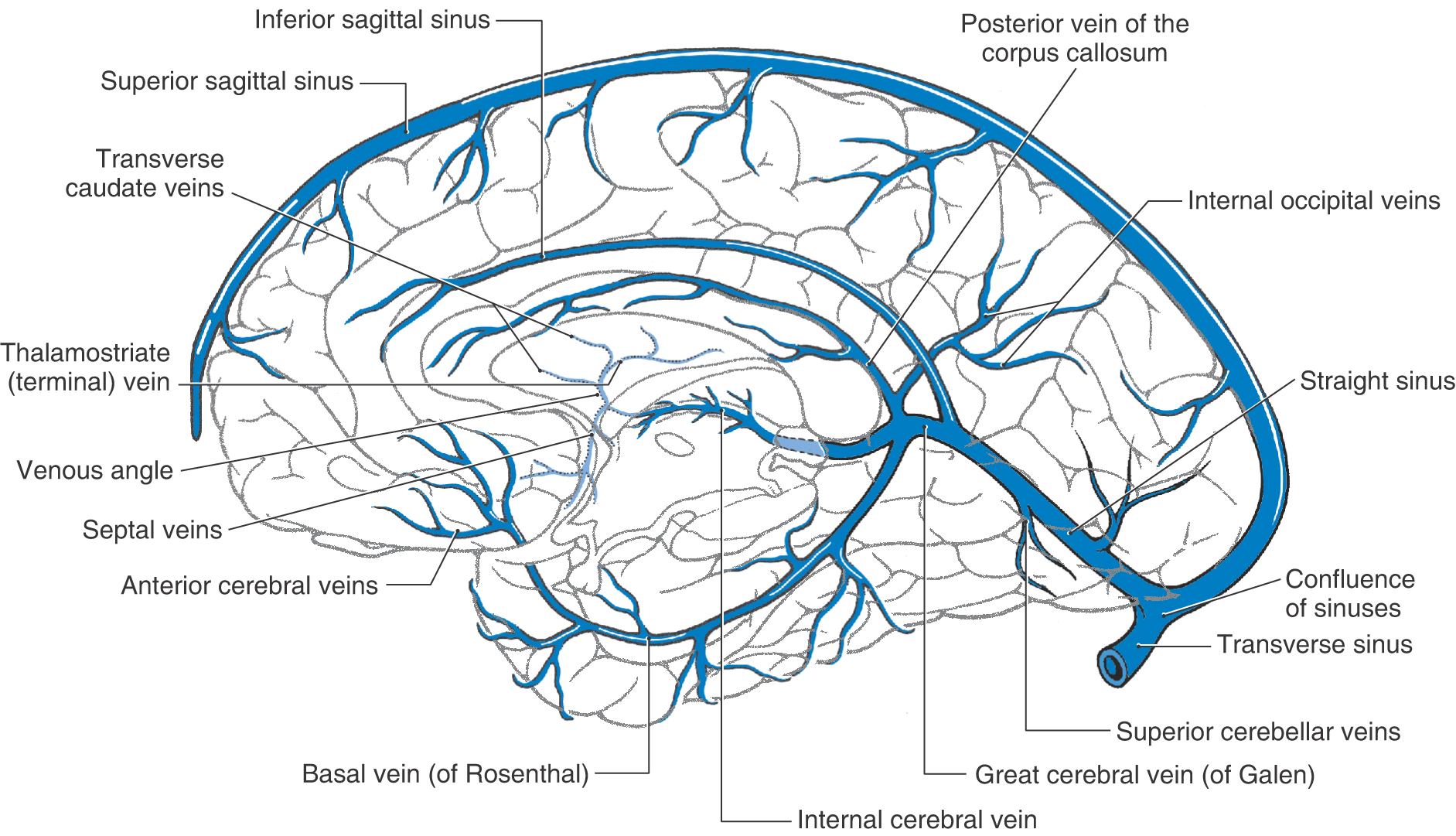
The main venous channels draining internal structures of the hemisphere are the internal cerebral veins (Figs. 8-20 and 8-24). They course along the dorsomedial edge of the thalamus and are located in the tela choroidea of the third ventricle. The principal tributaries of the internal cerebral veins and their relationships to adjacent structures are shown in Figure 8-24. Of these, the thalamostriate vein (also called the terminal vein) merits comment. It is found in association with the stria terminalis and drains the caudate nucleus (via the transverse caudate veins) and internal regions of the hemisphere dorsal and lateral to the caudate nucleus.
Figure 8-24. Veins draining internal areas of the hemisphere and the tributaries of the great cerebral vein and straight sinus.
The two internal cerebral veins join to form the great cerebral vein (of Galen). This large venous channel has several tributaries (Figs. 8-20 and 8-24) and is caudally continuous with the straight sinus.
Cerebral and spinal veins and dural sinuses lack valves. Consequently, pathologic processes may alter normal venous flow patterns and result in the transport of material into the brain. For example, a tumor or infection in the orbit may cause venous blood to flow toward the cavernous sinus rather than away from it. In this way, infectious material or tumor cells may pass from the orbit into the cavernous sinus and, through its connecting channels, to other parts of the brain.
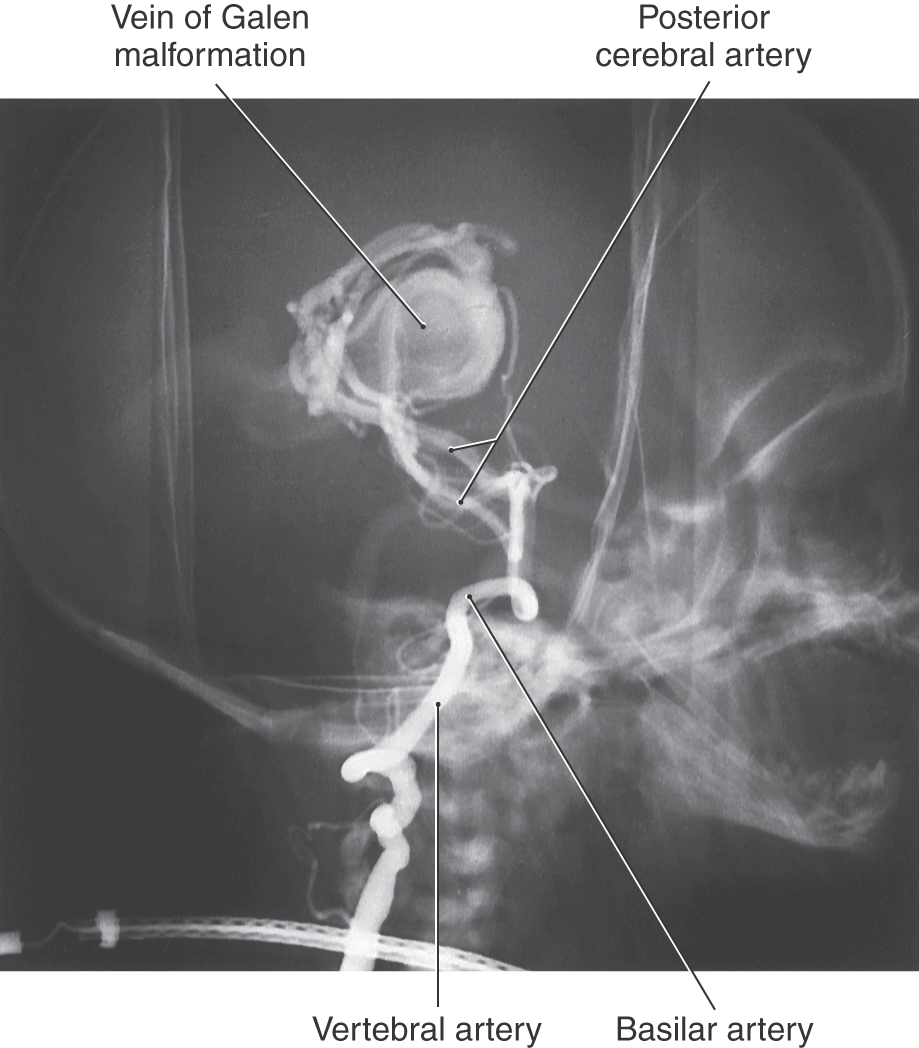
Malformations of the great cerebral vein of Galen are sometimes described as a special type of AVM or as an aneurysm. This lesion is usually seen in newborns or infants (Fig. 8-25). In these cases, the great cerebral vein is grossly enlarged and fed by large and abnormal branches of the cerebral and cerebellar arteries. Bulging fontanelles, progressive hydrocephalus (resulting from occlusion of the cerebral aqueduct), and dilated veins in the face and scalp are characteristic findings.
Figure 8-25. Vein of Galen malformation in an infant. This lesion is served by the posterior cerebral arteries.
Brainstem and Cerebellum
The brainstem is drained by a loosely organized network of venous channels located on its surface. In general, these vessels enter larger veins or venous sinuses located in the immediate vicinity. For example, veins of the midbrain enter the great cerebral and basal veins, whereas those of the pons and medulla may enter the petrosal sinuses and the cerebellar veins.
Venous drainage from the cerebellum is straightforward. The superior cerebellar veins enter the straight, transverse, or superior petrosal sinuses. The inferior cerebellar surface is drained by inferior cerebellar veins, which enter the inferior petrosal, transverse, or straight sinuses.
Cerebral Venous Thrombosis
The occurrence of blood clots within venous structures of the CNS is commonly called cerebral venous thrombosis. The veins involved are usually the larger ones, such as the superficial veins on the cerebral cortex or the internal cerebral veins. When sinuses are involved (dural sinus thrombosis), the clots may be found in the superior or inferior sagittal sinus, the straight sinus, the cavernous sinus, or in some cases more than one sinus.
The potential causes of cerebral venous thrombosis are numerous and variable and may be seen as a sequela to a primary disease process not related to the CNS, such as severe dehydration, cachexia related to systemic or neoplastic diseases, a hypercoagulable condition, use of birth control pills, sickle cell disease, or diabetes mellitus. On the other hand, cerebral venous thrombosis may occur after brain surgery, meningitis, sinus infections, traumatic head injuries, or gunshot injury to the head, especially those that may involve the sinuses.
The occlusion of a venous structure reduces or blocks venous return and results in a cascade of events. Upstream to the blockage, the veins and sinuses are engorged, the surrounding white matter becomes edematous, hemorrhage from the increased venous pressure is possible, and there are signs and symptoms of increased intracranial pressure (headache, nausea, vomiting, lethargy). The infarct created is called a venous infarct because of its origin from venous structures. The deficits experienced by the patient may reflect the general location of the venous thrombosis. For example, thrombosis of the middle portions of the superior sagittal sinus may result in increased tone and weakness of the opposite lower extremity or both lower extremities because of the proximity of the occluded sinus to the motor cortex. Thrombosis of the caudal portions of the superior sagittal sinus may result in visual deficits because of venous stasis of the superior sagittal sinus.
ARTERIES OF THE SPINAL CORD
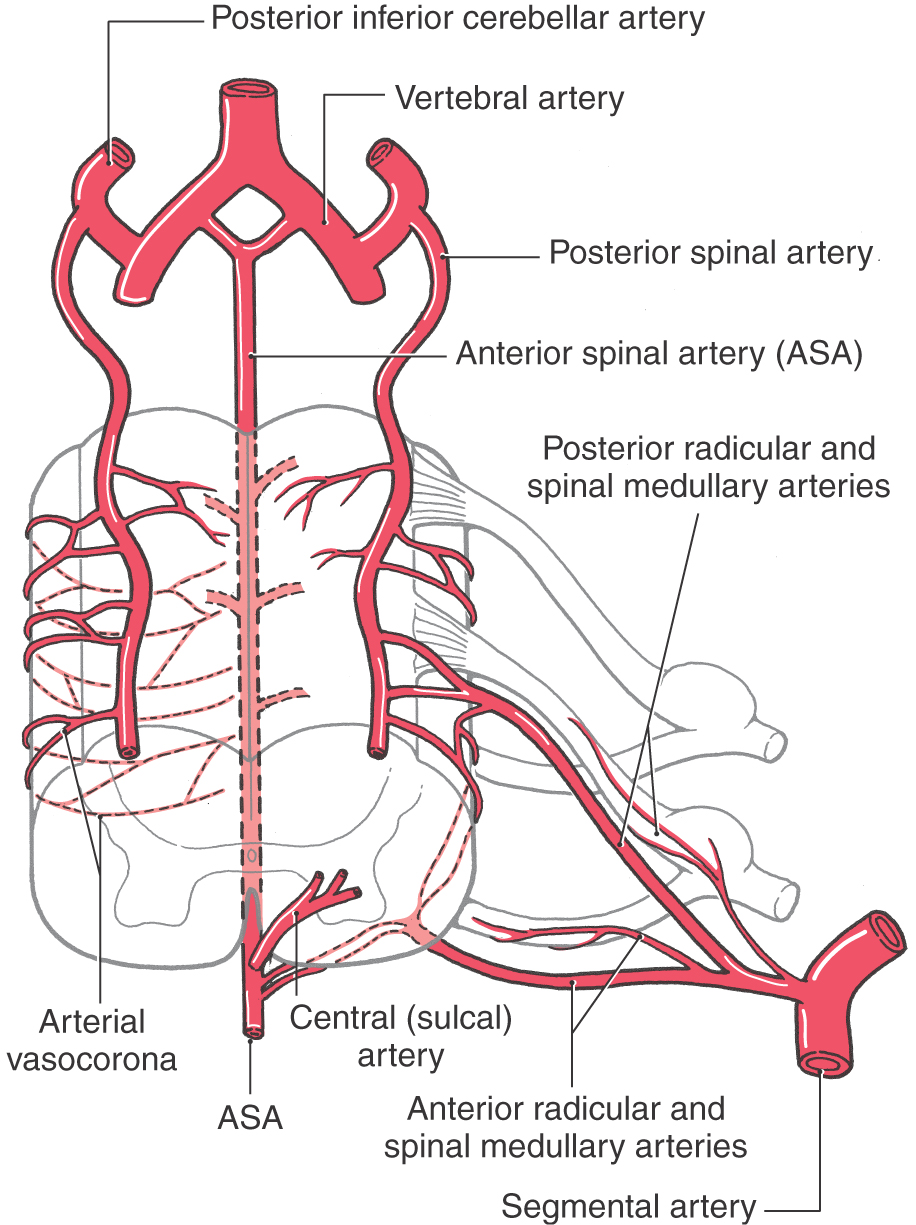
The blood supply to the spinal cord comes from the anterior and posterior spinal arteries and from spinal branches of segmental arteries (Fig. 8-26). The anterior spinal artery gives off central (or sulcal) branches, which pass alternately to the right and to the left to serve central regions of the spinal cord. The posterior spinal arteries course on the surface of the spinal cord medial to the posterior root entry zone. These arteries serve the posterior columns and contribute to the arterial vasocorona located on the surface of the spinal cord (Fig. 8-26).
Figure 8-26. Arteries serving the spinal cord.
The blood supply to the spinal cord is supplemented at most spinal levels by branches of segmental arteries. These spinal branches enter the intervertebral foramina and divide into posterior (dorsal) and anterior (ventral) radicular and spinal medullary arteries (Fig. 8-26). Because the radicular arteries supply the posterior and anterior roots, each spinal level has these branches. However, the spinal medullary arteries serve to supplement the blood supply to the spinal cord and are therefore not present at every spinal level. The terminal branches of the medullary arteries contribute to the formation of the arterial vasocorona. At level T12, L1, or L2, one spinal medullary (or possibly a radicular) artery, usually on the left, is especially large. This is the artery of Adamkiewicz. Surgery in this area of the lower back should avoid compromise of this vessel as it is a major source of blood to lower thoracic and upper lumbar cord levels.
VEINS OF THE SPINAL CORD
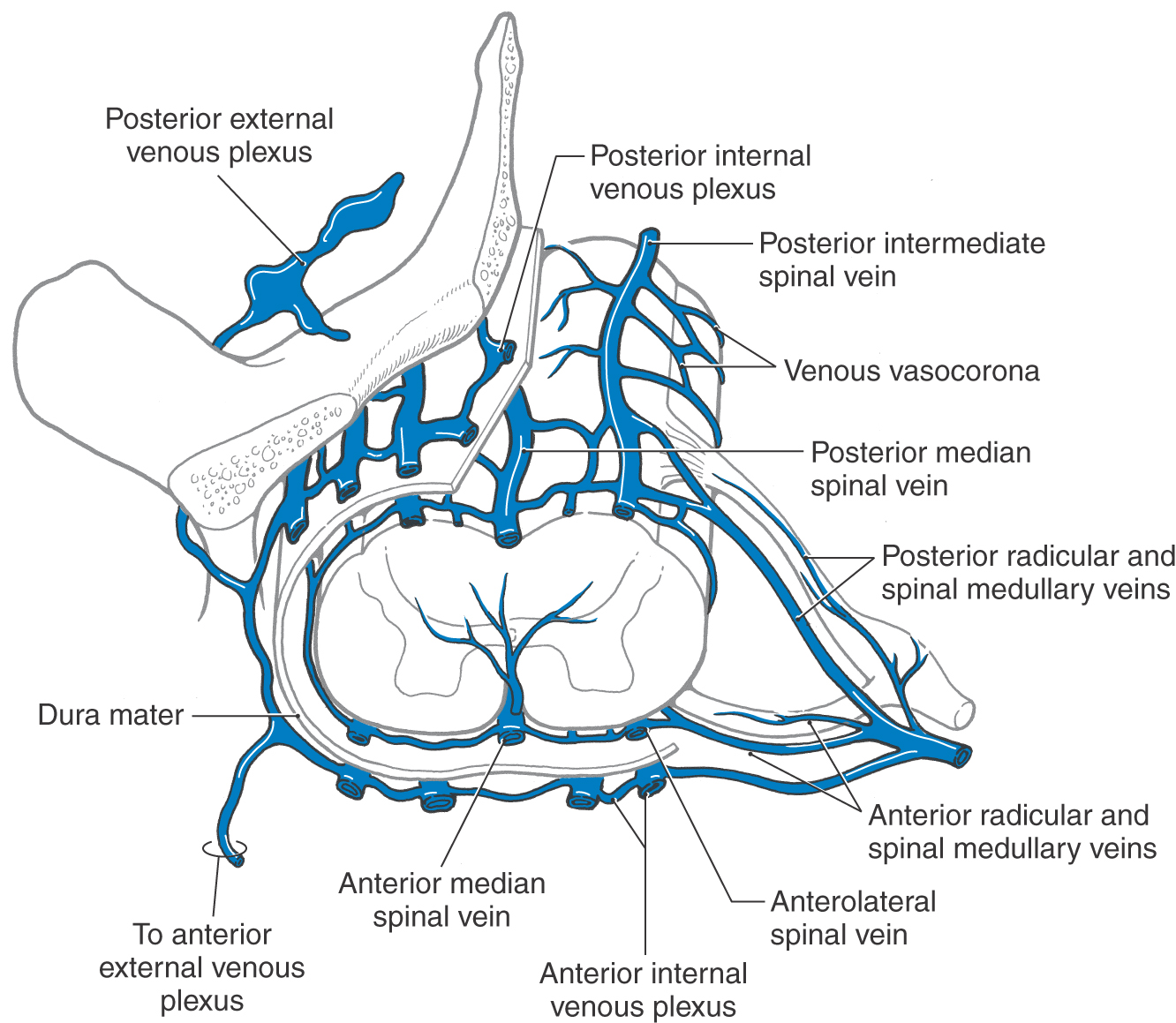
In general, the venous drainage of the spinal cord mirrors its arterial supply. The location of anterior and posterior spinal veins and their relationship to other spinal venous structures are shown in Figure 8-27. There is extensive communication between spinal veins and the internal and external venous plexuses found adjacent to the dural sac and vertebral bodies. The veins forming these plexuses apparently lack valves, and the flow in these channels is easily reversed. This represents an important conduit through which metastases from the pelvis, kidney, or lung may spread to the vertebral bodies or into the CNS.
Figure 8-27. Veins draining the spinal cord and the general relationships of internal and external venous plexuses.
Spinal Arteriovenous Malformations
Spinal AVMs, although comparable to those of the cranial cavity, do have some unique features. In adults, these lesions are usually served by branches of one segmental artery, whereas in children, spinal AVMs are usually much larger and have several feeding arteries. Spinal AVMs bleed less frequently than their cranial counterparts and may give rise to more localizing signs and symptoms. For example, in addition to low back pain and occasional sensory or motor problems, patients frequently experience impaired micturition.
BLOOD-BRAIN BARRIER
Although this chapter is primarily concerned with the distribution of vessels on the surface of the CNS, it is appropriate to mention briefly the blood-brain barrier (Fig. 8-28). Although this is a physiologic barrier to the movement of many substances into or out of the brain, the blood-brain barrier has anatomic features that correlate with its function.
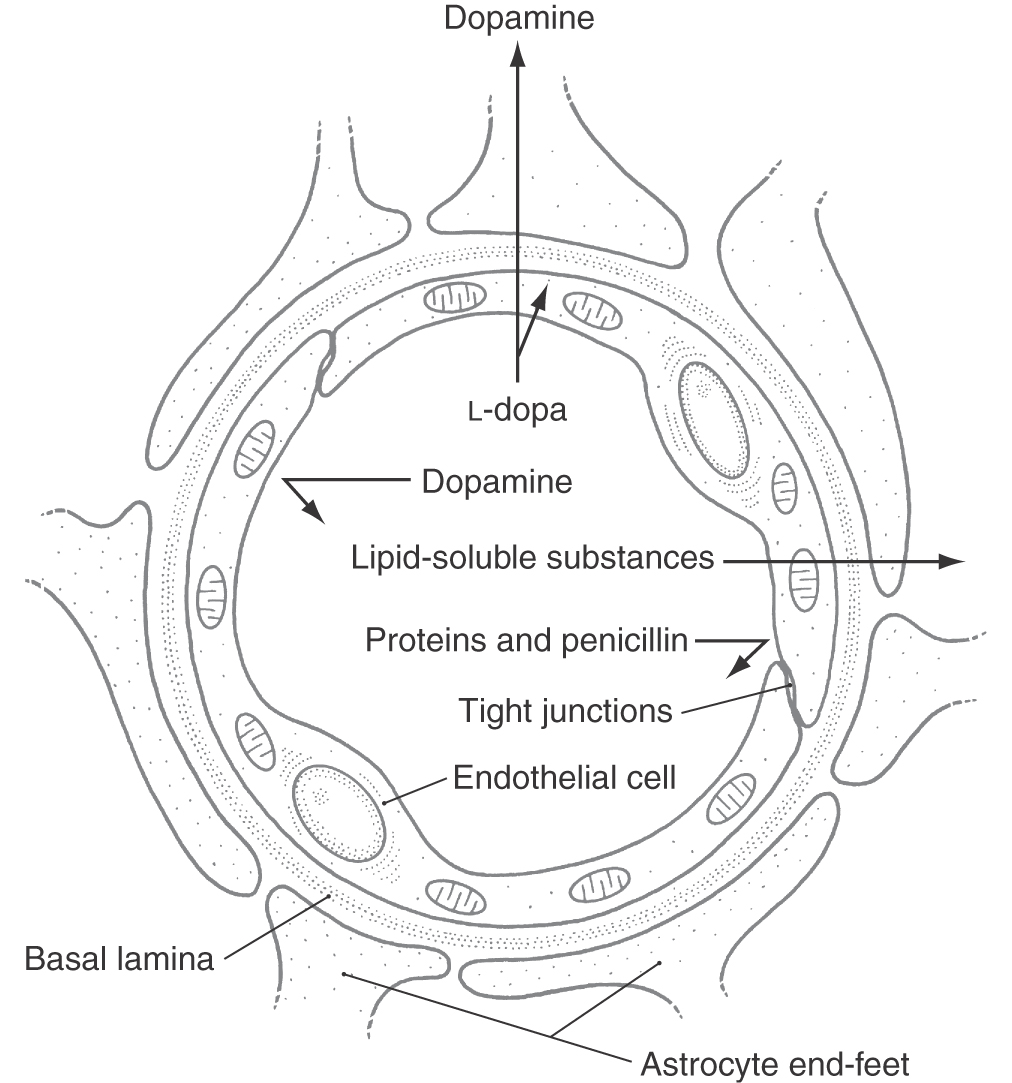
Figure 8-28. Basic structure of the blood-brain barrier.
The endothelial cells of brain capillaries form a continuous lining membrane; they are joined by numerous tight (occluding) junctions and have no fenestrations (Fig. 8-28). In contrast, capillaries of the general circulation have fenestrations and often lack tight junctions. Endothelial cells of brain capillaries rest on a continuous basement membrane (basal lamina), which in turn is surrounded by the end-feet of astrocytes (Fig. 8-28). Including the endothelium and its tight junctions, the subjacent basal lamina, and the astrocyte perivascular end-feet is a more inclusive view of the structure of the blood-brain barrier.
Under normal (healthy) conditions, the blood-brain barrier prohibits the movement of high-molecular-weight substances (such as proteins, penicillin, and dopamine) and vital dyes into the brain. On the other hand, L-dopa, a precursor for dopamine production, will cross the blood-brain barrier. In some disease states, however, the barrier breaks down. In the case of a brain tumor, the new capillaries that proliferate into the lesion do not have a close apposition to astrocytes. As a result, the endothelium of these tumor capillaries develops fenestrations but no tight junctions. These can provide a mechanism for diagnosis. For example, a radioactive amino acid injected intravascularly in such a patient will exit the capillaries in the tumor and will localize therein, but it will not be found in other (normal) parts of the brain.
Sources and Additional Reading
AbuRahma AF, Bergans JJ. Noninvasive Vascular Diagnosis. London: Springer-Verlag; 2000.
Duvernoy HM. Human Brainstem Vessels. Berlin: Springer-Verlag; 1978.
Hassler O. Blood supply to the human spinal cord. Arch Neurol. 1966;15:302–307.
Hassler O. Venous anatomy of human hindbrain. Arch Neurol. 1967;16:404–409.