A Primer on Vaccines
At the conclusion of the chapter, the reader should be able to:
• Identify the federal agency that regulates vaccine products.
• Describe vaccine policy and the role of vaccines in public safety.
• Briefly describe the history and use of several specific vaccines.
• Explain some new targets and technologies for vaccines.
• Identify at least three essential characteristics of a vaccine.
• Based on immunologic principles, describe the host response to vaccination.
• Analyze the problems associated with AIDS vaccine development and use.
• Describe the development and application of human papillomavirus vaccine.
• Compare and contrast the applications of at least four vaccines.
• Correctly answer case study related multiple choice questions.
• Be prepared to participate in a discussion of critical thinking questions.
• Describe the principle and clinical application of the tetanus antibodies assay.
Applications of Vaccines
• A boom in scientific discovery and the production of vaccines
• A desire to protect children from significant outbreaks of infectious diseases, including polio, measles, mumps, rubella, and pertussis (whooping cough)
• An increase in the birth rate among more educated and affluent parents, who accepted the use of vaccines
Despite public fears, American children now receive vaccinations to numerous diseases that were once common childhood infectious diseases. In the United States, the recommended childhood immunization schedule now includes vaccines to protect against 15 diseases, including seasonal influenza. Immunization schedules vary by age and by country (Tables 16-1 to 16-3, A and B).
Table 16-1
Childhood Vaccination Schedule, South Africa, 2011
| Age | Vaccine (No. of Doses) |
| At birth | BCG, vaccine against tuberculosis; trivalent oral polio vaccine (TOPV) |
| 6 wk | TOPV (one); rotavirus vaccine (RV) oral (one); DTaP-IPV/Hib vaccine (one); hepatitis B vaccine (one); PCV7 pneumococcal vaccine (one) |
| 10 wk | DTaP-IPV/Hib vaccine (two); DTaP (two); hepatitis B vaccine (two) |
| 14 wk | RV, oral rotavirus vaccine (two); DTaP-IPV/Hib vaccine (three); hepatitis B vaccine (three); PCV7, pneumococcal vaccine (two) |
| 9 mo | Measles vaccine (one); PCV7, pneumococcal vaccine (three) |
| 18 mo | DTaP-IPV/Hib vaccine (four); measles vaccine (two) |
| 6 yr | Td vaccine |
| 12 yr | Td vaccine |
From South African Vaccination and Immunisation Centre: www.savic.ac.za.
Table 16-2
Recommended Immunizations for Children, Birth Through 6 Years Old, United States, 2011
| Age | Vaccine |
| Birth | Hepatitis B (HepB) 11 |
| 1 mo | HepB 22 |
| 2 mo | HepB 2, if not given at 1 mo; rotavirus vaccine (RV)2; diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP)3; Haemophilus influenzae type b conjugate vaccine (Hib)4; pneumococcal vaccine (PCV)5; inactivated poliovirus vaccine (IPV)6 |
| 4 mo | RV2; DTaP3; Hib4; PCV5; IPV6 |
| 6 mo | HepB 3 (6-18 mo)1; RV2; DTaP3; Hib4; PCV5; IPV (6-18 mo)6; influenza yearly7 (6 mo-6 yr) |
| 12 mo | Hib4; PCV5; measles, mumps, and rubella (MMR) (12-15 mo)8; varicella (12-15 mo)9; hepatitis A (HepA)10 (12-23 mo); second dose should be given 6-18 mo later |
| 15 mo | DTaP3 |
| 18 mo | Influenza yearly7 |
| 2-3 yr | Influenza yearly7 |
| 4-6 yr | Influenza yearly7; DTaP3; IPV6; MMR8; varicella9 |
Note: Meningococcal conjugate vaccine, quadrivalent (MCV4), minimum age, 2 yr.
• Administer two doses of MCV4 at least 8 wk apart, children aged 2-10 yr with persistent complement component deficiency and anatomic or functional asplenia, and one dose every 5 yr thereafter.
• Persons with human immunodeficiency virus (HIV) infection who are vaccinated with MCV4 should receive two doses at least 8 wk apart.
• Administer one dose of MCV4 to children aged 2-10 yr who travel to countries with highly endemic or epidemic disease and during outbreaks caused by a vaccine serogroup.
• Administer MCV4 to children at continued risk for meningococcal disease who were previously vaccinated with MCV4 or meningococcal polysaccharide vaccine after 3 yr if first dose was administered at age 2-6 yr.
1Hepatitis B vaccine (HepB) (minimum age, birth).
•Administer monovalent HepB to all newborns before hospital discharge.
•If mother is hepatitis B surface antigen (HBsAg)-positive, administer HepB and 0.5 mL of hepatitis B immune globulin (HBIG) within 12 hr of birth.
•If mother’s HBsAg status is unknown, administer HepB within 12 hours of birth. Determine mother’s HBsAg status as soon as possible and, if HBsAg-positive, administer HBIG (no later than age 1 wk).
Doses following the birth dose:
•The second dose should be administered at age 1 or 2 mo. Monovalent HepB should be used for doses administered before age 6 wk .
•Infants born to HBsAg-positive mothers should be tested for HBsAg and antibody to HBsAg 1-2 mo after completion of at least three doses of the HepB series, at age 9-18 mo (generally at the next well-child visit).
•Administration of four doses of HepB-infants is permissible when a combination vaccine containing HepB is administered after the birth dose.
•Infants who did not receive a birth dose should receive three doses of HepB on a schedule of 0, 1, and 6 mo.
•The final (third or fourth) dose in the HepB series should be administered no earlier than age 24 wk.
3Diptheria and tetanus toxides and acellular perfusion vaccine (DTaP) (minimum age, 6 wk).
•The fourth dose may be administered as early as age 12 mo, provided at least 6 mo have elapsed since the third dose.
4Hemophilus influenzae type b-conjugate vaccine (Hib) (minimum age, 6 wk).
•If PRP-OMP (PedvaxHIB or Comvax [HepB-Hib]) is administered at ages 2 and 4 mo, a dose at age 6 mo is not indicated.
•Hiberix should not be used for doses at ages 2, 4, or 6 mo for the primary series but can be used as the final dose in children aged 12 mo-4 yr.
5Pneumococcal vaccine (minimum age, 6 wk for pneumococcal conjugate vaccine [PCV]; 2 yr for pneumococcal polysaccharide vaccine [PPSV]).
•PCV is recommended for all children <5 yr. Administer one dose of PCV to all healthy children aged 24-59 mo who are not completely vaccinated for their age.
•A PCV series begun with 7-valent PCV (PCV7) should be completed with 13-valent PCV (PCV13).
•A single supplemental dose of PCV13 is recommended for all children aged 14-59 mo who have received an age-appropriate series of PCV7.
•A single supplemental dose of PCV13 is recommended for all children aged 60-71 mo with underlying medical conditions who have received an age-appropriate series of PCV7.
•The supplemental dose of PCV13 should be administered at least 8 wk after the previous dose of PCV7. See MMWR 2010:59(No. RR-11).
•Administer PPSV at least 8 wk after last dose of PCV to children aged 2 yr or older with certain underlying medical conditions, including a cochlear implant.
6Inactivated poliovirus vaccine (IPV) (minimum age, 6 wk).
•If 4 or more doses are administered prior to age 4 yr an additional dose should be administered at age 4-6 yr.
•The final dose in the series should be administered on or after the fourth birthday and at least 6 mo following the previous dose.
7Influenza vaccine (seasonal); (minimum age, 6 mo for trivalent inactivated influenza vaccine [TIV]; 2 yr for live attenuated influenza vaccine [LAIV]).
•For healthy children aged 2 yr and older (i.e., those who do not have underlying medical conditions that predispose them to influenza complications), LAIV or TIV may be used, except that LAIV should not be given to children aged 2-4 yr who have had wheezing in the past 12 mo.
•Administer two doses (separated by at least 4 wk) to children aged 6 mo-8 yr who are receiving seasonal influenza vaccine for the first time or who were vaccinated for the first time during the previous influenza season but only received one dose.
•Children aged 6 mo-8 yr who received no doses of monovalent 2009 H1N1 vaccine should receive two doses of 2010–2011 seasonal influenza vaccine.
8Measles, mumps, and rubella vaccine (MMR) (minimum age, 12 mo). See MMWR 2010;59(No. RR-8):33–34.
•The second dose may be administered before age 4 yr, provided at least 4 wk have elapsed since the first dose.
•The second dose may be administered before age 4 yr, provided at least 3 mo have elapsed since the first dose.
•For children aged 12 mo-12 yr, the recommended minimum interval between doses is 3 mo. However, if the second dose was administered at least 4 wk after the first dose, it can be accepted as valid.
9Varicella vaccine (minimum age, 12 mo).
•The second dose may be administered before age 4 yr, provided at least 3 mo have elapsed since the first dose.
•For children aged 12 mo-12 yr, the recommended minimum interval between doses is 3 mo. However, if the second dose was administered at least 4 wk after the first dose, it can be accepted as valid.
10Hepatitis A vaccine (HepA) (minimum age, 12 mo).
•Administer two doses at least 6 mo apart.
•HepA is recommended for children >23 mo who live in areas in which vaccination programs target older children, who are at increased risk for infection, or for whom immunity against hepatitis A is desired.
From Centers for Disease Control: www.cdc.gov/vaccines
Immunization Schedules (www.cdc.gov/vaccines) Retrieved October 31, 2011.
Centers for Disease Control: ___________Prevention of Pneumococcal Disease Among Infants and Children — Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine
December 10, 2010 / 59(RR11);1-18 MMWR Morb Mortal Wkly Rep 59(RR-11) 2010; and
Prevention and Control of Influenza with Vaccines
Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010
August 6, 2010 / 59(rr08);1-62 Centers for Disease Control: MMWR Morb Mortal Wkly Rep 59(RR-8):33–34, 2010.
Table 16-3A
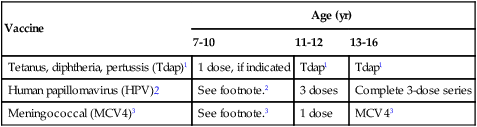
Recommended Immunization Schedule for Persons 7-18 years, United States, 2011
| Vaccine | Age (yr) | ||
| 7-10 | 11-12 | 13-16 | |
| Tetanus, diphtheria, pertussis (Tdap)1 | 1 dose, if indicated | Tdap1 | Tdap1 |
| Human papillomavirus (HPV)2 | See footnote.2 | 3 doses | Complete 3-dose series |
| Meningococcal (MCV4)3 | See footnote.3 | 1 dose | MCV43 |
1Tetanus and diphtheria toxoids and acellular pertussis vaccine (Tdap, minimum age, 10 yr for Boostrix and 11 yr for Adacel).
• Persons aged 11-18 yr who have not received Tdap should receive a dose followed by Td booster doses every 10 yr thereafter.
• Persons aged 7-10 yr who are not fully immunized against pertussis (including those never vaccinated or with unknown pertussis vaccination status) should receive a single dose of Tdap. Refer to the catch-up schedule if additional doses of tetanus and diphtheria toxoid–containing vaccine are needed.
• Tdap can be administered regardless of the interval since the last tetanus and diphtheria toxoid–containing vaccine.
2Human papillomavirus (HPV) vaccine. HPV4 (Gardasil) and HPV2 (Cervarix). (minimum age, 9 yr).
• Either HPV4 or HPV2 is recommended in a three-dose series for females aged 11 or 12 yr. HPV4 is recommended in a three-dose series for males aged 11 or 12 yr.
• The vaccine series can be started beginning at age 9 yr.
• Administer the second dose 1 to 2 mo after the first dose and the third dose 6 mo after the first dose (at least 24 wk after the first dose).
• See MMWR 2010;59:626-32, available at http://www.cdc.gov/mmwr/pdf/wk/mm5920.pdf
3Meningococcal conjugate vaccine/MCV4, quadrivalent (minimum age, 2 yr).
• Administer MCV4 at age 11-12 yr with a booster dose at age 16 yr.
• Administer one dose at age 13-18 yr if not previously vaccinated.
• Persons who received first dose at age 13-15 yr should receive a booster dose at age 16-18 yr with a minimum interval of at least 8 wk after the preceding dose.
• If the first dose is administered at age 16 yr or older, a booster dose is not needed.
• Administer two doses at least 8 wk apart to previously unvaccinated persons with persistent complement component deficiency and anatomic or functional asplenia, and one dose every 5 yr thereafter.
• Adolescents aged 11-18 with human immunodeficiency virus (HIV) infection should receive a two-dose primary series of MCV4, at least 8 wk apart.
• See MMWR 2011;60:72-76, available at http://www.cdc.gov/mmwr/pdf/wk/mm6003.pdf and Vaccines for Children Program.
Reference: www.cdc.gov/vaccines, retrieved October 1, 2012.
From Centers for Disease Control: Vaccines and immunizations, 2012 (www.cdc.gov/vaccines).
Table 16-3B
Recommended Immunization Schedule for Persons aged 7-18 years, United States, 2012
| Vaccine | Age: 7-18 yr |
| Influenza4 | Yearly for all children |
| Pneumococcal (PCV13)5 | See footnote.5 |
| Hepatitis A (Hep A)6 | Complete 2-dose series |
| Hepatitis (Hep B)7 | Complete 3-dose series |
| Inactivated poliovirus (IPV)8 | Complete 3-dose series |
| Measles, mumps, rubella (MMR)9 | Complete 2-dose series |
| Varicella10 | Complete 2-dose series |
4Influenza vaccine (trivalent inactivated influenza vaccine (TIV) and live, attenuated influenza vaccine (LAIV)).
•For most healthy nonpregnant persons, either LAIV or TIV may be used, except LAIV should not be used for some persons, including those with asthma or any other underlying medical conditions that predispose them to influenza complications. For all other contraindications to use of LAIV, see MMWR 59(RR-8), 2010, available at http://www.cedc.gov/mmwr/pdf/rr/rr5908.pdf.
•Administer one dose to persons aged 9 yr and older.
•For children 6 mo-8 yr of age:
–For the 2011-2012 season, administer two doses (separated by at least 4 wk) to those who did not receive at least one dose of the 2010-2011 vaccine. Those who received at least one dose of the 2010-2011 vaccine require one dose for the 2011-2012 season.
–For the 2012-2013 season, follow dosing guidelines in the 2012 ACIP influenza vaccine recommendations.
5Pneumococcal vaccines (Pneomococcal conjugate vaccine (PCV) and pneumococcal polysaccharide vaccine (PPSV)).
•A single dose of PCV may be administered to children aged 6-18 yr who have functional or anatomic asplenia, HIV infection or other immunocompromising condition, cochlear implant or CSF leak. See MMWR 59(No. RR-11), 2010.
•Administer PPSV at least 8 wk after the last dose of PCV to children aged 2 yr or older with certain underlying medical conditions, including a cochlear implant. A single revaccination should be administered after 5 yr to children with anatomic/functional asplenia or an immunocompromising condition.
•HepA is recommended for children aged older than 23 mo who live in areas where vaccination programs target older children, or who are at increased risk for infection, or for whom immunity against hepatitis A is desired.
•Administer two doses at least 6 mo apart to unvaccinated persons.
•Administer the three-dose series to those not previously vaccinated. For those with incomplete vaccination, follow the catch-up schedule.
•A two-dose series (separated by at least 4 mo) of adult formulation Recombivax HB is licensed for children aged 11-15 yr.
8Inactivated poliovirus vaccine (IPV).
•The final dose in the series should be administered on or after the fourth birthday and at least 6 mo following the previous dose.
•If both oral polio vaccine (OPV) and IPV were administered as part of a series, a total of four doses should be administered, regardless of the child’s current age.
•IPV is not routinely recommended for U.S. residents aged 18 yr or older.
9Measles, mumps, and rubella (MMR) vaccine.
•For persons without evidence of immunity (see MMWR 56[No. RR-4], 2007), administer two doses if not previously vaccinated or the second dose if only one dose has been administered.
•For persons aged 7–12 yr, the recommended minimum interval between doses is 3 mo. However, if the second dose was administered at least 4 wk after the first dose, it can be accepted as valid.
•For persons aged 13 yr and older, the minimum interval between doses is 4 wk.
Reference: www.cdc.gov/vaccines, retrieved October 1, 2012.
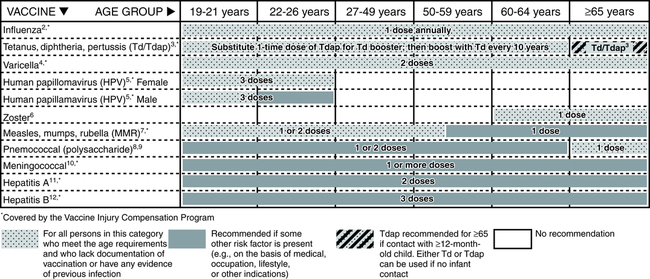
Adults require updates of certain vaccinations (Fig. 16-1). Especially serious diseases for adults age 65 years and older include diphtheria, herpes zoster (shingles), influenza, pneumococcus, and tetanus (lockjaw). In 2006, a vaccine was approved for adults older than 60 years to reduce the risk of shingles (reactivation of varicella virus) in those who had chickenpox in childhood. International travelers frequently require vaccination to endemic diseases in a particular country (e.g., hepatitis A, yellow fever). Health care professionals are now protected against hepatitis B by vaccines. Also, each year, many adults prepare for winter and the flu season by receiving flu vaccine.
Vaccine Approval
Safety Issues
A U.S. Food and Drug Administration (FDA)–approved vaccine (Table 16-4) must meet specific requirements, as follows:
Table 16-4
Examples of Available Vaccines Licensed for Immunization and Distribution in the United States
| Vaccine Name | Trade Name | Manufacturer |
| BCG Live BCG Live BCG Live |
BCG Vaccine Mycobax TICE BCG |
Organon Teknika Corp LLC Sanofi Pasteur, Ltd Organon Teknika Corp LLC |
| Diphtheria & tetanus toxoids adsorbed Diphtheria & tetanus toxoids & acellular pertussis vaccine adsorbed Diphtheria & tetanus toxoids & acellular pertussis vaccine adsorbed Diphtheria & tetanus toxoids & acellular pertussis vaccine adsorbed Diphtheria & tetanus toxoids & acellular pertussis adsorbed, hepatitis B (recombinant) and inactivated poliovirus vaccine combined Diphtheria & tetanus toxoids & acellular pertussis adsorbed and inactivated poliovirus vaccine Diphtheria & tetanus toxoids & acellular pertussis adsorbed, inactivated poliovirus and Haemophilus b conjugate (tetanus toxoid conjugate) vaccine |
Trepedia Infanrix DAPTACEL Pediarix KINRIX Pentacel |
Sanofi Pasteur, Inc. Sanofi Pasteur, Inc. GlaxoSmithKline Biologicals Sanofi Pasteur, Ltd. GlaxoSmithKline Biologicals GlaxoSmithKline Biologicals Sanofi Pasteur, Ltd |
| Haemophilus b conjugate vaccine (meningococcal protein conjugate) Haemophilus b conjugate vaccine (tetanus toxoid conjugate) Haemophilus b conjugate vaccine (tetanus toxoid conjugate) Haemophilus b conjugate vaccine (meningococcal protein conjugate) & Hepatits B vaccine (recombinant) |
PedvaxHIB ActHIB Hiberix Comvax |
Merck & Co., Inc. Sanofi Pasteur, SA GlaxoSmithKline Biologicals, S.A. Merck & Co., Inc. |
| Hepatitis A, inactivated Hepatitis A, inactivated |
Havrix VAQTA |
GlaxoSmithKline Biologicals Merck & Co., Inc. |
| Hepatitis A inactivated and hepatitis B (recombinant) vaccine | Twinrix | GlaxoSmithKline Biologicals |
| Hepatitis B vaccine (recombinant) Hepatitis B vaccine (recombinant) |
Recombivax HB Engerix-B |
Merck & Co., Inc. GlaxoSmithKline Biologicals |
| Human papillomavirus quadrivalent (types 6, 11, 16, 18) vaccine, recombinant Human papillomavirus bivalent (types 16, 18) vaccine, recombinant |
Gardasil Cervarix |
Merck & Co., Inc. GlaxoSmithKline Biologicals |
| Influenza A (H1N1) 2009 monovalent vaccine Influenza A (H1N1) 2009 monovalent vaccine Influenza A (H1N1) 2009 monovalent vaccine Influenza A (H1N1) 2009 monovalent vaccine Influenza A (H1N1) 2009 monovalent vaccine |
No trade name No trade name No trade name No trade name No trade name |
CSL Limited MedImmune, LLC ID Biomedical Corp of Quebec Novartis Vaccines and Diagnostics Limited Sanofi Pasteur, Inc. |
| Influenza virus vaccine Influenza virus vaccine, live, intranasal Influenza virus vaccine, H5N1 Influenza virus vaccine, trivalent, types A and B Influenza virus vaccine, trivalent, types A and B Influenza virus vaccine, trivalent, types A and B Influenza virus vaccine, trivalent, types A and B Influenza virus vaccine, trivalent, types A and B |
Afluria FluMist No trade name FluLaval Fluarix Fluvirin Agriflu Fluzone and Fluzone High-Dose |
CSL Limited MedImmune, LLC Sanofi Pasteur, Inc. ID Biomedical Corp of Quebec GlaxoSmithKline Biologicals Novartis Vaccines and Diagnostics S.r.l. Novartis Vaccines and Diagnostics S.r.l. Sanofi Pasteur, Inc. |
| Japanese encephalitis virus vaccine, inactivated, adsorbed Japanese encephalitis virus vaccine, inactivated |
Ixiaro JE-Vax |
Intercell Biomedical Research Foundation for Microbial Diseases of Osaka University |
| Measles virus vaccine, live Measles, mumps, and rubella virus vaccine, live Measles, mumps, rubella and varicella virus vaccine, live |
Attenuvax M-M-R-II ProQuad |
Merck & Co, Inc. Merck & Co, Inc. Merck & Co, Inc. |
| Meningococcal (groups A, C, Y, and W-135) Oligosaccharide diphtheria CRM197 conjugate vaccine Meningococcal polysaccharide (serogroups A, C, Y, and W-135) diphtheria toxoid conjugate vaccine Meningococcal polysaccharide (serogroups A, C, Y, and W-135 combined |
Menveo
Menactra |
Novartis Vaccines and Diagnostics, Inc. Sanofi Pasteur, Inc. Sanofi Pasteur, Inc. |
| Mumps virus vaccine, live | Mumpsvax | Merck & Co, Inc. |
| Pneumococcal vaccine, polyvalent Pneumococcal 7-valent conjugate vaccine Pneumococcal 13-valent conjugate vaccine |
Pneumovax 23 Prevnar Prevnar 13 |
Merck & Co, Inc. Wyeth Pharmaceuticals Inc. Wyeth Pharmaceuticals Inc. |
| Poliovirus vaccine inactivated (monkey kidney cell) | IPOL | Sanofi Pasteur, SA |
| Rabies vaccine Rabies vaccine |
Imovax RabAvert |
Sanofi Pasteur, SA Novartis Vaccines and Diagnostics |
| Rotavirus vaccine, live, oral Rotavirus vaccine, live, oral |
ROTARIX RotaTeq |
GlaxoSmithKline Biologicals Merck & Co., Inc. |
| Rubella virus vaccine, live | Meruvax II | Merck & Co., Inc. |
| Smallpox (vaccinia) vaccine, live | ACAM 2000 | Sanofi Pasteur Biologics Co. |
| Tetanus & diphtheria toxoids adsorbed for adult use Tetanus & diphtheria toxoids adsorbed for adult use |
No trade name DECAVAC |
MassBiologics Sanofi Pasteur, Inc. |
| Tetanus toxoid Tetanus toxoid adsorbed Tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine, adsorbed Tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine, adsorbed |
No trade name No trade name Adacel Boostrix |
Sanofi Pasteur, Inc. Sanofi Pasteur, Inc. Sanofi Pasteur, Inc. GlaxoSmithKline Biologicals |
| Typhoid vaccine live oral Ty21a Typhoid VI polysaccharide vaccine |
Vivotif TYPHIM VI |
Berna Biotech, Ltd. Sanofi Pasteur, Inc. |
| Varicella virus vaccine, live Yellow Fever vaccine |
Varivax YF-Vax |
Merck & Co., Inc. Sanofi Pasteur, Inc. |
| Zoster vaccine, live | ZostaVax | Merck & Co., Inc. |
† Children ≥2 yr with certain medical conditions may need a dose of pneumococcal vaccine (PPSV) and meningococcal vaccine (MCV4). See vaccine-specific recommendations at http://www.cdc.gov/vaccines/pubs/ACIP-list.htm.
Adapted from U.S. Food and Drug Administration: Complete list of vaccines licensed for immunization and distribution in the U.S., 2012 (http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ ucm093833.htm).
Representative Vaccines
HIV-AIDS
The status of HIV vaccines to date is as follows:
1. There are no proven effective therapeutic or preventive HIV vaccines.
2. There is a lack of knowledge related to the ability of a vaccine to induce HIV-specific immune responses that are effective in preventing or treating HIV infection.
3. Therapeutic HIV vaccine research is still in its early stages.
Vaccine Problems
Researchers have identified the following specific problem areas:
• A lack of knowledge related to the critical components in the body’s immune response to HIV infection.
• The high risk of using the entire weakened or inactive HIV in a vaccine.
• The extensive rate of viral mutation as HIV replicates. Strains worldwide vary by as much as 35% in terms of the proteins that make up the outer coat of the virus. Even an infected person can experience a change in viral protein by as much as 10% over years. This genetic diversity may require an effective vaccine to be based on multiple viral strains.
• The protective effect of a vaccine may last only a short time and frequent mandatory booster vaccinations would be impractical and expensive.
• Vaccinated persons could become more susceptible to HIV infection because of vaccine-induced enhancement of infection.
• No vaccine clinical trial to date has demonstrated stimulation of the cellular components of the immune system in the way needed to destroy HIV.
• Animal models have severe limitations, including the possibility of integration of DNA into the human genome from monkeys.
• No research studies have successfully demonstrated which immune responses correlate with protection from HIV infection.
Vaccine Expectations
Reasons for optimism about HIV vaccine development include the following:
1. Nonhuman primates vaccinated with products based on HIV or simian immunodeficiency virus have shown complete or partial protection against infection with the wild-type virus.
2. Successful vaccines have been developed against the feline immunodeficiency virus, also a retrovirus.
3. Almost all humans develop some form of immune response that is protective or able to control the viral infection over a long period. Some individuals remain disease-free for up to 25 years, frequently with undetectable viral load levels.
4. Vaccines that present epitopes to the immune system in a conformationally precise manner may induce the body to produce neutralizing antibodies and provide a high level of protection against HIV infection.
5. In the future, microbial and viral genome sequencing will become increasingly rapid and less expensive. One approach, known as reverse vaccinology, involves cloning and expressing all proteins that are predicted based on a complete genome to be secreted or surface-associated, starting with the complete genome sequence. This approach allows for a small group of proteins from microorganisms (e.g., group B meningococcus, group B Streptococcus, extraintestinal pathogenic Escherichia coli) to be candidates for multivalent subunit vaccines. To date, however, these organisms have eluded vaccine development.
 Tetanus Antibodies (IgG)
Tetanus Antibodies (IgG)
Principle
Clinical Application
1. If the postvaccination concentration is less than 1.0 IU, the patient is considered a nonresponder.
2. If the postvaccination concentration is 1.0 IU or higher, a patient with a ratio of less than 1.5 is a nonresponder, a ratio of 1.5 to less than 3.0 is a weak responder, and a ratio of 3.0 or higher is a good responder.
3. If the prevaccination concentration is greater than 1.0, it may be difficult to assess the response based on a ratio alone. A postvaccination concentration above 2.5 IU in this case is usually adequate.
Chapter Highlights
• Vaccines provide artificially acquired active immunity to a specific disease.
• The CBER regulates vaccine products. According to the CDC, vaccines have reduced preventable infectious diseases to an all-time low. Vaccine development is an important focus of research for AIDS, malaria, and other devastating diseases.
• Pathogens or pathogen products adapted for biological warfare include smallpox, anthrax, plague, tularemia, brucellosis, Q fever, botulinum toxin, and staphylococcal enterotoxin B.
• Jenner discovered a fundamental principle of immunization with smallpox vaccine and paved the way for the development of rabies (Louis Pasteur) and other vaccines (e.g., diphtheria, typhoid).
• Children now receive vaccines for many childhood diseases (e.g., rubella). Adults require boosters (tetanus). A vaccine approved in 2006 for adults reduces the risk of shingles.
• International travelers frequently require vaccination to endemic diseases (e.g., hepatitis A) in a particular country. Health care professionals are now protected against hepatitis B through vaccines. Many adults receive the flu vaccine. Vaccines are also given to pets and livestock.
• A vaccine stimulates active immunity and creates an immune memory so that exposure to the active disease microorganism will stimulate an already primed immune system to fight the disease.
• Most vaccines can be divided into two categories, live attenuated vaccines and nonreplicating vaccines.
• A vaccine must produce protective immunity with minimal side effects, produce a strong immune response, and be stable during its shelf life.
• Classic preventive vaccines are designed to mimic the effects of natural exposure to microbes. The earliest host response to vaccination is called the innate immune response.
• Vaccines emphasize public health safety (anthrax) or prevent the return of epidemic diseases.
• Preventive AIDS vaccines are for HIV-negative individuals to prevent HIV infection. Therapeutic AIDS vaccines are for HIV-positive individuals to improve the immune system.
• Anthrax vaccine is for emergency use in the event of an anthrax-based attack on the U.S. population.
• No available vaccine can prevent congenital CMV disease, although a few CMV vaccines have been tested in humans.
• Experimental DNA-based vaccine to protect against hay fever after just six injections has been in development.
• Cancer vaccines such as Gardasil for HPV work by exposing the body’s immune cells to weakened forms of an antigen.
• FluLaval is an influenza vaccine for immunizing people 18 years of age and older. The FDA has licensed five flu vaccines.
• A therapeutic vaccine is directed at patients with AML.
• Polio has been reduced by more than 99% and the number of countries with endemic transmission has been reduced by more than 96%.
• The threat of bioterrorism with smallpox has led to high-risk individuals already being vaccinated.