CHAPTER 30 A Multimodal Approach to Transfusion Avoidance and Blood Loss Management in Partial Knee Arthroplasty
Introduction
Modern surgical techniques have reduced the amount of blood loss during total knee arthroplasty (TKA) procedures. Despite numerous advances, allogeneic transfusion rates still remain high. Transfusion rates following unilateral TKA range from 4% to 46%, while bilateral TKA results in transfusion rates between 31% and 72%.1,2 Acute postoperative anemia, risks of allogeneic transfusion, and wound complications remain of great concern to the patient and surgeon. Allogeneic transfusions traditionally have been used to ameliorate the occurrence of anemia, but complications following allogeneic transfusion remain. Incorrect blood component transfusion, disease transmission, allergic reactions, fluid overload, transfusion reactions, and immunosuppression from allogeneic transfusions are well described.3–8 Few data currently exist with regard to blood loss and transfusion rates in patients undergoing partial knee arthroplasty (PKA). In available studies regarding unicompartmental arthroplasty, average blood loss ranges from less than 200 to 240 ml, transfusion rates appear low, and postoperative hemoglobin drop ranges from 1.8 to 2.73 g/dl.9–12 While patient risks for transfusion are lower with PKA, our practice follows a similar protocol with TKA and PKA with regard to minimizing perioperative blood loss and transfusion avoidance. The protocol focuses on a multimodal approach. This approach needs to be utilized in the preoperative, intraoperative, and postoperative periods to optimize the patient prior to surgery and minimize blood loss during and following arthroplasty. Conservation techniques for the preoperative, intraoperative, and postoperative periods are reviewed, and blood management protocols are presented.
Why We Should Try To Avoid Transfusions
Historically, 50% of patients undergoing total joint arthroplasty receive an allogeneic blood transfusion. The risks of allogeneic transfusions are well described in the literature for TKA, but data following PKA are sparse.9,13–19 The main risks for direct transfusion-related morbidity and mortality include incorrect blood component transfusion (70%) and immunologic risks (28%), while transfusion-transmissible infection (2%) is of less significance.20 With regard to transmissible infection, West Nile, TT, parvovirus B19, and SEN virus transmission has been discussed as a potential source of infection after allogeneic transfusions.20,21 On a global scale, protozoa infections, including malaria and toxoplasmosis, remain some of the most common transfusion-transmitted infections.20 In addition, some cases of the blood-borne transmission of prions have been described.20
Perhaps more concerning to the surgeon, allogeneic transfusions have been implicated in the increased incidence of infection after surgery. Tang et al.22 performed a prospective series of 2809 consecutive colon resections. In this report, transfusion was the single most powerful risk factor for postoperative infection, with an odds ratio greater than 5. Kendall et al.23 described immunosuppression secondary to allogeneic transfusions in 34 patients undergoing total hip arthroplasty (THA). According to Kendall et al., the lymphocyte function is impaired, which may be the etiology of the increased risk of deep prosthesis infection.23 The effect of allogeneic transfusion on the immune system is now well documented in the literature.20 Authors believe that the immunomodulating effect of red blood cell transfusion may be responsible for the better outcome in transplantation patients receiving transfusion, the higher risk of recurrence following malignancy resection in patients receiving transfusion, and the higher risk of postoperative infection following transfusion.20 The incriminated pathomechanism of these effects of blood transfusion is called transfusion-related immunomodulation.20
The increased risk of infection following allogeneic transfusion is fairly well established in the joint arthroplasty literature. Pulido et al.24 looked at predisposing factors for developing periprosthetic infection in a series of 9245 arthroplasty patients. They demonstrated a 2.1-fold increase in the rate of periprosthetic infection following allogeneic transfusion. Similarly, Shaunder et al.25 demonstrated a 3.6 times greater relative risk for developing postoperative infection after cardiac and orthopaedic surgery following allogeneic blood transfusion. Similarly, Murphy et al.26 showed an increased rate of confirmed or suspected infections in a series of 84 patients undergoing THA. They compared patients receiving autologous blood transfusion with those receiving allogeneic blood. Although the numbers were small in this series, the infection rate in the group receiving allogeneic transfusion was 32% compared to 3% in the autologous group. Other complications have also been linked to transfusion. In a recent population-based review of 28,087 THA patients, Pedersen et al.27 compared 2254 patients receiving allogeneic transfusion with 2254 nontransfused, matched patients. In this series, transfused patients demonstrated a higher 90-day mortality and increased odds of pneumonia (odds ratio 2.2 and 2.1, respectively). Bierbaum et al.1 evaluated 9482 patients undergoing major orthopedic surgery. This study demonstrated an increase in overall complication rate in patients requiring transfusion. Complications associated with transfusion in this series included an increase in infection, fluid overload, and a longer hospital stay.
Risk Factors For Transfusion
Preoperative blood values remain the best way to predict which patients will require perioperative allogeneic transfusion.28–31 Checking the hemoglobin and hematocrit before scheduling the indicated procedure identifies those patients at risk for requiring transfusion. In one of Cushner et al.’s original papers, they described factors that influence transfusion rates following TKA.32 In this study, they found preoperative hematocrit values were the best predictor for transfusion needs. Nuttall et al.33 as well as Boettnner et al.28 had similar findings, also noting the importance of preoperative hemoglobin. To minimize postoperative transfusion requirements, the patient’s hemoglobin must be maximized during the preoperative period. Patients average a 10% hematocrit loss routinely after total joint arthroplasty. Therefore, those who begin with higher preoperative hemoglobin concentrations are better able to tolerate the loss. Patients who are anemic preoperatively will be anemic during the postoperative period and may require transfusion. Guerin et al.34 performed a prospective review of 162 consecutive hip and knee arthroplasties. In this series, patients with preoperative hemoglobin levels less than 13 g/dl were four times more likely to receive a transfusion than those patients with preoperative hemoglobin levels greater than 15 g/dl.
Nuttall et al.33 evaluated 299 patients who underwent primary or revision THA in an attempt to predict the risk factors for allogeneic transfusion. In this study, risk factors for transfusion included preoperative hemoglobin, weight, age, anticipated blood loss, and aspirin use. Interestingly, they noted that predonated blood was often not transfused, leading to blood wastage and an increase in cost. Nuttall and colleagues33 concluded that identifying patients who were unlikely to require transfusion could avoid the unnecessary autologous predonation. Similar results were recently published by Boettner et al.28 In their study of 283 patients undergoing THA, not only was preoperative autologous donation (PAD) not beneficial in nonanemic patients, but PAD increased the overall transfusion rate, as our practice has demonstrated previously.
The preoperative hemoglobin concentrate has been shown to be the best predictor for postoperative transfusion in several other studies as well. Cabibbo et al.35 recently reviewed their autologous blood donation program in 1198 orthopaedic patients over the past 10 years. In this series, a preoperative hemoglobin level greater than 14 g/dl was a strong predictor for not requiring postoperative transfusion. Sculco and Gallina36 evaluated 1405 patients who underwent total joint arthroplasty. Again, preoperative hemoglobin level was inversely related to the frequency of perioperative allogeneic transfusions. In a large multicenter study, Bierbaum et al.1 evaluated 9482 patients undergoing major orthopaedic surgery. An increased transfusion rate for patients with a preoperative hemoglobin value of less than 13 g/dl was demonstrated. These studies suggest that the patient’s hemoglobin should be checked and optimized prior to surgery to minimize postoperative transfusion and complication rates. In our practice, we feel that preoperative optimization of the patient’s hemoglobin/hematocrit is the most valuable step in reducing perioperative transfusion rates.
Preoperative Blood Management
Preoperative Autologous Donations
In the late 1980s, the standard of care for a patient undergoing TKA was the preoperative donation of autologous blood. Although commonplace in the United States, PAD is uncommon in other areas of the world. PAD has several limitations. As discussed previously, patients who are most anemic in the preoperative period are at greatest risk for postoperative transfusion. These same patients are often excluded from PAD due to the preoperative anemia. This alone inhibits the ability of a PAD program to helping those patients with the greatest need. In addition, PAD may actually increase the risk of transfusion in the remaining patients by reducing their preoperative hemoglobin level, making a previously low-risk patient now at higher risk for transfusion.32,33,37 The assumption cannot be made that, after donating blood, the patients return to their predonation status. Several studies have shown that PAD will lower the patient’s preoperative blood levels.28,38–40 The patient donates the blood, does not return to baseline, and arrives for the surgery in an anemic state. The patient is now more at risk for allogeneic transfusion because of the autologous donation.
In our experience, too many patients donated blood too close in time to the scheduled surgery to recover the blood lost with PAD. In addition, a 1- to 2-unit PAD program does not cause a significant erythropoietic response. Because no erythropoietic response occurs, patients do not return to their baseline level. This is demonstrated by the literature. For example, Hatzidakis and coworkers41 performed a retrospective analysis of 489 consecutive patients undergoing total joint arthroplasty. A decrease in hemoglobin concentration from the time of the donation to the time of surgery was reported; the average decrease was 1.22 g/dl. The authors did not recommend PAD for patients with predonation status greater than 13 g/dl. We evaluated our PAD program previously with regard to preoperative hemoglobin levels. Between 1993 and 1995, 2 units of PAD were obtained on average compared with 1 unit of PAD obtained in the years between 1995 and 1997. Our studies showed a 3% decrease in hematocrit values for every unit donated before surgery.42 When 1 unit was donated, a 3% decrease in hematocrit was noted before surgery. When 2 units were donated, a 6% decrease from baseline was noted; 2 units of PAD resulted in more anemia before the surgical procedure.39
Our practice tried to address the issue of wastage with automatic infusion of the autologous blood. We reviewed our results of a 1-unit PAD program with automatic infusion of the donated blood. All patients were given their PAD immediately after surgery, resulting in 0% wastage. No numerical transfusion triggers were utilized and subsequent allogeneic transfusion was based on symptoms. Despite ordering the PAD unit 1 month before surgery, significant preoperative anemia was noted. In this retrospective review of 148 patients undergoing unilateral TKA, a 1.3-g/dl decrease in hemoglobin was noted between predonation and presurgical testing.43 We refer to this occurrence as “orthopaedic-induced anemia.” Whereas only 26.2% of patients were in the high-transfusion-risk group (hemoglobin >10 g/dl and ≤13 g/dl) before surgery, 55.7% of patients were in this high-risk category after PAD. The patients did not recover from the autologous donations that occurred 4 weeks before surgery. A mean hemoglobin level of 14.0 g/dl was seen before donation, whereas the mean preoperative hemoglobin level decreased to 12.6 g/dl. As documented by others, the use of PAD resulted in anemia, and the patients did not return to the predonation hemoglobin and hematocrit values. Although the allogeneic infusion rate was low in this series, we believe this reflects our lower trigger point for transfusion in the immediate postoperative period. Had historical transfusion triggers been followed (hemoglobin concentration < 10 g/dl), a transfusion rate of 30% would have been found. Fifty percent of our patients were discharged with hemoglobin concentrations less than 9 g/dl.
The low allogeneic transfusion rate should not be misconstrued as efficacy of a 1-unit PAD program, but rather a change in our transfusion practice. PAD programs are limited by significant wastage if automatic transfusion is not followed. In a review of 1198 patients enrolled in an autologous blood donation program, Cabibbo et al.35 demonstrated a high degree of autologous blood wastage in patients with an optimized preoperative hemoglobin. In this series, a preoperative hemoglobin level greater than 14 g/dl was a strong predictor for not requiring postoperative transfusion. In this subgroup of patients, autologous blood wastage was over 90%. Even in the subgroup of patients with preoperative hemoglobin levels between 13 and 14 g/dl, autologous blood wastage was still over 50%. Even in a recent study that concluded autologous blood donation was a cost-effective measure in reducing allogeneic transfusion rates following arthroplasty, Green et al.44 demonstrated overall autologous blood wastage of 38% in 356 patients. When looking at TKA alone, the autologous blood wastage was 51%. These findings are similar to those of Bierbaum et al.1 In their large prospective series including 9482 patients, the autologous blood wastage was 45%. Our institution has abandoned the aforementioned protocol based on the apparent lack of efficacy. In addition, this type of protocol places patients at an additional risk. A protocol with a 100% autologous rate exposes patients to the added risk of donation error. Goldman et al. reviewed autologous error rates in Canada and found an error rate of 6 in 149.45 The majority of these errors were related to labeling error (48%) or error in component preparation (25%). One patient received the wrong unit of donated blood, which is not an uncommon occurrence. According to the College of American Pathologists,46 0.9% of the 3852 institutions studied had at least 1 unit of PAD given to the wrong patient.
Cost is the final issue of PAD, for this is not an inexpensive process. The costs of a PAD program are related to procurement and the cost connected with giving the blood. Billote et al.38 evaluated PAD in patients who were receiving THA and found no benefit in PAD for nonanemic patients undergoing primary hip replacement. Each patient donated 2 units of autologous blood, with an additional cost of $758 per patient despite frequent blood wastage. Etchason et al.42 studied the cost of the PAD program and concluded that increased protection afforded by autologous blood is limited and may not justify the increased cost. However, a more recent study comparing the cost of erythropoietin versus autologous and allogenic blood donation in total joint arthroplasty showed that autologous blood donation might be cost effective.44 In 356 unilateral total joint arthroplasties performed during an 11-month period, a combination of autologous blood donation and allogeneic blood was the least costly approach at $856 and $892 per patient for THA and TKA, respectively. The most costly strategy was allogeneic blood only at $1769 and $1352 per THA and TKA patient, respectively.
Use of Erythropoietins
The importance of preoperative hemoglobin concentrations was discussed earlier. The surgeon is often limited in his or her ability to maximize the patient’s predonation hemoglobin values. In the past, we participated in a PAD program. Unhappy with the anemia caused by donations, we put our patients on a new protocol. We began incorporating a patient-specific protocol to utilize epoetin alfa (EPO) (Procrit; Ortho Biotech, PA) into our busy knee practice.39 We obtain a preoperative hemoglobin and hematocrit prior to surgical booking. Patients with a hemoglobin level greater than 10 and less than 13 g/dl are indicated to receive EPO injections prior to surgery. Patients receive 40,000 units at 3 weeks, 2 weeks, and 1 week prior to surgery, with an average rise in hemoglobin of 1.5 g.39 We compared 50 patients who received EPO injections with 50 patients participating in the autologous program with automatic reinfusion.43 The patients receiving EPO had higher blood parameters preoperatively, postoperatively, and on discharge than the patients who participated in the autologous program. Additionally, our overall cost was reduced because the autologous program was used in 25% of the patients with EPO compared with 100% in previous protocols. Due to the success of this protocol, our indications have expanded. We now employ the same protocol for all arthroplasty patients, including those undergoing PKA, unilateral arthroplasty, bilateral arthroplasty, revision arthroplasty, and two-stage revisions for infections.
The success of this protocol is best demonstrated with discussion of our more complicated cases. By utilizing EPO between stages during the two-stage treatment of an infected total joint arthroplasty, our allogeneic transfusion rates were decreased from a high of 88% to 33%.47 On evaluation of blood loss and transfusion rates following revision TKA, we found that 75% of female patients scheduled for revision were in the high-risk group (hemoglobin > 10 and <13 g/dl).48 In our practice, approximately 25–30% of patients fall into the high-risk group and require EPO injections. This means that 75% required no preoperative intervention. This saves our office the time and inconvenience of routinely scheduling autologous blood donations for all patients, for many of whom it would be unnecessary. Treating 25% of patients with EPO injections is significantly less costly than using an autologous donation program for 100% of the patients, especially in light of potential risks with an automatic reinfusion protocol. A similar protocol was recently studied by Moonen et al.49 and compared to re-transfusion of autologous shed blood. All patients in this study had a preoperative hemoglobin level between 10 and 13 g/dl. In 100 arthroplasty patients randomized to preoperative erythropoietin injection or re-transfusion of autologous shed blood, the allogeneic transfusion rate for the group receiving preoperative injections of erythropoietin was 4% compared to 28% for the group with a reinfusion drain. A second recent study compared the use of erythropoietin to a PAD program.50 In this series, 121 arthroplasty patients with a preoperative hemoglobin between 11 and 14 g/dl either received weekly EPO injections or underwent PAD. The EPO group demonstrated higher hemoglobin levels, lower transfusion rates, and a significant increase in postoperative vigor.50
Insall-Scott Institute Protocol
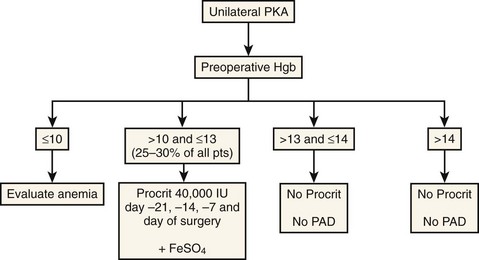
We check hemoglobin values before surgery and identify patients at increased risk; approximately 25% of patients receive the EPO injections (Fig. 30–1). The other patients, with a preoperative hemoglobin greater than 13 g/dl, are scheduled for surgery without any PAD or recombinant human EPO prescriptions. The patients with a hemoglobin between 10 and 13 g/dl receive their EPO injections before surgery from our office. Patients with a hemoglobin less than 10 g/dl should be referred to a hematologist for thorough evaluation. This approach maximizes the hemoglobin and hematocrit levels for the high-risk patients and saves cost by allowing low-risk patients to proceed without intervention.
Intraoperative Blood Management
Acute Normovolemic Hemodilution
Acute normovolemic hemodilution involves simultaneous removal of whole blood from a patient immediately before surgery and replacement with acellular fluids (crystalloid or colloid) to maintain normal blood volume. This technique is recommended when the potential for blood loss may exceed 20% in patients with hemoglobin greater than 10 g/dl. It has a cost and preoperative time commitment similar to PAD, with the possibility of clerical error or bacterial contamination. It is often impractical in most total joint programs because of the relatively short duration of the procedure and lesser blood loss.51 Acute normovolemic hemodilution is not necessary when blood loss is expected to be less than 500–1000 ml and therefore has little role in PKA.
Hypotensive Anesthesia
Hypotensive anesthesia is a technique for reducing intraoperative blood loss by significantly lowering the mean arterial pressure during surgery. Hypotensive anesthesia may reduce intraoperative blood loss, but it depends on the relative decrease in pressure and the type of anesthesia.52–54 The reduction in blood loss does not seem to be related to cardiac output.55 Hypotensive anesthesia is associated with tissue hypoperfusion, and complications can occur, including death. Patients with underlying cardiac, renal, cerebral, or peripheral vessel disease are at increased risk. Two recent studies have shown hypotensive anesthesia during total joint arthroplasty to be safe in patients with aortic stenosis and chronic renal dysfunction, but these series are severely limited by the number of patients evaluated (22 and 54 patients, respectively).54,56 The possibility of increased deep vein thrombosis events also has been raised. During PKA, the reduced amount of expected blood loss and the use of an intraoperative tourniquet reduces the benefit of hypotensive anesthesia. Our practice currently feels the risks outweigh the benefits in PKA.
Tissue Hemostasis
At our institution, we advocate the use of a 30-ml lidocaine-with-epinephrine injection along the arthrotomy site prior to the arthrotomy. For a medial unicompartmental arthroplasty, a small midvastus split (1 cm) is performed. For patellofemoral or bicompartmental arthroplasty, a smaller quad-sparing incision in conjunction with the lidocaine-with-epinephrine injection is performed. This is based on our experience with TKA. When both the minimally invasive incision and the epinephrine injections were used, a decrease in blood loss was noted. In our series of 236 patients, the preoperative-to-postoperative drop in hemoglobin averaged 2.05 g/dl in the study group versus 3.37 g/dl in the control group (p < .01).57
Another option is fibrin tissue adhesives. The fibrin tissue adhesive is sprayed on the internal aspects of the operating field before skin closure. In addition to the direct mechanism of action, the fibrin sealants contain various amounts of antifibrinolytics, increasing their efficacy.58 In several randomized controlled trials, fibrin sealants reduced the rate of exposure to allogeneic red blood cell transfusions by approximately 54%.59 The use of fibrin tissue adhesive significantly reduced mean postoperative blood loss from 800 to 360 ml in a study of 58 patients undergoing joint surgery.58 Molloy et al. compared three groups in a randomized, controlled trial of 150 patients undergoing TKA.60 One group received a topical fibrin spray applied intraoperatively. One group received a tranexamic acid (TXA) bolus. The last group was the control. Both TXA and the topical fibrin spray were shown to be effective in decreasing blood loss compared to the control. There was no difference seen in the groups receiving TXA versus the fibrin spray. Everts et al. looked at utilizing an autologous platelet gel and fibrin sealant compared to a control group in 165 unilateral TKAs.61,62 The hemoglobin following discharge was 11.3 g/dl for the platelet gel and fibrin sealant group versus 8.9 g/dl for the control group. They demonstrated a decrease in the rate of allogeneic transfusion, fewer wound complications, and a 1.4-day decrease in length of hospital stay with use of the platelet gel and fibrin sealant. Carless et al. published a meta-analysis looking at the benefit of fibrin sealant in minimizing perioperative allogeneic transfusion.59 Although the analysis demonstrated an absolute risk reduction of 19%, conclusions were limited as the trials included were small, uncontrolled, and unblinded. Gardner et al. looked at platelet gels.63 In a retrospective review of 98 TKAs, they found less narcotic use, better range of motion, and a shortened hospital stay with use of the platelet gel versus control. The decrease in hemoglobin was 2.7 g/dl with the platelet gel versus 3.2 g/dl in the control group.
Another option is the use of specialized cautery units that enhance operative hemostasis. An example of such technology includes the Aquamantys system (Salient Surgical Technologies, Portsmouth, NH), which improves hemostasis via collagen shrinking at cooler temperatures over broader fields and with less tissue destruction than standard electrocautery devices. Using bipolar radiofrequency energy combined with saline, high-risk areas for bleeding can be coagulated to obtain hemostasis with less tissue damage. These high-risk areas include exposed surfaces of bone, the posterolateral corner and posterior capsule, branches of the geniculate artery, and the synovium in the suprapatellar pouch. Rosenberg reviewed the orthopaedic literature and recommended bipolar sealing technology highly to decrease postoperative blood complications, pain, and swelling while possibly improving intraoperative visualization.64 Marulanda et al. compared the use of bipolar sealing technology with conventional electrocautery in a randomized, prospective study of 50 consecutive patients undergoing primary unilateral TKA.65 The authors report that patients in the bipolar sealing group experienced a significant reduction in mean total blood loss (296.0 vs. 424.5 ml; p = .02) and mean postoperative drainage (203.8 vs. 312.5 ml; p = .05) versus the control group. Isabell and Weeden recently reported on the results of a retrospective, controlled cohort study of 100 patients undergoing primary minimally invasive unilateral TKA, comparing the use of conventional electrocautery with bipolar sealing technology.66 Patients in the bipolar sealing group had a significantly lower mean decline in hemoglobin compared with the control group (3.3 ± 1.1 vs. 3.9 ± 1.2 g/dl; p = .0085). The authors reported that the prevalence of autologous transfusion was significantly lower (16% vs. 44%; p < .001) for the bipolar sealing group compared with the control group. The prevalence of allogeneic transfusion was also reported to be significantly lower for the bipolar sealing group compared with the control group (8% vs. 22%; p < .001). Pierson et al. conducted a prospective, randomized, controlled study designed to evaluate the efficacy of a saline-coupled bipolar sealing device versus conventional therapy in patients undergoing unilateral TKA.67 Ninety patients were randomized during the study, with the control and treatment groups each consisting of 45 patients. Preoperative hemoglobin levels were similar in both groups. The authors reported that the mean decline in hemoglobin was significantly lower for the bipolar sealing group compared with the control group (3.3 ± 1.0 vs. 3.8 ± 1.5 g/dl; p = .01). Further studies are needed to predict the device’s effect on decreasing transfusion rates and maximizing postoperative blood values. The device costs approximately $500 per use; a cost-benefit analysis needs to be done to determine whether the increased expense is worthwhile for PKA. We currently employ the use of bipolar cautery and fibrin sprays in PKA due to our anecdotal experience of improved wound appearance and lower incidence of significant hemarthrosis.
Pharmacologic Strategies
Pharmacologic strategies to decrease transfusion requirements in patients undergoing surgery have been studied extensively.51,68 One such drug class is the antifibrinolytics. Fibrinolysis is stimulated by surgical trauma and further augmented by the use of a tourniquet. Antifibrinolytics act to increase hemostasis within a surgical site by enhancing the clotting mechanism. A Cochrane review was performed to evaluate the use of antifibrinolytics to minimize perioperative blood transfusion.69 Despite notable heterogeneity in the various trials, aprotinin seems to reduce the need for red blood cell transfusion and the need for reoperation because of bleeding. Mostly in cardiac surgery, aprotinin was found to reduce the rate of allogeneic blood transfusions by 30%. Similar trends in efficacy were seen with TXA and aminocaproic acid. Trials directly comparing TXA with aprotinin have been done, and no significant difference has been reported, though a trend toward an increased risk of transfusion with TXA over aprotinin was seen (relative risk increase of 21%).70 However, aprotinin was withdrawn from the market in May 2008 due to concerns of complications and even death. It is now used restrictedly for research only.
Tranexamic Acid
Tranexamic acid inhibits fibrinolysis by blocking the lysine-bonding sites of plasminogen to fibrin. Numerous studies have documented the efficacy of TXA in reducing postoperative blood loss and transfusion requirements of TKA70–76and THA1,77; blood loss reductions of 25–50% have been noted.70–76,78,79 In direct comparison studies, TXA has proved more efficacious than acute normovolemic hemodilution in patients undergoing TKA.80,81 Optimal timing of administration and dosing are not yet defined. Several clinical studies have shown the efficacy of TXA boluses when given before surgery74,78 at deflation of the tourniquet,70,71,73 and for various times after surgery at variable doses.9,81 Most frequently, dosing is 10–20 mg/kg. In our institution, we have had success with 10 mg/kg before surgery and at the time of the tourniquet deflation.
Some more recent studies of TXA are available. Rajesparan et al. looked at a standard bolus of 1 g of TXA in THA patients.82 The bolus was given at anesthesia induction. With this protocol the TXA group demonstrated a significant decrease in early postoperative blood loss, total blood loss, and transfusion rate. Importantly, no increase in deep vein thrombosis was noted in the group receiving TXA.82 Johansson et al. did a randomized, double-blind study in 100 THA patients using a 15-mg/kg dose of TXA.83 Again, less bleeding was noted in the TXA group with no increase in venous thromboembolic events. Similar observations have been made in TKA patients. Camarasa et al. performed a double-blind, randomized study with 120 patients receiving TXA during TKA.84 The transfusion rate was 7.5% in the study group compared to 38.3% in those not receiving TXA. The hemoglobin drop seen at the time of discharge was 2.5 g/dl in the study group compared to 3.4 g/dl in the control group. Kagoma et al. performed a meta-analysis of the 29 available prospective studies in orthopaedic surgery.85 They concluded that patients receiving TXA demonstrate reduced transfusion need, reduced blood loss, and no increased incidence in DVT.85
Postoperative Management
Drain Usage
Drain usage has received little attention in the PKA literature. Confalonieri et al. have recently studied the necessity of closed-suction drainage following unicompartmental knee replacement.86 In their prospective, randomized trial, 78 patients were divided into two groups based on the use of a postoperative drain. This series failed to show a clinical benefit of postoperative drain usage with regard to pain, wound healing, range of motion, or length of hospital stay. Despite limited numbers, this is the only available series in the literature. Our practice continues to use postoperative drains in our partial knee replacement patients. Anecdotally, we feel we have fewer wound complications with postoperative drainage. This has been supported in the TKA literature. Holt et al.87 reported that blood loss was equal in patients with or without a postoperative drain. However, in the absence of a drain, the wounds exhibited greater ecchymosis and drainage. Niskanen et al.88 also showed more wound drainage when a postoperative drain was not used.
Most blood loss in a knee arthroplasty procedure occurs in the postoperative period, and techniques have been developed to salvage this shed blood. Numerous types of drains exist and basically differ on whether the cells are washed or unwashed and whether red cells are used for reinfusion. Faris31 studied the quality of shed blood and its reinfusion and found that shed blood was well tolerated, although a febrile reaction was noted in 2% of patients receiving the blood. Groh et al.89 and Majkowski et al.7 performed retrospective studies demonstrating the efficacy of postoperative drain usage. In a survey of American Association of Hip and Knee Surgeons members,48 most respondents were found to use drains in the postoperative period; 62% always used a drain compared with 24% who never used a drain. Occasional use of a drain also was reported. The survey found that 47% of respondents used a reinfusion type of drain, which was removed in approximately 24–36 hours. Jones et al.90 conducted a postoperative review of 43,000 hip replacements and 33,000 knee replacements performed in the United Kingdom. They evaluated the use of autologous salvage drains in hip and knee surgery patients. The authors concluded that reinfusion drains seemed to be a cost-effective means of reducing the requirement of allogeneic blood in hip and knee arthroplasty. In this series, allogeneic transfusion was required in 21% of patients with a reinfusion drain compared to 45.7% of patients with a suction drain. In another series, Grosvenor et al.91 looked at the efficacy of postoperative blood salvage after hip arthroplasty in patients with and without deposited autologous blood. The results of this study showed that postoperative blood salvage significantly reduced the risk of allogeneic transfusions. The patients who were treated with postoperative blood salvage were approximately 10 times more likely to avoid allogeneic transfusions than patients who had a non-reinfusion drain. In a more recent randomized prospective review, Smith et al. demonstrated the benefit of reinfusion drains in THA.92 In a series of 158 patients, reinfusion drains demonstrated fewer patients with a postoperative hemoglobin of less than 9 g/dl, a lower transfusions rate, and a slight overall cost savings. Friederichs et al. compared reinfusion drains with preoperative autologous blood donation in 200 consecutive joint arthroplasties.77 In patients with a preoperative hematocrit of greater than 37%, the use of a reinfusion drain lowered the allogeneic transfusion rate to 1.2%. They concluded that perioperative blood salvage is safe and effective and makes it possible to discontinue the practice of predonating blood in arthroplasty patients with a preoperative hematocrit greater than 37%. This is the practice at our institution because PAD has been abandoned for patients with hemoglobin values greater than 13 g/dl.
If reinfusion is to be utilized, the decision must be made whether to use washed or unwashed cells. For our TKA patients, we switched to the OrthoPAT system (Zimmer Inc, Warsaw, IN) in an attempt to improve the quality of blood returned to the patient. Such a system has the benefits of a washed cell device without the additional costs of a cell-saver type system. We believe that a lower volume of better quality packed red blood cells is advantageous. Several studies have looked at the efficacy of the OrthoPAT system. Clark et al. evaluated the OrthoPAT system in 398 patients.93 In this series, primary or revision hip arthroplasties with no preoperative autologous blood donation, knee arthroplasties with no preoperative autologous blood donation, and unilateral primary hip arthroplasties were 2.7, 2.3, and 2 times less likely (p < .05), respectively, to use allogeneic blood with OrthoPAT. Similarly, Del Trujillo et al. demonstrated a significant reduction in the allogeneic transfusion rate from 48% to 15% in 108 patients undergoing THA with the use of the OrthoPAT system compared to controls.94
Mont et al.95 recommended a new approach, designing a study to evaluate the efficacy of an intraoperative surgeon decision to use a reinfusion drain. In the TKA group, 84% of the patients in the standard group had reinfusion, similar to the 85% in the reinfusion drain group. This demonstrated clearly that the surgeon could not predict intraoperatively whether a patient would require a reinfusion drain. However, in more than 94% of the cases, by 90 minutes postoperatively, a decision could be made as to whether a reinfusion drain was necessary based on early drain collection. This study concluded that a drain could be placed and converted to a reinfusion drain based on early postoperative blood loss. This approach may be reasonable in partial knee replacement patients, where fewer patients will actually benefit from a reinfusion drain.
Most surgeons use a drain after elective TKA. Although this practice can debated based on current literature, the studies about TKA show a significant decrease in the transfusion rates when postoperative salvage devices are used. It is likely that reinfusion drainage is not cost effective in partial knee replacement due to the reduced perioperative blood loss. Moonen et al. studied re-transfusion in 438 arthroplasty patients.96 They concluded that re-transfusion of filtered shed blood following unicompartmental knee arthroplasty is doubtfully cost efficient due to the minimal bone resection required and the low risk of allogeneic transfusion.
Conclusion
The number one factor preventing allogeneic transfusion is the preoperative hemoglobin and hematocrit. To minimize transfusion, the surgeon must take a preemptive role and optimize patients prior to surgery. The practice of autologous blood donations should be questioned. Cushner et al.43 highlighted orthopaedic surgery–induced anemia and questioned the autologous donation process. A patient-specific approach that identifies at-risk patients is likely most cost effective. Pierson et al.67 reviewed a patient-specific approach based on the patient’s preoperative blood status. Higher preoperative blood status not only reduces the risk of transfusion, but also improves patient outcome. For example, Keating et al.97 assessed patient vigor after joint arthroplasty. Among the objective criteria studied, there was a significant correlation between vigor and hematocrit value: the higher the postoperative blood levels, the higher the vigor scale.
1 Bierbaum BE, Callaghan JJ, Galante JO, et al. Analysis of blood management in patients having total hip or knee arthroplasty. J Bone Joint Surg [Am]. 1999;81:2.
2 Keating EM, Ranawat CS, Cats-Baril W. Assessment of postoperative vigor in patients undergoing elective total joint arthroplasty: a concise patient- and caregiver-based instrument. Orthopedics. 1999;22:s119.
3 Brunson ME, Alexander JW. Mechanisms of transfusion-induced immunosuppression. Transfusion. 1990;30:651.
4 Cascinu S, Fedeli A, Del Ferro E, et al. Recombinant human erythropoietin treatment in cisplatin-associated anemia: a randomized double-blind trial with placebo. J Clin Oncol. 1994;12:1058.
5 Dodd RY. The risk of transfusion-transmitted infection. N Engl J Med. 1992;327:419.
6 Goodnough LT, Skikne B, Brugnara C. Erythropoietin, iron, and erythropoiesis. Blood. 2000;96:823.
7 Majkowski RS, Currie IC, Newman JH. Postoperative collection and reinfusion of autologous blood in total knee arthroplasty. Ann R Coll Surg Engl. 1991;73:381.
8 Walker R. Transfusion risks. Am J Clin Pathol. 1987;88:371.
9 Heck DA, Marmor L, Gibson A, Rougraf BT, Unicompartmental knee arthroplasty: a multicenter investigation with long-term follow-up evaluation. Clin Orthop Relat Res, 286, 1993, 154-159.
10 Jeer P, Cossey A, Keene G. Haemoglobin levels following unicompartmental knee arthroplasty: influence of transfusion and surgical approach. Knee. 2005;12:358-361.
11 Mullaji AB, Sharma A, Marawar S. Unicompartmental knee arthroplasty: functional recovery and radiographic results with a minimally invasive technique. J Arthoplasty. 2007;22(4 Suppl 1):7-11.
12 Zohar E, Fredman B, Ellis M, et al. A comparative study of the postoperative allogeneic blood-sparing effect of tranexamic acid versus acute normovolemic hemodilution after total knee replacement. Anesth Analg. 1999;89:1382.
13 Alter HJ, Nakatsuji Y, Melpolder J, et al. The incidence of transfusion-associated hepatitis G virus infection and its relation to liver disease. N Engl J Med. 1997;336:747.
14 Alter HJ, Purcell RH, Shih JW, et al. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989;321:1494.
15 Ammann AJ, Cowan MJ, Wara DW, et al. Acquired immunodeficiency in an infant: possible transmission by means of blood products. Lancet. 1983;1:956.
16 Kleinman S, Busch MP, Schreiber GB. The incidence/window period model and its use to assess the risk of transfusion-transmitted human immunodeficiency virus and hepatitis C virus infection. Transfus Med Rev. 1997;11:155.
17 Lackritz EM, Satten GA, Aberle-Grasse J, et al. Estimated risk of transmission of the human immunodeficiency virus by screened blood in the United States. N Engl J Med. 1995;333:1685.
18 Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ. The risk of transfusion-transmitted viral infections. N Engl J Med. 1996;334:1685.
19 Stevens CE, Aach RD, Hollinger FB, et al. Hepatitis B virus antibody in blood donors and the occurrence of non-A, non-B hepatitis in transfusion recipients: an analysis of the Transfusion-Transmitted Viruses Study. Ann Intern Med. 1984;101:733.
20 Buddeberg F, Schimmer BB, Spahn DR. Transfusion-transmissible infections and transfusion-related immunomodulation. Best Pract Res Clin Anaesthesiol. 2008;22:503-517.
21 Biggerstaff BJ, Petersen LR. Estimated risk of West Nile virus transmission through blood transfusion during an epidemic in Queens, New York City. Transfusion. 2002;42:1019.
22 Tang R, Chen HH, Wang YL, et al. Risk factors for surgical site infection after elective resection of the colon and rectum: a single-center prospective study of 2,809 consecutive patients. Ann Surg. 2001;234:181-189.
23 Kendall SJ, Weir J, Aspinall R, et al, Erythrocyte transfusion causes immunosuppression after total hip replacement. Clin Orthop Relat Res, 381, 2000, 145.
24 Pulido L, Ghanem E, Joshi A, et al, Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res, 466, 2008, 1710-1715.
25 Shander A, Spence RK, Adams D, et al. Timing and incidence of postoperative infections associated with blood transfusion: analysis of 1,489 orthopedic and cardiac surgery patients. Surg Infect (Larchmt). 2009;10:277-283.
26 Murphy P, Heal JM, Blumberg N. Infection or suspected infection after hip replacement surgery with autologous or homologous blood transfusions. Transfusion. 1991;31:212.
27 Pedersen AB, Mehnert F, Overgaard S, Johnsen S. Allogeneic blood transfusion and prognosis following total hip replacement: a population-based follow up study. BMC Musculoskel Disord. 2009;10:167-179.
28 Boettner F, Altneu EI, Williams BA, et al. Nonanemic patients do not benefit from autologous blood donation before total hip replacement. Hosp Special Surg J. 2010;6:66-70.
29 Canadian Orthopedic Peri-operative Erythropoietin Study Group. Effectiveness of peri-operative recombinant human erythropoietin in elective hip replacement. Lancet. 1993;341:1227.
30 De Andrade JR, Jove M, Landon G, et al. Baseline hemoglobin as a predictor of risk of transfusion and response to epoetin alfa in orthopedic surgery patients. Am J Orthop. 1996;8:533.
31 Faris PM. Unwashed filtered shed blood collected after knee and hip arthroplasty. J Bone Joint Surg [Am]. 1991;73:1169.
32 Cushner FD, Friedman RJ, Blood loss in total knee arthroplasty. Clin Orthop Relat Res, 269, 1991, 98.
33 Nuttall GA, Santrach PJ, Oliver WCJr, et al. The predictors of red cell transfusions in total hip arthroplasties. Transfusion. 1996;36:144.
34 Guerin S, Collins C, Kapoor H, et al. Blood transfusion requirement prediction in patients undergoing primary total hip and knee arthroplasty. Transfus Med. 2007;17:37-43.
35 Cabibbo S, Garozzo G, Antolino A, et al. Continuous improvement of our autologous blood donation program carried out during 10 years in 1198 orthopaedic patients. Transfus Apher Sci. 2009;40(1):13-17.
36 Sculco TP, Gallina J. Blood management experience: relationship between autologous blood donation and transfusion in orthopedic surgery. Orthopedics. 1999;22:s129.
37 Faris PM, Ritter MA, Ables RI, the American Erythropoietin Study Group. The effects of recombinant human erythropoietin on peri-operative transfusion requirements in patients having a major orthopaedic operation. J Bone Joint Surg [Am]. 1993;78:62.
38 Billote DB, Glisson SN, Green D, Wixson RL. A prospective, randomized study of preoperative autologous donation for hip replacement surgery. J Bone Joint Surg [Am]. 2002;84:1299.
39 Cushner FD, Scott WN. Evolution of blood transfusion management for a busy knee practice. Orthopedics. 1999;22:s145.
40 Stowell CP, Chandler H, Jove M, et al. An open-label, randomized study to compare the safety and efficacy of peri-operative epoietin alfa with pre-operative autologous blood donation in total joint arthroplasty. Orthopedics. 1999;22:s105.
41 Hatzidakis AM, Mendlick RM, McKillip T, et al. Preoperative autologous donation for total joint arthroplasty: an analysis of risk factors for allogeneic transfusion. J Bone Joint Surg [Am]. 2000;82:89.
42 Etchason J, Petz L, Keeler E, et al. The cost effectiveness of preoperative autologous blood donations. N Engl J Med. 1995;332:740.
43 Cushner FD, Hawes T, Kessler D, et al, Orthopedic-induced anemia: the fallacy of autologous donation programs. Clin Orthop Relat Res, 431, 2005, 145-149.
44 Green WS, Toy P, Bozic KJ. Cost minimization analysis of preoperative erythropoietin vs autologous and allogeneic blood donation in total joint arthroplasty. J Arthroplasty. 2008. December 2. [Epub ahead of print]
45 Goldman M, Remy-Prince S, Trepanier A, Decary F. Autologous donation error rates in Canada. Transfusion. 1997;37:523.
46 Cooper ES, Walker RH, Schmidt PJ, Polesky HF. The 1990 comprehensive blood bank surveys of the College of American Pathologists. Arch Pathol Lab Med. 1993;117:125.
47 Pagnano M, Cushner FD, Hansen A, et al, Blood management in two-stage revision knee arthroplasty for deep prosthetic infection. Clin Orthop Relat Res, 367, 1999, 238.
48 Cushner FD, Scott WN, Scuderi GR, et al. Blood loss and transfusion in bilateral total knee arthroplasty. J Knee Surg. 2005;28:102-107.
49 Moonen AF, Thomassen BJ, Knoors NT, et al. Pre-operative injections of epoetin-alpha versus post-operative retransfusion of autologous shed blood in total hip and knee replacement: a prospective randomised clinical trial. J Bone Joint Surg [Br]. 2008;90:1079-1083.
50 Keating EM, Callaghan JJ, Ranawat AS, et al. A randomized, parallel-group, open-label trial of recombinant human erythropoietin vs preoperative autologous donation in primary total joint arthroplasty: effect on postoperative vigor and handgrip strength. J Arthroplasty. 2007;22:325-333.
51 Keating EM, Meding JB. Perioperative blood management practices in elective orthopaedic surgery. J Am Acad Orthop Surg. 2002;10:393.
52 An HS, Mikhail WE, Jackson WT, et al. Effects of hypotensive anesthesia, nonsteroidal anti-inflammatory drugs, and polymethylmethacrylate on bleeding in total hip arthroplasty patients. J Arthroplasty. 1991;6:245.
53 Niemi TT, Pitkanen M, Syrjala M, Rosenberg PH. Comparison of hypotensive epidural anesthesia and spinal anesthesia on blood loss and coagulation during and after total hip arthroplasty. Acta Anesth Scand. 2000;44:457.
54 Sharrock NE, Beksac B, Flynn E, et al. Hypotensive epidural anaesthesia in patients with preoperative renal dysfunction undergoing total hip replacement. Br J Anaesth. 2006;96:207-212.
55 Sharrock NE, Mineo R, Go G. The effect of cardiac output on intraoperative blood loss during total hip arthroplasty. Reg Anesth. 1993;18:24.
56 Ho MC, Beathe JC, Sharrock NE. Hypotensive epidural anesthesia in patients with aortic stenosis undergoing total hip replacement. Reg Anesth Pain Med. 2008;33:129-133.
57 Cushner FD, Kim R, Scuderi GR, et al. Use of lidocaine with epinephrine injection to reduce blood loss in minimally invasive total knee arthroplasty. Transfus Alternat. Transfus Med. 2007;9(Suppl 1):59.
58 Levy O, Martinowitz U, Oran A, et al. The use of fibrin tissue adhesive to reduce blood loss and the need for blood transfusion after total knee arthroplasty: a prospective, randomized, multicenter study. J Bone Joint Surg [Am]. 1999;81:1580.
59 Carless PA, Henry DA, Anthony DM. Fibrin sealant use for minimising peri-operative allogeneic blood transfusion. Cochrane Database Syst Rev 2003;(1):CD004171.
60 Molloy DO, Archbold HAP, Ogonda L, et al. Comparison of topical fibrin spray and tranexamic acid on blood loss after total knee replacement: a prospective, randomized controlled trial. J Bone Joint Surg [Br]. 2007;89:306-309.
61 Everts P, Devilee R, Mahoney B, et al. Platelet gel and fibrin sealant reduce allogeneic blood transfusions in total knee arthroplasty. Acta Anaesthesiol Scand. 2006;50:593-599.
62 Everts P, Devilee R, Oosterbos C, et al. Autologous platelet gel and fibrin sealant enhance the efficacy of total knee arthroplasty: improved range of motion, decreased length of stay, and reduced incidence of arthrofibrosis. Knee Surg Sports Traumatol Arthrosc. 2007;15:888-894.
63 Gardner MJ, Demetrakopoulos D, Klepchick PR, et al. The efficacy of autologous gel in pain control and blood loss in total knee arthroplasty: an analysis of the haemoglobin, narcotic requirement and range of motion. Int Orthop. 2007;31:309-313.
64 Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplasty. 2007;22(4 Suppl 1):82-85.
65 Marulanda GA, Ragland PS, Seyler TM, et al. Reduction in blood loss with use of a bipolar sealer for hemostasis in primary total knee arthroplasty. Surg Technol Int. 2005;14:281.
66 Isabell G, Weeden S. Hemodynamic efficacy of a bipolar sealing device in primary total knee arthroplasty [abstract]. In: Proceedings of the Annual Meeting of the Texas Orthopaedic Association. Houston, TX: Texas Orthopaedic Association; 2006.
67 Pierson JL, Hellman EJ, Earles DR, et al. Randomized, prospective trial to examine the hemostatic efficacy of a bipolar sealing device in TKA [abstract]. Poster at AAOS, March 2006.
68 Porte RJ, Leebeek FWG. Pharmacological strategies to decrease transfusion requirements in patients undergoing surgery. Drugs. 2002;2:2193.
69 Henry DA, Moxey AJ, Carless PA, et al. Anti-fibrinolytic use for minimizing perioperative allogeneic blood transfusion. Cochrane Database Syst Rev 2007;(3):CD001886.
70 Tenholder M, Cushner FD. Intraoperative blood management in joint replacement surgery. Orthopedics. 2004;27(6 Suppl):s663.
71 Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomized, double-blind study of 86 patients. J Bone Joint Surg [Br]. 1996;78:434.
72 Hiippala S, Strid L, Wennerstrand M. Tranexamic acid (Cyklokapron) reduces perioperative blood loss associated with total knee arthroplasty. Br J Anaesth. 1995;74:534.
73 Hiippala ST, Strid LJ, Wennerstrand MI, et al. Tranexamic acid radically decreases blood loss and transfusions associated with total knee arthroplasty. Anesth Analg. 1997;84:839.
74 Jansen AJ, Andreica S, Claeys M, et al. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83:596.
75 Tanaka N, Sakahashi H, Sato E, et al. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg [Br]. 2001;83:702.
76 Veien M, Sorensen JV, Madsen F, Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anesth Scand. 2002;46:1206.
77 Friederichs MG, Mariani EM, Bourne MH. Perioperative blood salvage as an alternative to predonating blood for primary total knee and hip arthroplasty. J Arthroplasty. 2002;17:298.
78 Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta Orthop. 2001;72:442.
79 Ekback G, Axelsson K, Ryttberg L, et al. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesth Analg. 2000;91:1124.
80 Zohar E, Fredman B, Ellis M, et al. A comparative study of the postoperative allogeneic blood-sparing effect of tranexamic acid versus acute normovolemic hemodilution after total knee replacement. Anesth Analg. 1999;89:1382.
81 Zohar E, Fredman B, Ellis MH, et al. A comparative study of the postoperative allogeneic blood-sparing effects of tranexamic acid and of desmopressin after total knee replacement. Transfusion. 2001;41:1285.
82 Rajesparan K, Biant LC, Ahmed M, et al. The effect of an intravenous bolus of transexamic acid on blood loss in total hip replacement. J Bone Joint Surg [Br]. 2009;91:776-783.
83 Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76:314-319.
84 Camarasa MA, Ollé G, Serr-Prat M, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96:576-582.
85 Kagoma YK, Crowther MA, Douketis J, et al. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res. 2009;123:687-696.
86 Confalonieri N, Manzotti A, Pullen C. Is closed-suction drain necessary in unicompartmental knee replacement? A prospective randomized study. Knee. 2004;11:399-402.
87 Holt BT, Parks NL, Engh GA, Lawrence JM. Comparison of closed-suction drainage and no drainage after primary total knee arthroplasty. Orthopedics. 1997;20:1121.
88 Niskanen RO, Korkala OL, Haapala J, et al. Drainage is of no use in primary uncomplicated cemented hip and knee arthroplasty for osteoarthritis: a prospective randomized study. J Arthroplasty. 2000;15:567.
89 Groh GI, Buchert PK, Allen WC. A comparison of transfusion requirements after total knee arthroplasty using the Solcotrans Autotransfusion System. J Arthroplasty. 1990;3:281.
90 Jones HW, Savage L, White C, et al. Postoperative autologous blood salvage drains—are they useful in primary uncemented hip and knee arthroplasty? A prospective study of 186 cases. Acta Orthop Belg. 2004;70:466.
91 Grosvenor D, Goyal V, Goodman S. Efficacy of postoperative blood salvage following total hip arthroplasty in patients with and without deposited autologous units. J Bone Joint Surg [Am]. 2000;82:951.
92 Sharrock NE, Mineo R, Urquhart B, Salvati EA. The effect of two levels of hypotension on intraoperative blood loss during total hip arthroplasty performed under lumbar epidural anesthesia. Analg Anesth. 1993;76:580.
93 Clark CR, Sprat KF, Blondin M, et al. Perioperative autotransfusion in total hip and knee arthroplasty. J Arthroplasty. 2006;21:23-35.
94 Del Trujillo MM, Carrero A, Munoz M. The utility of the perioperative autologous transfusion system OrthoPAT in total hip replacement surgery: a prospective study. Arch Orthop Trauma Surg. 2008;128:1031-1038.
95 Mont MA, Low K, LaPorte DM, et al. Reinfusion drains after primary total hip and total knee arthroplasty. J South Orthop Assoc. 2000;9:193.
96 Moonen AF, Thomassen BJ, van Os JJ, et al. Retransfusion of filtered shed blood in everyday orthopaedic practice. Transfus Med. 2008;18:355-359.
97 Keating EM, Ritter MA. Transfusion options in total joint arthroplasty. J Arthroplasty. 2002;17:125.