Chapter 4 A Multidisciplinary Approach to Cancer
A Radiation Oncologist’s View
For many disease sites, the combination of radiation and chemotherapy has also been demonstrated to improve local disease control, enhance the effectiveness of organ-sparing approaches, and improve the curative potential of local treatments, presumably by sterilizing micrometastatic disease that would otherwise lead to the appearance of distant metastases. Randomized trials have proved that addition of concurrent chemotherapy to radiation improves local control and survival in patients with cervical, head and neck, lung, gastrointestinal, and other types of cancer. The use of chemoradiotherapy in the treatment of anal cancer has dramatically reduced the need for radical resection with colostomy for this disease.1–4
Radiation Biology
Another type of radiation-induced cell death is referred to as apoptosis or programmed cell death. Apoptosis can occur before or after mitosis.5 The plasma membrane and nuclear DNA may be important targets for apoptotic injury. Apoptosis appears to play an important role in the radiation response of some tumors and in certain normal tissues such as salivary glands and lymphocytes.
In vitro studies of the relationship between radiation dose and cell survival demonstrate that mammalian cells differ widely in their inherent radiosensitivity. These differences contribute to the wide range of doses required to cure tumors of different cell types. Even bulky lymphomas can typically be controlled with doses of 35 to 40 Gy, whereas 2- to 3-cm squamous carcinomas usually require doses of more than 60 Gy. Melanomas and most sarcomas require even higher doses and usually cannot be controlled with tolerable radiation doses if there is more than microscopic residual disease after surgery.
A number of factors influence cellular radiosensitivity and tumor responsiveness:
• Repair capacity: Cells differ in their ability to accumulate and repair sublethal injury. In general, normal tissues have a greater repair capacity than tumors. It is because of this difference that tumors can be controlled without unacceptable damage to irradiated normal tissues. However, some normal cells and tissues—particularly those that are rapidly proliferating, such as bone marrow and intestinal crypt cells—have relatively little repair capacity, and some tumors, such as prostate cancer, are able to accumulate and repair damage as effectively as most normal tissues. These variations influence the approaches used to treat various tumor types and sites.
• Although cells may also differ in their rate of repair, two-dose experiments and clinical experience suggest that most repair is accomplished within 4 to 6 hours. For this reason, schedules that involve more than one daily fraction of radiation are usually designed to require a minimum interfraction interval of approximately 6 hours to maximize repair of sublethal injury to normal tissues.
• Cell-cycle distribution: Cells are usually most sensitive during mitosis and in the late G2 phase of the cell cycle and most resistant in the mid- to late S and early G1 phases. The cell-cycle redistribution of cells after a dose of radiation may influence their overall sensitivity to a second dose.
• Hypoxia and reoxygenation: The dose of sparsely ionizing radiation required to effect a given level of cell killing is about three times greater under anoxic conditions than under fully oxygenated conditions. Although regions of hypoxia are present in many solid tumors and may have a role in the response to therapy, the clinical importance of hypoxia is diminished by reoxygenation that occurs as initially hypoxic cells become better oxygenated during a course of fractionated radiation therapy.6 To reduce the potential influence of hypoxia, radiation oncologists try to maintain patients’ hemoglobin levels at 10 to 12 g/dL or greater.
• Repopulation: The effect of cellular proliferation that occurs during a course of radiation therapy depends on the doubling time of the neoplastic cells and the total duration of treatment. Although the acute side effects of radiation usually limit the weekly dose of radiation to 900 to 1000 cGy/wk, many studies demonstrate that unnecessary protraction compromises local control and must be compensated for by increasing the dose of radiation.7–9 In addition, evidence suggests that radiation therapy as well as other cytotoxic treatment and even surgery can induce accelerated repopulation, increasing the detrimental effects of treatment protraction. Prolonged delays between surgical resection and initiation of radiation therapy may significantly compromise the efficacy of adjuvant radiation therapy.
Normal Tissue Effects of Radiation
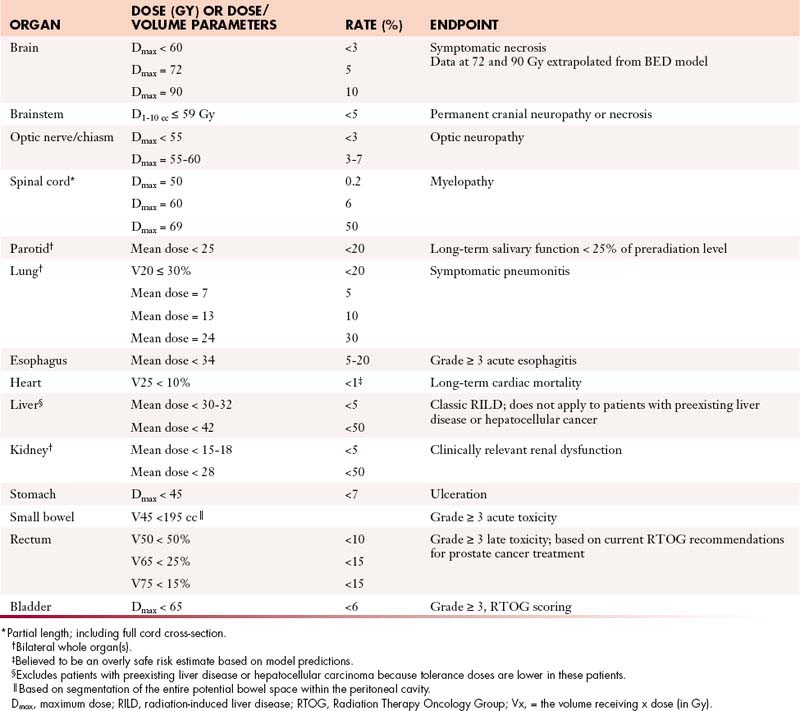
Tissues that are more slowly proliferating are referred to as late-responding tissues and tend to manifest side effects weeks or months after radiation therapy. These effects may reflect direct damage to parenchymal cells or damage to vascular stroma, and the dose-response relationship varies according to the tissue irradiated and other factors. Table 4-1 presents some of the conclusions of a 1991 task force10 charged with summarizing relevant data concerning the effect of ionizing radiation on normal tissues. A more detailed update was subsequently published in 2010.11 The duration of a course of radiation therapy has little impact on the incidence of late complications, but the dose per fraction has a major impact. In general, radiation schedules that involve fractional doses of 2 Gy or less permit maximal recovery of sublethal damage to normal tissues. For this reason and because acute side effects usually limit the weekly dose of radiation to no more than approximately 10 Gy, radiation therapy is most commonly delivered with a schedule of 1.8 to 2 Gy per fraction, five times per week. Most tumors repair cellular damage less effectively than late-responding normal tissues; as a result, the differential effect on tumor versus normal tissues is increased when a dose of radiation is fractionated. This is referred to as the fractionation effect.
Table 4-1 Approximate Dose/Volume/Outcome Data for Several Organs after Conventionally Fractionated Radiation Therapy
Under certain circumstances, alternate fractionation schedules may be used to reduce the overall duration of a course of radiation therapy. Hypofractionation, the use of daily fractional doses of more than 2 Gy per fraction, is routinely used for palliative radiation therapy to optimize convenience, cost, and the rapidity of symptom relief. Common schedules used for palliation include 30 Gy in 10 fractions, 20 Gy in 5 fractions, and in some cases, 8 to 10 Gy in a single dose of radiation. Recently, the development of highly conforming radiation technique has led investigators to explore the value of hypofractionated radiation therapy for curative radiation therapy in certain situations. This approach is most effective if adjacent critical structures receive a significantly lower dose and dose per fraction than the target. Accurate target delineation, precise patient positioning, and clear understanding of internal target motion are critical to successful treatment. Stereotactic body radiation therapy is a form of ultra-hypofractionated radiation therapy in which very large daily doses of radiation are delivered with great precision under image guidance. In contrast, hyperfractionation is the delivery of small doses of radiation two or more times daily (usually with a minimum interfraction interval of 5-6 hr). This approach is most useful when repopulation is considered to be an important factor in tumor curability but proximate late-reacting normal tissues prohibit the use of hypofractionation.
Therapeutic Gain
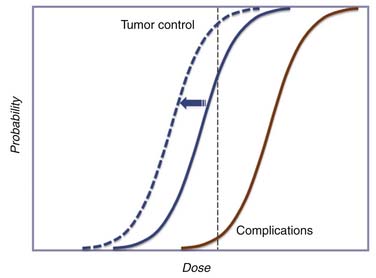
The goal of radiation therapy is to sterilize tumor with the fewest possible side effects. The difference between the rate of tumor control and the rate of normal tissue complications is referred to as the therapeutic gain or therapeutic ratio (Figure 4-1). The probabilities of tumor control and late normal tissue effects can generally be described by two sigmoid dose-response relationships. Below a threshold dose, the probability of tumor control is very low; it then rises steeply to a dose above which little additional benefit can be achieved by further increases in dose. The shape and slope of the curve are related to the type and size of the tumor and other factors including the use of concurrent systemic treatments. The likelihood of complication-free tumor control is determined by the separation between the tumor control and the late effects dose-response curves. The most successful treatment strategies are those that maximize the separation between these curves. Strategies that combine surgery or chemotherapy with radiation in a way that shifts the tumor control dose-response curve to the left without a commensurate shift in the complication curve probability increase the likelihood of a good result. Conversely, multidisciplinary treatments that increase the risk of complications without significant improvement in the probability of tumor control should be avoided.
Surgery and Radiation Therapy
In many cases, the close proximity of critical structures prohibits delivery of a dose of radiation sufficient to eradicate gross disease. In other cases, surgical resection leaves microscopic disease that could lead to future recurrence. In cases such as these, judicious combinations of surgical resection and radiation therapy may significantly improve local control and survival and preserve organ function. Postoperative radiation therapy is often used to prevent local recurrence after gross total resection.12,13 In some cases, preoperative radiation therapy is used to “downstage” tumor, improve local control, or enable the surgeon to use organ-sparing operations. This approach has been particularly effective in the treatment of rectal cancers.14 However, unnecessary multimodality treatment can increase complications, and poorly chosen surgical procedures can, in some cases, compromise the ability to deliver curative radiation therapy.
Chemotherapy and Radiation Therapy
Concurrent or sequential combinations of drugs and radiation may also improve cure through spatial cooperation. For example, chemotherapy may be used to sterilize minimal microscopic disease in distant sites while radiation is used to treat areas of gross or high-risk microscopic local and regional disease. The use of neoadjuvant chemotherapy before radiation therapy has been explored in a number of settings. Unfortunately, when neoadjuvant chemotherapy followed by radiation therapy has been tested in clinical trials, impressive chemotherapy responses have rarely translated into significant improvements in survival. Large meta-analyses of outcome in patients with head and neck or cervical cancers suggest much smaller benefits with this approach than with concurrent chemoradiation.2,15 However, neoadjuvant chemotherapy has been used effectively in patients with breast cancer and continues to be explored in other settings.16
Radiation Techniques
External Beam Radiation Therapy
Photons
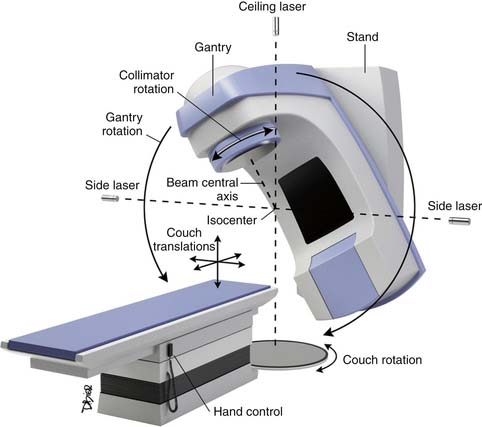
The photon source is located in the head of a gantry that rotates around the treatment table (Figure 4-2). Collimators in the treatment head govern the radiation field size and rotation; a secondary, electronically controlled, multileaved collimator or blocks inserted between the internal collimator and the patient are used to shape the field to the irregular contours of a treatment target volume. The energy of the beam, the distance from the source to the patient, the depth of the target, and the density of intervening tissue also determine the doses delivered to the targets and normal tissues. The depth of penetration and the amount of skin sparing are correlated with the energy of the photons.
Electrons and Protons
Protons are positively charged particles that deposit most of their energy at a tissue depth determined by the energy of the protons. The rapid deposition of energy is referred to as a Bragg peak. For most clinical applications, the proton energy is modulated to spread out the peak, creating a dose distribution characterized by a relatively low entrance dose, homogeneous dose within the target, and very little exit dose. Although proton beams have been studied in a small number of facilities for several decades, there has been a dramatic increase in interest in proton therapy and in the number of facilities providing this therapy as the cost of proton accelerators has declined somewhat during the past 5 or 6 years. The tumors that have so far been of greatest interest are prostate, skull base, ocular, and pediatric tumors.17 However, there are as yet no randomized trials comparing proton therapy with more conventional treatments. Proton therapy continues to be a very expensive, highly complex technology that requires highly skilled physics and technical support. The best applications for proton beam therapy are still to be determined.18
Other Particles
Several other types of particle beams, including neutrons, carbon ions, and pi-mesons, have been explored for their clinical potential but currently are not in common use.19
Treatment Planning
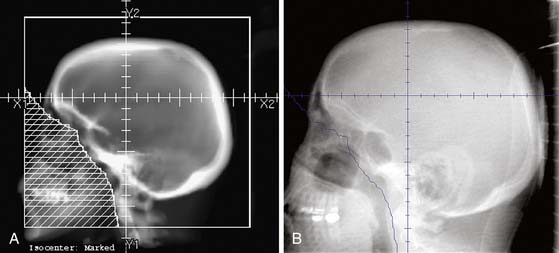
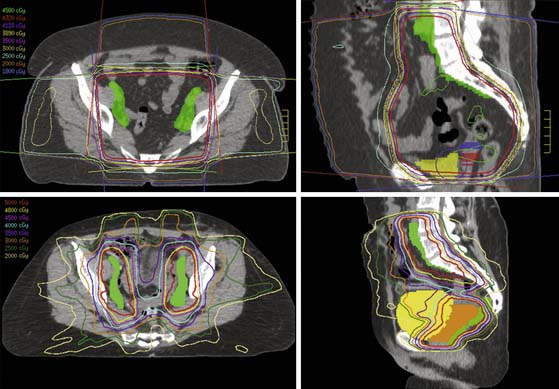
Before the early to mid 2000s, nearly all external beam radiation therapy was “forward-planned.” In other words, the radiation oncologist drew shaped fields on orthogonal plain x-rays, using bony landmarks as a guide (Figure 4-3). These x-ray films were produced using a “simulator” that mimicked the specifications of a treatment accelerator but had a diagnostic-energy beam in the rotating head. Using the radiation oncologist’s fields and a template of the patient’s external contour, the radiation dosimetrist calculated the length of time the machine needed to be on to deliver the appropriate dose. Techniques and field designs were tailored on the basis of findings on diagnostic imaging studies but were often relatively standard, based on years of feedback from studies of patterns of disease recurrence. Whenever possible, multiple-field techniques were used to minimize the volume of uninvolved tissue treated to a high dose (Figure 4-4). However, treatment fields that were designed without direct visualization of soft tissue structures were necessarily relatively simple and collateral treatment of uninvolved structures was often substantial.
These modern approaches can produce highly conforming treatment plans that often permit greater dose to targets or greater protection of normal structures than is possible with simpler techniques. However, the opportunity for error is also greater with these very complex treatments. The demands upon the radiation oncologist to understand normal anatomy and to correctly transmit the findings of diagnostic studies to contoured target volumes are much greater than with forward-planned techniques. Incorrectly defined target volumes lead to underdosage of the target, risking unnecessary tumor recurrences. Patient positioning and an understanding of internal organ motion also become more important when these very highly conforming treatments are used. In addition, because treatments are entirely dependent on accurate computer control of the treatment machine, meticulous quality assurance methods are required to ensure that treatments are being delivered as prescribed.
Brachytherapy
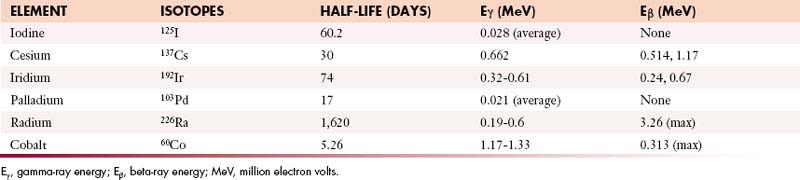
A number of different isotopes have been used in brachytherapy (Table 4-2). Radium (half-life 1620 yr) was once the primary isotope used for brachytherapy but has, for the most part, been abandoned in favor of safer isotopes with shorter half-lives. Iridium-192 has a half-life of 74 days and is used for most high dose-rate and pulsed dose-rate brachytherapy. As radium was phased out, cesium-137 became the primary source used for gynecologic treatments, but it is becoming difficult to obtain; as a result, many radiation oncologists are turning to iridium-based alternatives for treatment of gynecologic cancers. Iodine-125 and palladium-103 are frequently used for brachytherapy for prostate cancer.
Role of Imaging in Radiation Therapy
As a result, close, effective communication between radiation oncologist and diagnostic imager has never been more critical than it is today. Even small misunderstandings about the location or significance of radiographic abnormalities can result in serious errors in treatment design. Diagnostic imagers greatly assist their radiation oncology colleagues by providing specific information about the location, size, and relevance of abnormal findings. Communication can be improved by specifying the series and slice number corresponding to the best views of each finding. Additional anatomic information about the laterality, vertebral level, and proximity to easily identifiable structures often helps the radiation oncologist to accurately transfer diagnostic imaging information to treatment planning studies. Radiation oncologists also often depend on their imaging colleagues’ suggestions for ways of obtaining the most accurate depiction of disease. However, in very difficult cases, no amount of written communication can replace direct discussion between the imager and the radiation oncologist through tumor boards, face-to-face reviews, or telephone discussions while both the radiation oncologist and the diagnostic imager review the patient’s images. In this context, radiation oncologists have a responsibility to ask for help and to confirm that their understanding of images and reports is accurate.
1. Eifel P.J., Winter K., Morris M., et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of Radiation Therapy Oncology Group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872-880.
2. Pignon J.P., le Maitre A., Maillard E., et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4-14.
3. O’Connell M.J., Martenson J.A., Wieand H.S., et al. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331:502-507.
4. Cummings B.J., Keane T.J., O’Sullivan B., et al. Epidermoid anal cancer: treatment by radiation alone or by radiation and 5-fluorouracil with and without mitomycin C. Int J Radiat Oncol Biol Phys.. 1991;21:1115-1125.
5. Dewey W.C., Ling C.C., Meyn R.E. Radiation-induced apoptosis: relevance to radiotherapy. Int J Radiat Oncol Biol Phys.. 1995;33:781-796.
6. Kallman R.F. The phenomenon of reoxygenation and its implications for fractionated radiotherapy. Radiology. 1972;105:135-142.
7. Fyles A., Keane T.J., Barton M., et al. The effect of treatment duration in the local control of cervix cancer. Radiother Oncol. 1992;25:273-279.
8. Suwinski R., Sowa A., Rutkowski T., et al. Time factor in postoperative radiotherapy: a multivariate locoregional control analysis in 868 patients. Int J Radiat Oncol Biol Phys.. 2003;56:399-412.
9. Koukourakis M., Hlouverakis G., Kosma L., et al. The impact of overall treatment time on the results of radiotherapy for nonsmall cell lung carcinoma. Int J Radiat Oncol Biol Phys.. 1996;34:315-322.
10. Emami B., Lyman J., Brown A., et al. Tolerance of normal tissue to therapeutic radiation. Int J Radiat Oncol Biol Phys.. 1991;21:109-122.
11. Marks L.B., Ten Haken R.K., Martel M.K., et al. Guest editor’s introduction to QUANTEC: a users guide. Int J Radiat Oncol Biol Phys.. 2010;76:S1-2.
12. Mendenhall W.M., Amdur R.J., Hinerman R.W., et al. Postoperative radiation therapy for squamous cell carcinoma of the head and neck. Am J Otolaryngol. 2003;24:41-50.
13. Rotman M., Sedlis A., Piedmonte M.R., et al. A phase III randomized trial of postoperative pelvic irradiation in stage IB cervical carcinoma with poor prognostic features: follow-up of a Gynecologic Oncology Group study. Int J Radiat Oncol Biol Phys.. 2006;65:169-176.
14. Ceelen W., Fierens K., Van Nieuwenhove Y., et al. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer: a systematic review and meta-analysis. Int J Cancer. 2009;124:2966-2972.
15. Kirwan J.M., Symonds P., Green J.A., et al. A systematic review of acute and late toxicity of concomitant chemoradiation for cervical cancer. Radiother Oncol. 2003;68:217-226.
16. Mauri D., Pavlidis N., Ioannidis J.P. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:188-194.
17. Brada M., Pijls-Johannesma M., De Ruysscher D. Current clinical evidence for proton therapy. Cancer J. 2009;15:319-324.
18. Terasawa T., Dvorak T., Ip S., et al. Systematic review: charged-particle radiation therapy for cancer. Ann Intern Med. 2009;151:556-565.
19. Weber U., Kraft G. Comparison of carbon ions versus protons. Cancer J. 2009;15:325-332.