Chapter 3 A Multidisciplinary Approach to Cancer
A Medical Oncologist’s View
Epidemiology
As medicine, nutrition, and improved sanitation continue to further extend the average life expectancy, more people are surviving long enough to develop a malignancy. Thankfully, this is being tempered with an overall decrease in incidence and mortality from the most common cancers.1
The Rationale for Chemotherapy
Most cancers have 20% to 40% of cells in active cycling at any one time, which explains why the doubling time for a tumor is significantly longer than the cell cycle. Tumor growth would be exponential if all cells were dividing or constant if the fraction of actively cycling cells remained fixed; however, this does not correspond to clinically observed tumor doubling time. In 1825, Benjamin Gompertz described the nonexponential growth pattern he observed of disease in cancer patients. He noted the doubling time increased steadily as the tumor grew larger, a phenomenon now described as Gompertzian growth. This has been postulated to occur owing to decreased cell production, possibly related to relative lack of oxygen and of growth factors in the central portion of the large mass.2 A smaller tumor, conversely, would have a larger portion of actively cycling cells and, thus, be potentially more sensitive to cytotoxic chemotherapy.
Cytotoxic chemotherapy has the ability to kill more cancer cells than normal tissue, likely due to impaired DNA damage repair mechanisms in the former. This is relevant because most cytotoxic agents damage actively cycling cells. Typically, the more aggressive the cancer, the higher the proportion of its tumor cells that are in active phases of cell cycle.
Early studies of the ability of chemotherapy to kill cancer cells were conducted on leukemia cell lines in the 1960s.3 These studies noted log-kill kinetics, meaning if 99% of cells were killed, tumor mass would decrease from 1010 to 108 or from 105 to 103. The fraction of cells killed was proportional, regardless of tumor size; thus, even though a given treatment would appear to have eradicated the tumor, both clinically and radiographically, there would be a high probability of residual cells that would eventually proliferate and show up as a clinically evident tumor (relapse). One explanation for the achievement of sustainable complete remission following this argument would be that other factors such as host immune response may be important at low levels of residual tumoral cells.4
Indications for Chemotherapy
Adjuvant Chemotherapy
Surgical and radiotherapeutic treatments have made miraculous progress in the treatment for localized disease; however, many cancers have metastatic spread at diagnosis. Surgically or radiotherapeutically treated tumors may fail locally, but they often recur at distant sites. When considering the previously discussed undetectable period of tumor growth, it becomes apparent how a completely resected tumor may have significant occult residual disease, either locally or distant spread. In this situation, chemotherapy is given as an adjuvant to augment the effect of surgery; hence, the name adjuvant chemotherapy. Many patients who receive adjuvant therapy are without evidence of disease after local therapy. The pathologic margins of surgical specimens may be negative, and imaging may reveal no abnormality; however, significant relapse potential from residual local disease or micrometastases may exist. Adjuvant therapy aims to eradicate this subclinical disease before it reaches a critical threshold at which cure becomes difficult. Breast, lung, and colon cancer are a few examples of the many cancers that benefit from adjuvant therapy.5–7
Neoadjuvant Chemotherapy
Treating with chemotherapy before surgery is a newer concept. With more effective chemotherapy, neoadjuvant treatment approaches are occasionally used in appropriate-stage breast, lung, and resectable metastatic colorectal cancers.8–10
Neoadjuvant chemotherapy has three main advantages. Micrometastases are exposed to chemotherapy earlier in the treatment course, which may more effectively lead to eradication prior to becoming clinically apparent (based on log-kill theory). Second, a primary lesion that fails to respond indicates micrometastatic disease that is also likely resistant, allowing a change in therapy.11 Without neoadjuvant therapy, this knowledge would become apparent only after micrometastatic disease becomes clinically apparent and, thus, the potential for cure at that time would be very unlikely. Third, a primary tumor may regress sufficiently to facilitate a less morbid surgical procedure or occasionally obviate the need for surgical resection.12,13
Chemotherapy for Metastatic Cancer
The large portion of chemotherapy is given for clinically evident metastatic cancer, often as part of a palliation strategy in order to prolong survival and improve quality of life. However, some malignancies, including lymphoma and testicular cancers, may be cured even when they present with advanced metastatic disease. Other cancers, including ovarian and breast cancer, may demonstrate great sensitivity to chemotherapy in the metastatic setting with long-term disease control or even transient disappearance of all disease. An additional number of cancers, such as lung or pancreatic, may have brief stabilization or minor response to therapy, but long-term control is uncommon.
Chemotherapy Schedules
Combination Chemotherapy
The rationale for combination chemotherapy was adapted from observations of tuberculosis therapy in the 1950s. If a single agent was used, resistance would eventually develop. If multiple agents with different mechanisms of action were used concurrently, resistance was less common. Frei and coworkers14 published a study of combinatorial chemotherapy for leukemia in 1958, one of the first randomized clinical trials. They demonstrated transient responses in adult and pediatric leukemia patients achieved by combining methotrexate and 6-mercaptopurine, thus ushering in the modern era of combinatorial chemotherapy. Today, most patients treated with curative intent are given combination chemotherapy.
Intermittent Chemotherapy
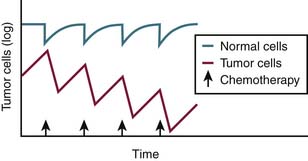
Because tumors have impaired DNA repair mechanisms and are thus more sensitive than normal tissue, they take longer to recover from the insult of chemotherapy. As a result, cytotoxic chemotherapy given at the appropriate interval will allow normal tissue, including hematopoietic stem cells, to recover, whereas tumor will not have sufficient time to recuperate (Figure 3-1). Most cytotoxic therapies are dosed every 2 or 3 weeks based on this observation.
Route of Administration
The majority of chemotherapy is administered intravenously, in an attempt to eliminate erratic gastrointestinal absorption as well as compliance issues. Chemotherapeutic agents can be given as a bolus (given over a very short interval), short infusion (given over a period of hours), or continuous infusion (usually administered as an inpatient or with a preprogrammed pump for home administration). The advantages of intravenous administration include more standardized absorption, documented compliance, and use of concurrent intravenous hydration or supportive medications.
Clinical Development
Phase I studies are the first in human studies of a new agent and have the goal of determining the maximum tolerated dose (MTD) by using dose escalation and dose-limiting toxicities (DLTs). Response rates are typically low because adequate dosing is unknown and patients typically have refractory disease, but a significant portion of patients do receive benefit.15 Pharmacokinetic and pharmacodynamic studies are performed during early clinical development to better understand the in vivo properties of the drug. Phase II studies use doses and schedules from the phase I data to assess efficacy with response as the primary endpoint. Patients are typically less heavily pretreated and must have measurable disease for response monitoring. Phase II trials typically enroll 20 to 50 patients, are designed for early termination if a significant number of responses are not seen, and may evaluate a new agent alone or in combination with standard chemotherapy.
1. Edwards B.K., Ward E., Kohler B.A., et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544-573.
2. Watson J.V. The cell proliferation kinetics of the EMT6/M/AC mouse tumour at four volumes during unperturbed growth in vivo. Cell Tissue Kinet. 1976;9:147-156.
3. Skipper H.E., Schabel F.M.Jr., Wilcox W.S. Experimental evaluation of potential anticancer agents. XIII. On the criteria and kinetics associated with “curability” of experimental leukemia. Cancer Chemother Rep. 1964;35:1-111.
4. Rockall T., Lowndes S., Johnson P., et al. Multidisciplinary treatment of cancer: surgery, chemotherapy and radiotherapy. In: Husband J.E., Reznek R.H. Imaging in Oncology. London: Taylor & Francis; 2004:43-63.
5. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687-1717.
6. Pignon J.-P., Tribodet H., Scagliotti G.V., et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552-3559.
7. Sun W., Haller D.G. Adjuvant therapy of colon cancer. Semin Oncol. 2005;32:95-102.
8. Carlson R.W., Allred D.C., Anderson B.O., et al. Breast cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2009;7:122-192.
9. Gray J., Sommers E., Alvelo-Rivera M., et al. Neoadjuvant chemotherapy for resectable non-small-cell lung cancer. Oncology (Huntingt). 2009;23:879-886.
10. Chau I., Chan S., Cunningham D. Overview of preoperative and postoperative therapy for colorectal cancer: the European and United States perspectives. Clin Colorectal Cancer. 2003;3:19-33.
11. Rosen G., Caparros B., Huvos A.G., et al. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49:1221-1230.
12. Jacquillat C., Weil M., Baillet F., et al. Results of neoadjuvant chemotherapy and radiation therapy in the breast-conserving treatment of 250 patients with all stages of infiltrative breast cancer. Cancer. 1990;66:119-129.
13. The Department of Veterans Affairs Laryngeal Cancer Study Group. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324:1685-1690.
14. Frei E.III, Holland J.F., Schneiderman M.A., et al. A comparative study of two regimens of combination chemotherapy in acute leukemia. Blood. 1958;13:1126-1148.
15. Wheler J., Tsimberidou A.M., Hong D., et al. Survival of patients in a phase 1 clinic. Cancer. 2009;115:1091-1099.