A
A severity characterisation of trauma (ASCOT). Trauma scale derived from the Glasgow coma scale, systolic BP, revised trauma score, abbreviated injury scale and age. A logistic regression equation provides a probability of mortality. Excludes patients with very poor or very good prognoses. Has been claimed to be superior to the trauma revised injury severity score system, although is more complex.
Champion HR, Copes WS, Sacco WJ, et al (1996). J Trauma; 40: 42–8
Abbott, Edward Gilbert, see Morton, William
Abbreviated injury scale (AIS). Trauma scale first described in 1971 and updated many times since. Comprises a classification of injuries with each given a six-digit code (the last indicating severity, with 1 = minor and 6 = fatal). The codes are linked to International Classification of Diseases codes, thus aiding standardisation of records. The anatomical profile is a refinement in which the locations of injuries are divided into four categories; the AIS scores are added and the square root taken to minimise the contribution of less severe injuries.
Copes WS, Lawnick M, Champion HR, Sacco WJ (1988). J Trauma; 28: 78–86
Abciximab. Monoclonal antibody used as an antiplatelet drug and adjunct to aspirin and heparin in high-risk patients undergoing percutaneous coronary intervention. Consists of Fab fragments of immunoglobulin directed against the glycoprotein IIb/IIIa receptor on the platelet surface. Inhibits platelet aggregation and thrombus formation; effects last 24–48 h after infusion. Careful consideration of risks and benefits should precede use since risk of bleeding is increased. Licensed for single use only.
Abdominal compartment syndrome. Combination of increased intra-abdominal pressure and organ dysfunction (e.g. following abdominal trauma or extensive surgery) resulting from haemorrhage or expansion of the third space fluid compartment. May also follow liver transplantation, sepsis, burns and acute pancreatitis. Intra-abdominal pressures above 20–25 cmH2O may impair ventilation and be associated with reduced venous return, cardiac output, renal blood flow and urine output. Increased CVP may lead to raised ICP. Diagnosed by clinical features and intra-abdominal pressure measurement (performed via a bladder catheter or nasogastric tube, in combination with a water column manometer).
Management includes laparotomy ± silastic material to cover the abdominal contents. Paracentesis may be effective if raised intra-abdominal pressure is due to accumulation of fluid, e.g. ascites. Full resuscitation must be performed before decompression as rapid release of pressure may result in sudden washout of inflammatory mediators from ischaemic tissues, causing acidosis and hypotension. Mortality of the syndrome is 25–70%.
Abdominal field block. Technique using 100–200 ml local anaesthetic agent, involving infiltration of the skin, subcutaneous tissues, abdominal muscles and fascia. Provides analgesia of the abdominal wall and anterior peritoneum, but not of the viscera. Now rarely used. Rectus sheath block, transversus abdominis plane block, iliac crest block and inguinal hernia field block are more specific blocks.
Abdominal sepsis, see Intra-abdominal sepsis
Abdominal trauma. May be blunt (e.g. road traffic accidents) or penetrating (e.g. stabbing, bullet wounds). Often carries a high morbidity and mortality because injuries may go undetected. Massive intra-abdominal blood loss or abdominal compartment syndrome may follow. The abdomen can be divided into three areas:
 intrathoracic: protected by the bony thoracic cage. Contains the spleen, liver, stomach and diaphragm. Injury may be associated with rib fractures. The diaphragm may also be injured by blows to the lower abdomen (which impart pressure waves to the diaphragm) or by penetrating injuries of the chest.
intrathoracic: protected by the bony thoracic cage. Contains the spleen, liver, stomach and diaphragm. Injury may be associated with rib fractures. The diaphragm may also be injured by blows to the lower abdomen (which impart pressure waves to the diaphragm) or by penetrating injuries of the chest.
 basic resuscitation as for trauma generally.
basic resuscitation as for trauma generally.
 initial assessment: examination of the anterior abdominal wall, both flanks, back, buttocks, perineum (and in men, the urethral meatus) for bruises, lacerations, entry and exit wounds. Signs may be masked by unconsciousness, spinal cord injury or the effects of alcohol or drugs. Abdominal swelling usually indicates intra-abdominal haemorrhage; abdominal guarding or rigidity usually indicates visceral injury. Absence of bowel sounds may indicate intraperitoneal haemorrhage or peritoneal soiling with bowel contents. Colonic or rectal injuries may cause blood pr. A high index of suspicion is required for retroperitoneal injuries since examination is difficult.
initial assessment: examination of the anterior abdominal wall, both flanks, back, buttocks, perineum (and in men, the urethral meatus) for bruises, lacerations, entry and exit wounds. Signs may be masked by unconsciousness, spinal cord injury or the effects of alcohol or drugs. Abdominal swelling usually indicates intra-abdominal haemorrhage; abdominal guarding or rigidity usually indicates visceral injury. Absence of bowel sounds may indicate intraperitoneal haemorrhage or peritoneal soiling with bowel contents. Colonic or rectal injuries may cause blood pr. A high index of suspicion is required for retroperitoneal injuries since examination is difficult.
 imaging: abdominal X-ray may reveal free gas under the diaphragm (erect or semi-erect; may also be visible on CXR) or laterally (lateral decubitus X-ray); other investigations include pelvic X-ray and urological radiology if indicated (e.g. iv urogram), CT and MRI scanning and ultrasound.
imaging: abdominal X-ray may reveal free gas under the diaphragm (erect or semi-erect; may also be visible on CXR) or laterally (lateral decubitus X-ray); other investigations include pelvic X-ray and urological radiology if indicated (e.g. iv urogram), CT and MRI scanning and ultrasound.
 peritoneal lavage is indicated in blunt abdominal trauma associated with:
peritoneal lavage is indicated in blunt abdominal trauma associated with:
– altered pain response (head injury, spinal cord injury, drugs, etc.).
– unexplained hypovolaemia following multiple trauma.
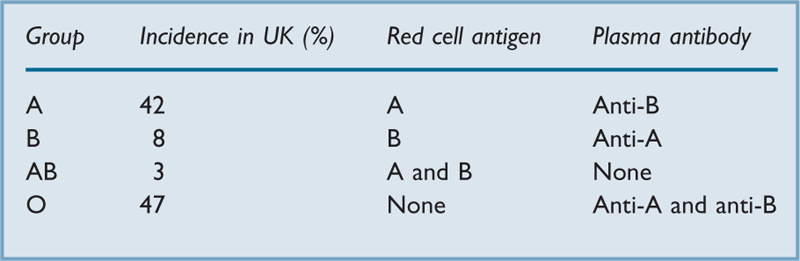
ABO blood groups. Discovered in 1900 by Landsteiner in Vienna. Antigens may be present on red blood cells, with antibodies in the plasma (Table 1). The antibodies, mostly type-M immunoglobulins, develop within the first few months of life, presumably in response to naturally occurring antigens of similar structure to the blood antigens. Infusion of blood containing an ABO antigen into a patient who already has the corresponding antibody may lead to an adverse reaction; hence the description of group O individuals as universal donors, and of group AB individuals as universal recipients.
[Karl Landsteiner (1868–1943), Austrian-born US pathologist]
See also, Blood compatibility testing; Blood groups; Blood transfusion
Abruption, see Antepartum haemorrhage
Absolute risk reduction. Indicator of treatment effect in clinical trials, representing the decrease in risk of a given treatment compared with a control treatment, i.e. the inverse of the number needed to treat. For a reduction in incidence of events from a% to b%, it equals (a – b)%.
See also, Meta-analysis; Odds ratio; Relative risk reduction
Abuse of anaesthetic agents. May occur because of easy access to potent drugs by operating theatre or ICU staff. Opioid analgesic drugs are the most commonly abused agents, but others include benzodiazepines and inhalational anaesthetic agents. Abuse may be suggested by behavioural or mood changes, or excessive and inappropriate requests for opioids. Main considerations include the safety of patients, counselling and psychiatric therapy for the abuser and legal aspects of drug abuse. May be associated with alcoholism.
Bryson EO, Silverstein JH (2008). Anesthesiology; 109: 905–17
See also, Misuse of Drugs Act; Sick doctor scheme; Substance abuse
Acarbose. Inhibitor of intestinal alpha glucosidases and pancreatic amylase; used in the treatment of diabetes mellitus, usually in combination with a biguanide or sulphonylurea. Delays digestion and absorption of starch and sucrose and has a small blood glucose-lowering effect.
Accessory nerve block. Performed for spasm of trapezius and sternomastoid muscles (there is no sensory component to the nerve). 5–10 ml local anaesthetic agent is injected 2 cm below the mastoid process into the sternomastoid muscle, through which the nerve runs.
Accident, major, see Incident, major
ACD-CPR, Active compression decompression CPR, see Cardiac massage; Cardiopulmonary resuscitation
ACE inhibitors, see Angiotensin converting enzyme inhibitors
Acetaminophen, see Paracetamol
Acetazolamide. Carbonic anhydrase inhibitor. Reduces renal bicarbonate formation and hydrogen ion excretion at the proximal convoluted tubule, thereby inducing a metabolic acidosis. A weak diuretic, but rarely used as such. Also used to treat glaucoma, metabolic alkalosis, altitude sickness and childhood epilepsy. Useful in the treatment of severe hyperphosphataemia as it promotes urinary excretion of phosphate. May be used to lower ICP (e.g. in benign intracranial hypertension) by reducing CSF production. Has been used to alkalinise the urine in tumour lysis syndrome or to enhance excretion in drug intoxications, e.g. with salicylates.
Acetylcholine (ACh). Neurotransmitter, the acetyl ester of choline (Fig. 1). Synthesised from acetylcoenzyme A and choline in nerve ending cytoplasm; the reaction is catalysed by choline acetyltransferase. Choline is actively transported into the nerve and acetylcoenzyme A is formed in mitochondria. ACh is stored in vesicles.
 parasympathetic postganglionic nerve endings.
parasympathetic postganglionic nerve endings.
 sympathetic postganglionic nerve endings at sweat glands and some muscle blood vessels.
sympathetic postganglionic nerve endings at sweat glands and some muscle blood vessels.
 many parts of the CNS, where it has a prominent role in learning.
many parts of the CNS, where it has a prominent role in learning.
Actions may be broadly divided into either muscarinic or nicotinic, depending on the acetylcholine receptors involved. ACh is hydrolysed to choline and acetate by acetylcholinesterase on the postsynaptic membrane. Other esterases also exist, e.g. plasma cholinesterase.
See also, Acetylcholine receptors; Neuromuscular transmission; Parasympathetic nervous system; Sympathetic nervous system; Synaptic transmission
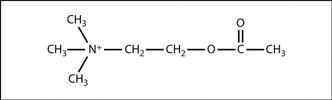
Fig. 1 Structure of acetylcholine
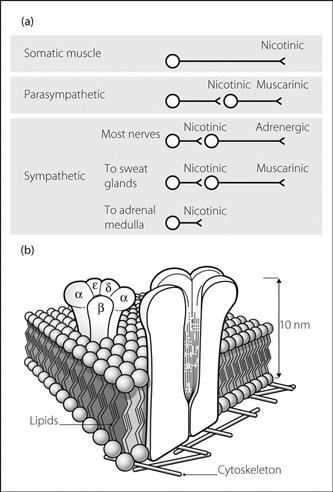
Acetylcholine receptors. Transmembrane receptors activated by acetylcholine (ACh). Classified according to their relative sensitivity to nicotine or muscarine (Fig. 2a).
• Nicotinic receptors: ligand-gated ion channels present at numerous sites within the nervous system; notable examples include the neuromuscular junction (NMJ) and autonomic ganglia. Each receptor consists of five glycosylated protein subunits that project into the synaptic cleft. The adult receptor consists of 2 α, β, δ and ε units. The ε subunit is replaced by a γ subunit in the neonate. The subunits span the postsynaptic membrane, forming a cylinder around a central ion channel (Fig. 2b). The two α subunits of each receptor carry the binding sites for ACh. Occupation of these sites opens the ion channel, allowing cations (mainly sodium, potassium and calcium) to flow into the cell down their concentration gradients; this produces an excitatory postsynaptic potential. If these summate and exceed the threshold potential, an action potential is generated. Non-depolarising neuromuscular blocking drugs are reversible competitive antagonists of these receptors at the NMJ.
• Muscarinic receptors: G protein-coupled receptors, largely coupled to either adenylate cyclase or phospholipase C, via Gi and Gq proteins respectively. Mediate postganglionic neurotransmission via parasympathetic neurones, as well as sympathetic outflow to sweat glands (Fig. 2a). Classified according to structural subtype, distribution and function:
 M1: Gq-coupled; stomach (stimulates acid secretion) and brain (memory formation).
M1: Gq-coupled; stomach (stimulates acid secretion) and brain (memory formation).
 M2: Gi-coupled; heart; decreases heart rate, contractility and atrioventricular nodal conduction.
M2: Gi-coupled; heart; decreases heart rate, contractility and atrioventricular nodal conduction.
 M4/5: brain and adrenal medulla.
M4/5: brain and adrenal medulla.
Muscarinic receptor agonists include bethanechol, carbachol and pilocarpine (in the eye); antagonists include hyoscine, atropine and ipratropium bromide.
The activation threshold of muscarinic receptors is lower than that of nicotinic receptors. Injection of ACh or poisoning with anticholinesterases thus causes parasympathetic stimulation and sweating at lower doses, before having effects at autonomic ganglia and the NMJ at higher doses.
See also, Neuromuscular transmission; Parasympathetic nervous system; Sympathetic nervous system; Synaptic transmission
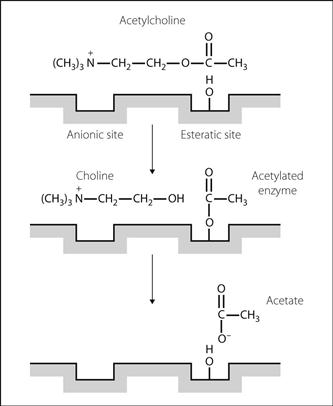
Acetylcholinesterase. Enzyme present at the synaptic membranes of cholinergic synapses and neuromuscular junctions. Also found in red blood cells and the placenta. Metabolises acetylcholine (ACh) to acetate and choline, thus terminating its action. The N(CH3)3+ moeity of ACh binds to the anionic site of the enzyme, and the acetate end of ACh forms an intermediate bond at the esteratic site. Choline is liberated, and the intermediate substrate/enzyme complex is then hydrolysed to release acetate (Fig. 3).
See also, Acetylcholinesterase inhibitors; Neuromuscular transmission; Synaptic transmission
Acetylcholinesterase inhibitors. Substances that increase acetylcholine (ACh) concentrations by inhibiting acetylcholinesterase (AChE). Used clinically for their action at the neuromuscular junction in myasthenia gravis and in the reversal of non-depolarising neuromuscular blockade. Concurrent administration of an antimuscarinic agent, e.g. atropine or glycopyrronium, reduces unwanted effects of increased ACh concentrations at muscarinic receptors. Effects at ganglia are minimal at normal doses. Central effects may occur if the drug readily crosses the blood–brain barrier, e.g. physostigmine (used to treat the central anticholinergic syndrome).
Have also been used to treat tachyarrhythmias.
• Classified according to mechanism of action:
– neostigmine, physostigmine (few hours).
Acetylcholinesterase inhibitors augment depolarising neuromuscular blockade and may cause depolarising blockade in overdose. They may also cause bradycardia, hypotension, agitation, miosis, increased GIT activity, sweating and salivation.
Centrally acting acetylcholinesterase inhibitors (e.g. donepezil, rivastigmine, galantamine) are used for symptomatic treatment of Alzheimer’s dementia. Of anaesthetic relevance because of their side effects (including nausea, vomiting, fatigue, muscle cramps, increased creatine kinase, convulsions, bradycardia, confusion), enhancement of the actions of suxamethonium, and possible antagonism of non-depolarising neuromuscular blocking drugs.
[Alois Alzheimer (1864–1915), German neurologist and pathologist]
See also, Neuromuscular transmission; Organophosphorus poisoning
N-Acetylcysteine. Derivative of the naturally occurring amino acid, L-cysteine. A free radical scavenger, licensed as an antidote to paracetamol poisoning. Acts by restoring depleted hepatic stores of glutathione and providing an alternative substrate for a toxic metabolite of paracetamol. Also used as an ocular lubricant and to prevent nephropathy due to radiological contrast media in patients with reduced renal function.
Has been investigated for the treatment of fulminant hepatic failure, MODS, acute lung injury and neuropsychiatric complications of carbon monoxide poisoning, as well as a possible role in protection against myocardial reperfusion injury. Also used as a mucolytic because of its ability to split disulphide bonds in mucus glycoprotein.
Achalasia. Disorder of oesophageal motility caused by idiopathic degeneration of nerve cells in the myenteric plexus or vagal nuclei. Results in dysphagia and oesophageal dilatation. A similar condition may result from American trypanosomal infection (Chagas’ disease). Aspiration pneumonitis or repeated chest infections may occur. Treated by mechanical distension of the lower oesophagus or by surgery. Heller’s cardiomyotomy (longitudinal myotomy leaving the mucosa intact) may be undertaken via abdominal or thoracic approaches. Preoperative respiratory assessment is essential. Patients are at high risk of aspirating oesophageal contents, and rapid sequence induction is indicated.
[Carlos Chagas (1879–1934), Brazilian physician; Ernst Heller (1877–1964), German surgeon]
See also, Aspiration of gastric contents; Induction, rapid sequence
Achondroplasia. Skeletal disorder, inherited as an autosomal dominant gene, although most cases arise by spontaneous mutation. Results in dwarfism, with a normal size trunk and shortened limbs. Flat face, bulging skull vault and spinal deformity may make tracheal intubation difficult, and the larynx may be smaller than normal. Obstructive sleep apnoea may occur. Foramen magnum and spinal canal stenoses may be present, the former resulting in cord compression on neck extension, the latter making neuraxial blockade difficult and reducing volume requirements for epidural anaesthesia.
Aciclovir. Antiviral drug; an analogue of nucleoside 2′-deoxyguanosine. Inhibits viral DNA polymerase; active against herpes viruses and used in the treatment of encephalitis, varicella zoster (chickenpox/shingles) and postherpetic neuralgia, and for prophylaxis and treatment of herpes infections in immunocompromised patients. Treatment should start at onset of infection; the drug does not eradicate the virus but may markedly attenuate the clinical infection.
Acid. Species that acts as a proton (H+) donor when in solution (Brønsted–Lowry definition).
[Johannes N Brønsted (1879–1947), Danish chemist; Thomas M Lowry (1874–1936), English chemist]
Acidaemia. Arterial pH < 7.35 or hydrogen ion concentration > 45 nmol/l.
Acid–base balance. Maintenance of stable pH in body fluids is necessary for normal enzyme activity, ion distribution and protein structure. Blood pH is normally maintained at 7.35–7.45 (hydrogen ion [H+] concentration 35–45 nmol/l); intracellular pH changes with extracellular pH. During normal metabolism of neutral substances, organic acids are produced that generate hydrogen ions.
• Maintenance of pH depends on:
 buffers in tissues and blood, which minimise changes in H+ concentration.
buffers in tissues and blood, which minimise changes in H+ concentration.
Because of the relationship between CO2, carbonic acid, bicarbonate (HCO3–) and H+, and the ability to excrete CO2 rapidly from the lungs, respiratory function is important in acid–base balance:
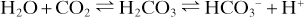
Thus hyper- and hypoventilation cause alkalosis and acidosis respectively. Similarly, hyper- or hypoventilation may compensate for non-respiratory acidosis or alkalosis respectively, by returning pH towards normal.
• The kidney can compensate for acid–base disturbances in three ways:
– filtered Na+ is exchanged for H+ across the tubule cell membrane.
– filtered HCO3– and excreted H+ form carbonic acid.
– carbonic acid is converted to CO2 and water by carbonic anhydrase on the cell membrane.
– carbonic acid dissociates into HCO3– and H+.
– HCO3– passes into the blood; H+ is exchanged for Na+, etc.
In acid–base disorders, the primary change determines whether a disturbance is respiratory or metabolic. The direction of change in H+ concentration determines acidosis or alkalosis. Renal and respiratory compensation act to restore normal pH, not reverse the primary change. For example, in the Henderson–Hasselbalch equation:
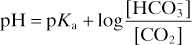
adjustment of the HCO3–/CO2 concentration ratio restores pH towards its normal value, e.g.:
 primary change: increased CO2; leads to decreased pH (respiratory acidosis).
primary change: increased CO2; leads to decreased pH (respiratory acidosis).
 compensation: HCO3– retention by kidneys; increased ammonium secretion, etc.
compensation: HCO3– retention by kidneys; increased ammonium secretion, etc.
An alternative approach, suggested by Stewart in 1983, focuses on the strong ion difference to explain the underlying processes rather than the above ‘traditional approach’, which concentrates more on interpretation of measurements. It is based on the degree of dissociation of ions in solution, in particular the effects of strong ions and weak acids, and the role of bicarbonate as a marker of acid–base imbalance rather than a cause.
[Peter Stewart (1921–1993), Canadian physiologist]
See also, Acid; Base; Blood gas interpretation; Breathing, control of; Davenport diagram; Siggaard-Andersen nomogram
Acid–citrate–dextrose solution, see Blood storage
Acidosis. A process in which arterial pH < 7.35 (or hydrogen ion > 45 mmol/l), or would be < 7.35 if there were no compensatory mechanisms of acid–base balance.
Acidosis, metabolic. Acidosis due to metabolic causes, resulting in an inappropriately low pH for the measured arterial PCO2.
– ketone bodies, e.g. in diabetes mellitus.
– lactate, e.g. in shock, exercise.
 acid ingestion: e.g. salicylate poisoning.
acid ingestion: e.g. salicylate poisoning.
 failure to excrete hydrogen ions (H+):
failure to excrete hydrogen ions (H+):
– distal renal tubular acidosis.
– carbonic anhydrase inhibitors.
 excessive loss of bicarbonate:
excessive loss of bicarbonate:
– proximal renal tubular acidosis.
• May be differentiated by the presence or absence of an anion gap:
• Primary change: increased H+/decreased bicarbonate.
 hyperventilation: plasma bicarbonate falls by about 1.3 mmol/l for every 1 kPa acute decrease in arterial PCO2, which usually does not fall below 1.3–1.9 kPa (10–15 mmHg).
hyperventilation: plasma bicarbonate falls by about 1.3 mmol/l for every 1 kPa acute decrease in arterial PCO2, which usually does not fall below 1.3–1.9 kPa (10–15 mmHg).
• Effects:
 hyperventilation (Kussmaul breathing).
hyperventilation (Kussmaul breathing).
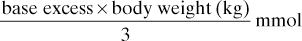
Half this amount is given initially.
 other agents under investigation include sodium dichloroacetate, Carbicarb (sodium bicarbonate and carbonate in equimolar concentrations) and THAM (2-amino-2-hydroxymethyl-1,3-propanediol).
other agents under investigation include sodium dichloroacetate, Carbicarb (sodium bicarbonate and carbonate in equimolar concentrations) and THAM (2-amino-2-hydroxymethyl-1,3-propanediol).
Morris CG, Low J (2008). Anaesthesia; 63: 294–301 and 396–411
Acidosis, respiratory. Acidosis due to increased arterial PCO2. Caused by alveolar hypoventilation.
Acquired immune deficiency syndrome (AIDS), see Human immunodeficiency viral infection
Acromegaly. Disease caused by excessive growth hormone secretion after puberty; usually caused by a pituitary adenoma but ectopic secretion may also occur. Incidence is 6–8 per million per year.
 respiratory obstruction, including sleep apnoea.
respiratory obstruction, including sleep apnoea.
 tendency towards diabetes mellitus, hypertension and cardiac failure (may be due to cardiomyopathy). Thyroid and adrenal impairment may occur.
tendency towards diabetes mellitus, hypertension and cardiac failure (may be due to cardiomyopathy). Thyroid and adrenal impairment may occur.
Treatment is primarily pituitary surgery with or without subsequent radiotherapy. Some patients respond to bromocriptine or somatostatin analogues.
Nemergut EC, Dumont AS, Barry UT, Laws ER (2007). Anesth Analg; 101: 1170–81
Acta Anaesthesiologica Scandinavica. Official journal of the Scandinavian Society of Anaesthesiology and Intensive Care Medicine, first published in 1957.
Actin. One of the protein components of muscle (mw 43 000). In muscle, arranged into a double strand of thin filaments (F-actin) with globular ‘beads’ (G-actin), to which myosin binds, along their length. Present in all cells as microfilaments.
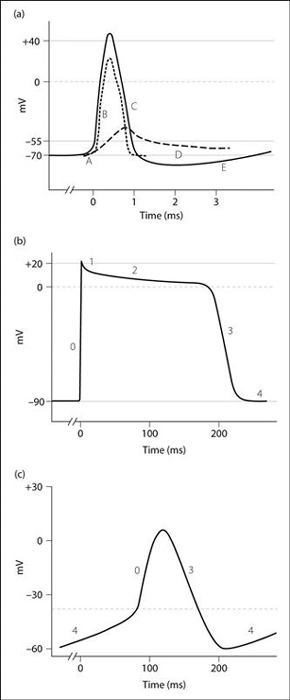
Action potential. Sequential changes in membrane potential that result in the propagation of electrical impulses in excitable cells. Neuronal, myocardial and cardiac nodal action potentials have distinct characteristics, determined by their underlying ionic fluxes (Fig. 4).
• Neuronal action potential (Fig. 4a):
 A: depolarisation of the membrane by 15 mV (threshold level).
A: depolarisation of the membrane by 15 mV (threshold level).
 B: rapid depolarisation to +40 mV.
B: rapid depolarisation to +40 mV.
Slow initial depolarisation causes opening of voltage-gated sodium channels (VGSCs) and influx of Na+ into the cell, which causes further rapid depolarisation. Na+ conductance then falls as the VGSCs enter an inactivated state. K+ efflux via voltage-gated potassium channels occurs more slowly and helps bring about repolarisation. Normal ion distribution (and hence the resting membrane potential) is restored by the action of the sodium/potassium pump. The action potential is followed by a refractory period.
• Myocardial action potential (Fig. 4b):
 phase 0: fast depolarisation and Na+ influx via VGSCs.
phase 0: fast depolarisation and Na+ influx via VGSCs.
 phase 1: onset of repolarisation due to sodium channel closure.
phase 1: onset of repolarisation due to sodium channel closure.
 phase 2: plateau due to Ca2+ influx via voltage-gated calcium channels (VGCCs).
phase 2: plateau due to Ca2+ influx via voltage-gated calcium channels (VGCCs).
 phase 3: repolarisation and K+ efflux.
phase 3: repolarisation and K+ efflux.
 phase 4: resting membrane potential.
phase 4: resting membrane potential.
The long plateau of phase 2 prolongs the refractory period, preventing tetanisation.
• Cardiac nodal action potential (Fig. 4c):
 phase 0: depolarisation caused by opening of L-type VGCCs and Ca2+ influx.
phase 0: depolarisation caused by opening of L-type VGCCs and Ca2+ influx.
Notably, there is no contribution by Na+ flux to the action potential in pacemaker cells.
Activated charcoal, see Charcoal, activated
Activated clotting time, see Coagulation studies
Activation energy. Energy required to initiate a chemical reaction. For ignition of explosive mixtures of anaesthetic agents the energy may be provided by sparks, e.g. from electrical equipment or build-up of static electricity. Combustion of cyclopropane requires less activation energy than that of diethyl ether. Activation energy is less for mixtures with O2 than with air, and least for stoichiometric mixtures of reactants.
Active compression/decompression cardiopulmonary resuscitation, see Cardiac massage; Cardiopulmonary resuscitation
Active transport. Energy-requiring transport of particles across cell membranes. Protein ‘pumps’ within the membranes utilise energy which is usually supplied by ATP metabolism, in order to move ions and molecules, often against concentration gradients. A typical example is the sodium/potassium pump.
Acupuncture. Use of fine needles (usually 30–33 G) to produce healing and pain relief. Originated in China thousands of years ago, and closely linked with the philosophy and practice of traditional Chinese medicine. Thus abnormalities in the flow of Qi (Chi: the life energy that circulates around the body along meridians, nourishing the internal organs) result in imbalance between Yin and Yang, the two polar opposites present in all aspects of the universe. Internal abnormalities may be diagnosed by pulse diagnosis (palpation of the radial arteries at different positions and depths). The appropriate organ is then treated by acupuncture at specific points on the skin, often along the meridian named after, and related to, that organ. Yin and Yang, and flow of Qi, are thus restored.
Modern Western acupuncture involves needle insertion at sites chosen for more ‘scientific’ reasons; e.g. around an affected area, at trigger points found nearby, or more proximally but within the appropriate dermatome. These may be combined with distant or local traditional points, although conclusive evidence for the existence of acupuncture points and meridians has never been shown. The needles may be left inserted and stimulated manually, electrically or thermally to increase intensity of stimulation. Pressure at acupuncture points (acupressure) may produce similar but less intense stimulation.
 local reflex pathways at spinal level.
local reflex pathways at spinal level.
 closure of the ‘gate’ in the gate control theory of pain.
closure of the ‘gate’ in the gate control theory of pain.
 central release of endorphins/enkephalins, and possibly involvement of other neurotransmitters.
central release of endorphins/enkephalins, and possibly involvement of other neurotransmitters.
Still used widely in China. Increasingly used in the West for chronic pain, musculoskeletal disorders, headache and migraine, and other disorders in which modern Western medicine has had little success. Claims that acupuncture may be employed alone to provide analgesia for surgery are now viewed with scepticism, although it has been used to provide analgesia and reduce PONV, e.g. 5 min stimulation at the point P6 (pericardium 6: 1–2 inches [2.5–5 cm] proximal to the distal wrist crease, between flexor carpi radialis and palmaris longus tendons).
Wang SM, Kain ZN, White P (2008). Anesth Analg; 106: 602–10 and 611–21
Acute coronary syndromes (ACS). Group of clinical conditions characterised by acute myocardial ischaemia; usually caused by acute thrombus formation within a coronary artery upon the exposed surface of a ruptured or eroded atheromatous plaque. May also occur due to coronary artery spasm, arteritis or sudden severe hypo- or hypertension.
 S-T segment elevation myocardial infarction (STEMI): ACS with ST segment elevation on 12-lead ECG. Suggestive of total coronary artery occlusion. Consistently associated with elevated plasma biomarkers of myocardial damage. Immediate reperfusion therapy (see below) significantly improves outcomes. ACS with new-onset left bundle branch block (LBBB) or evidence of posterior infarction is included in this category for treatment purposes.
S-T segment elevation myocardial infarction (STEMI): ACS with ST segment elevation on 12-lead ECG. Suggestive of total coronary artery occlusion. Consistently associated with elevated plasma biomarkers of myocardial damage. Immediate reperfusion therapy (see below) significantly improves outcomes. ACS with new-onset left bundle branch block (LBBB) or evidence of posterior infarction is included in this category for treatment purposes.
 non S-T segment elevation acute coronary syndromes (NSTEACS): suggestive of sub-total arterial occlusion. Immediate reperfusion therapy is not indicated, although early (within 24 h) percutaneous transluminal coronary angioplasty (PTCA) may be considered in high-risk patients. Further subdivided into:
non S-T segment elevation acute coronary syndromes (NSTEACS): suggestive of sub-total arterial occlusion. Immediate reperfusion therapy is not indicated, although early (within 24 h) percutaneous transluminal coronary angioplasty (PTCA) may be considered in high-risk patients. Further subdivided into:
– unstable angina: ACS without elevated cardiac biomarkers.
 pain as for myocardial ischaemia.
pain as for myocardial ischaemia.
 arrhythmias; cardiac arrest may occur.
arrhythmias; cardiac arrest may occur.
 anxiety, sweating, pallor, dyspnoea.
anxiety, sweating, pallor, dyspnoea.
 cardiac failure and cardiogenic shock.
cardiac failure and cardiogenic shock.
• Differential diagnosis: pain and ECG changes may occur with lesions of:
 heart/great vessels, e.g. aortic dissection, pericarditis.
heart/great vessels, e.g. aortic dissection, pericarditis.
 lung, e.g. PE, chest infection.
lung, e.g. PE, chest infection.
 oesophagus, e.g. spasm, inflammation, rupture.
oesophagus, e.g. spasm, inflammation, rupture.
 abdominal organs, e.g. peptic ulcer disease, pancreatitis, cholecystitis.
abdominal organs, e.g. peptic ulcer disease, pancreatitis, cholecystitis.
 12-lead ECG (see Myocardial infarction for characteristic features of STEMI and their localisation by ECG pattern). Bundle branch block may be evident. NSTEACS may coexist with a normal ECG or: S-T segment depression; S-T segment elevation insufficient to meet reperfusion therapy criteria (see below); T wave flattening or inversion; or biphasic T waves.
12-lead ECG (see Myocardial infarction for characteristic features of STEMI and their localisation by ECG pattern). Bundle branch block may be evident. NSTEACS may coexist with a normal ECG or: S-T segment depression; S-T segment elevation insufficient to meet reperfusion therapy criteria (see below); T wave flattening or inversion; or biphasic T waves.
– cardiac enzymes: largely replaced by troponins.
– regulatory proteins involved in cardiac and skeletal muscle contraction.
– may be elevated in myocardial damage due to other causes, e.g. myocarditis, contusion and also other non-cardiac critical illness (e.g. sepsis, renal failure, PE), probably reflecting myocardial injury but in most cases not related to coronary artery disease. Likely benefit of NSTEMI treatment in these cases should be assessed on an individual patient basis.
 echocardiography: may be used to assess regional and global ventricular function; regional wall motion abnormalities and loss of thickness suggest acute infarction. Also useful in diagnosing complications of MI (e.g. ventricular aneurysm, mitral regurgitation, mural thrombus).
echocardiography: may be used to assess regional and global ventricular function; regional wall motion abnormalities and loss of thickness suggest acute infarction. Also useful in diagnosing complications of MI (e.g. ventricular aneurysm, mitral regurgitation, mural thrombus).
• Immediate management of suspected ACS:
 O2 via facemask (if evidence of hypoxia, pulmonary oedema or ongoing ischaemia), cardiac monitoring, 12-lead ECG, iv access.
O2 via facemask (if evidence of hypoxia, pulmonary oedema or ongoing ischaemia), cardiac monitoring, 12-lead ECG, iv access.
 aspirin 300 mg orally.
aspirin 300 mg orally.
 analgesia (e.g. iv morphine in 2 mg increments).
analgesia (e.g. iv morphine in 2 mg increments).
 associated pulmonary oedema and arrhythmias should be treated in the usual way.
associated pulmonary oedema and arrhythmias should be treated in the usual way.
 consideration for immediate reperfusion therapy if:
consideration for immediate reperfusion therapy if:
– presentation < 12 h after symptom onset, unrelieved by GTN.
– S-T segment elevation > 0.1 mV in two or more contiguous chest leads or two adjacent limb leads.
– posterior infarction (dominant R wave and S-T depression in V1–V2 chest leads).
• Reperfusion strategies include:
 pharmacological thrombolytic therapy: agents include streptokinase, alteplase, tenecteplase and reteplase. Survival benefit is reduced with increasing delay, and is negligible from 12 h after onset of symptoms. Administration of thrombolysis within 1 h of the patient calling for professional help is a national audit standard. Contraindications include active bleeding, recent trauma (including surgery and CPR), previous haemorrhagic stroke or recent CVA, uncontrolled hypertension and pregnancy.
pharmacological thrombolytic therapy: agents include streptokinase, alteplase, tenecteplase and reteplase. Survival benefit is reduced with increasing delay, and is negligible from 12 h after onset of symptoms. Administration of thrombolysis within 1 h of the patient calling for professional help is a national audit standard. Contraindications include active bleeding, recent trauma (including surgery and CPR), previous haemorrhagic stroke or recent CVA, uncontrolled hypertension and pregnancy.
 emergency coronary artery bypass surgery.
emergency coronary artery bypass surgery.
Thrombolysis is generally only preferred if primary PTCA is unavailable or there would be delay of > 90 min in delivering it and the presentation is within 3 h of symptom onset. Primary PTCA is particularly superior if: there is cardiogenic shock; there are contraindications to thrombolysis; or the patient is at high risk of death (e.g. age > 75, previous MI, extensive anterior infarct). Emergency surgery is generally reserved for those known to have disease uncorrectable by PTCA or in whom primary PTCA fails.
Patients not meeting criteria for immediate reperfusion (i.e. those with NSTEACS) are managed either invasively (PTCA within 24 h plus abciximab iv) or conservatively (pharmacological management only). High-risk patients are most likely to benefit from invasive therapy.
• Pharmacological adjuncts include:
 clopidogrel and low-molecular weight heparin (e.g. enoxaparin): should be given to all patients (in the absence of contraindications) with definite or strongly suspected ACS, in addition to aspirin. Prasugrel and ticagrelor are newer alternatives to clopidogrel for certain patients.
clopidogrel and low-molecular weight heparin (e.g. enoxaparin): should be given to all patients (in the absence of contraindications) with definite or strongly suspected ACS, in addition to aspirin. Prasugrel and ticagrelor are newer alternatives to clopidogrel for certain patients.
 GTN sublingually or by iv infusion if pain persists.
GTN sublingually or by iv infusion if pain persists.
 glycoprotein IIB/IIIa inhibitors (e.g. abciximab and tirofiban): beneficial in patients undergoing PTCA, those at high risk of death, or both.
glycoprotein IIB/IIIa inhibitors (e.g. abciximab and tirofiban): beneficial in patients undergoing PTCA, those at high risk of death, or both.
 β-adrenergic receptor antagonists: reduce the rate of reinfarction and VF and should be commenced within 24 h if there are no contraindications, e.g. heart block, pulmonary oedema, hypotension. Those unable to receive β-blockers should receive one of the non-dihydropyridine calcium channel blocking drugs (e.g. verapamil).
β-adrenergic receptor antagonists: reduce the rate of reinfarction and VF and should be commenced within 24 h if there are no contraindications, e.g. heart block, pulmonary oedema, hypotension. Those unable to receive β-blockers should receive one of the non-dihydropyridine calcium channel blocking drugs (e.g. verapamil).
 ACE inhibitors: improve long-term survival after MI and should be commenced within 24 h, assuming no contraindications.
ACE inhibitors: improve long-term survival after MI and should be commenced within 24 h, assuming no contraindications.
 magnesium and potassium supplementation to maintain normal levels reduces the indidence of arrhythmias. Prophylactic administration of antiarrhythmic drugs is no longer recommended.
magnesium and potassium supplementation to maintain normal levels reduces the indidence of arrhythmias. Prophylactic administration of antiarrhythmic drugs is no longer recommended.
Kumar A, Cannon CP (2009). Mayo Clin Proc; 84: 917–38, 1021–36
Acute cortical necrosis, see Renal failure
Acute crisis resource management, see Crisis resource management
Acute demyelinating encephalomyelopathy, see Demyelinating diseases
Acute life-threatening events – recognition and treatment (ALERT). Multiprofessional course aimed at reducing the incidence of potentially avoidable cardiac arrests and admissions to ICU. Targeted especially at junior doctors and ward nurses. Sharing principles of many life-support training programmes (e.g. ALS, ATLS, APLS, CCrISP), its development embraces both clinical governance and multiprofessional education. Uses a structured and prioritised system of patient assessment and management to recognise and treat seriously ill patients or those at risk of deterioration.
Smith GB, Osgood VM, Crane S (2002). Resuscitation; 52: 281–6
See also, Early warning scores; Medical emergency team; Outreach team
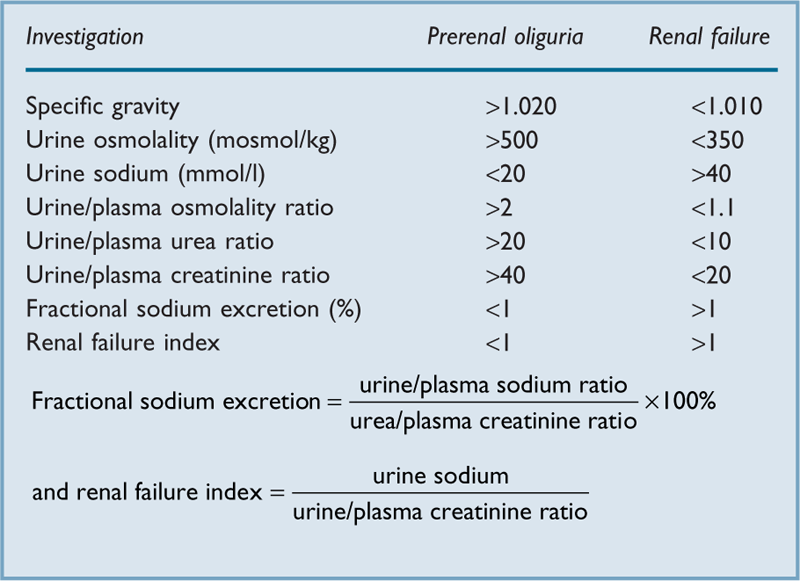
Acute kidney injury (AKI). Previously referred to as acute renal failure, describing a rapid deterioration (within 48 h) from baseline renal function; classified according to severity by the RIFLE criteria. An independent risk factor for in-hospital morbidity and mortality and a major cause of death in ICU, especially as part of MODS. May develop with or without pre-existing renal impairment. May follow any severe acute illness, dehydration, trauma or major surgery (especially involving the heart and great vessels), hepatic failure, obstetric emergencies, and any condition involving sustained hypotension.
 prerenal: caused by renal hypoperfusion, e.g. shock, hypovolaemia, cardiac failure, renal artery stenosis.
prerenal: caused by renal hypoperfusion, e.g. shock, hypovolaemia, cardiac failure, renal artery stenosis.
 renal: caused by renal disease:
renal: caused by renal disease:
– amyloid.
– acute tubular necrosis (ATN): accounts for 75% of hospital AKI. Caused by renal hypoperfusion or ischaemia and/or chemical toxicity, trauma or sepsis. Nephrotoxins include analgesics (e.g. chronic aspirin and paracetamol therapy), NSAIDs, aminoglycosides, immunosuppressive drugs, radiological contrast media and heavy metals. Usually (but not always) associated with oliguria (caused by tubular cell necrosis, tubular obstruction and cortical arteriolar vasoconstriction).
– acute cortical necrosis: typically associated with placental abruption, pre-eclampsia and septic abortion, but also with factors causing ATN. Confirmed by renal biopsy. Usually irreversible.
– tubulointerstitial nephritis/pyelonephritis.
– tubular obstruction, e.g. in myeloma, myoglobinuria.
– vascular, e.g. hypertension, connective tissue disease.
 postrenal: caused by obstruction in the urinary tract, e.g. bladder tumour, prostatic hypertrophy.
postrenal: caused by obstruction in the urinary tract, e.g. bladder tumour, prostatic hypertrophy.
 uraemia and accumulation of other substances (e.g. drugs): nausea, vomiting, malaise, increased bleeding and susceptibility to infection, decreased healing.
uraemia and accumulation of other substances (e.g. drugs): nausea, vomiting, malaise, increased bleeding and susceptibility to infection, decreased healing.
 reduced sodium and water excretion and oedema, hypertension, hyperkalaemia, acidosis.
reduced sodium and water excretion and oedema, hypertension, hyperkalaemia, acidosis.
• The following may aid diagnosis:
 analysis of urine: e.g. tubular casts may be seen in ATN, myoglobinuria may be present.
analysis of urine: e.g. tubular casts may be seen in ATN, myoglobinuria may be present.
 plasma and urine indices (Table 2).
plasma and urine indices (Table 2).
 flushing of the urinary catheter using aseptic technique.
flushing of the urinary catheter using aseptic technique.
 assessment of cardiac and volume status to exclude hypovolaemia.
assessment of cardiac and volume status to exclude hypovolaemia.
 diuretic administration, e.g. furosemide or mannitol: increased urine output may occur in incipient ATN but there is no evidence of a prophylactic or therapeutic effect; however reduction in renal O2 demand (furosemide) and scavenging of free radicals (mannitol) have been suggested as being theoretically beneficial.
diuretic administration, e.g. furosemide or mannitol: increased urine output may occur in incipient ATN but there is no evidence of a prophylactic or therapeutic effect; however reduction in renal O2 demand (furosemide) and scavenging of free radicals (mannitol) have been suggested as being theoretically beneficial.
 renal ultrasound or biopsy.
renal ultrasound or biopsy.
 directed at the primary cause with optimisation of renal blood flow.
directed at the primary cause with optimisation of renal blood flow.
 fluid restriction if appropriate, e.g. previous hour’s urine output + 30 ml/h whilst oliguric.
fluid restriction if appropriate, e.g. previous hour’s urine output + 30 ml/h whilst oliguric.
 H2 receptor antagonists are commonly administered to reduce GIT haemorrhage.
H2 receptor antagonists are commonly administered to reduce GIT haemorrhage.
 monitoring of drug levels, as clearance may be reduced considerably.
monitoring of drug levels, as clearance may be reduced considerably.
Acute lung injury (ALI). Syndrome of pulmonary inflammation and increased pulmonary capillary permeability associated with a variety of clinical, radiological and physiological abnormalities that cannot be explained by, but may coexist with, left atrial or pulmonary capillary hypertension. Associated with sepsis, major trauma, aspiration pneumonitis, blood transfusion, pancreatitis, cardiopulmonary bypass and fat embolism. Onset is usually within 2–3 days of the precipitating illness or injury, although direct lung insults usually have a shorter latency. Acute respiratory distress syndrome (ARDS) is now regarded to be the most severe form of ALI, with a mortality of 40–60%. The definitions of ALI and ARDS are increasingly being challenged and diagnostic criteria vary, but the most commonly used are those defined by the 1994 American-European Consensus Committee:
 bilateral diffuse infiltrates seen on the CXR.
bilateral diffuse infiltrates seen on the CXR.
 pulmonary wedge pressure ≤ 18 mmHg or absence of clinical evidence of left atrial hypertension.
pulmonary wedge pressure ≤ 18 mmHg or absence of clinical evidence of left atrial hypertension.
 arterial hypoxaemia resistant to oxygen therapy alone (PaO2/FIO2 < 39.9 kPa [300 mmHg] for definition of ALI; < 26.6 kPa [200 mmHg] for definition of ARDS), regardless of the level of ventilatory support.
arterial hypoxaemia resistant to oxygen therapy alone (PaO2/FIO2 < 39.9 kPa [300 mmHg] for definition of ALI; < 26.6 kPa [200 mmHg] for definition of ARDS), regardless of the level of ventilatory support.
 reduced respiratory compliance, lung volumes and increased work of breathing.
reduced respiratory compliance, lung volumes and increased work of breathing.

 mismatch with increased shunt.
mismatch with increased shunt.
 pulmonary vascular resistance may be raised.
pulmonary vascular resistance may be raised.
 in uncomplicated ALI, plasma oncotic pressure is normal.
in uncomplicated ALI, plasma oncotic pressure is normal.
 MODS may occur and is a common cause of death.
MODS may occur and is a common cause of death.
 direct insult to lung, e.g. aspiration, smoke inhalation.
direct insult to lung, e.g. aspiration, smoke inhalation.
 indirect result of an acute systemic inflammatory response involving both humoral (activation of complement, coagulation and kinin systems; release of mediators including cytokines, oxidants, nitric oxide) and cellular (neutrophils, macrophages and lymphocytes) components. Pulmonary infiltration by neutrophils leads to interstitial fibrosis, possibly as a result of damage caused by free radicals. Examples include sepsis, pancreatitis and fat embolism.
indirect result of an acute systemic inflammatory response involving both humoral (activation of complement, coagulation and kinin systems; release of mediators including cytokines, oxidants, nitric oxide) and cellular (neutrophils, macrophages and lymphocytes) components. Pulmonary infiltration by neutrophils leads to interstitial fibrosis, possibly as a result of damage caused by free radicals. Examples include sepsis, pancreatitis and fat embolism.
• Management involves prompt treatment of the underlying cause and supportive therapy:
 general support: nutrition, DVT prophylaxis, prevention of infection.
general support: nutrition, DVT prophylaxis, prevention of infection.
 O2 therapy, accepting SpO2 > 90%. CPAP is often helpful as it improves FRC.
O2 therapy, accepting SpO2 > 90%. CPAP is often helpful as it improves FRC.
 ventilatory support: IPPV may be necessary if CPAP is ineffective. Lung protection strategies improve survival in ARDS and consist of using low tidal volumes/inspiratory pressures (e.g. 4–6 ml/kg and PPlateau < 30 cmH2O) with moderate PEEP, tolerating a degree of respiratory acidosis (permissive hypercapnia). High-pressure recruitment manoeuvres and high PEEP are often beneficial in life-threatening hypoxaemia, but increase the risk of barotrauma and impaired cardiac output.
ventilatory support: IPPV may be necessary if CPAP is ineffective. Lung protection strategies improve survival in ARDS and consist of using low tidal volumes/inspiratory pressures (e.g. 4–6 ml/kg and PPlateau < 30 cmH2O) with moderate PEEP, tolerating a degree of respiratory acidosis (permissive hypercapnia). High-pressure recruitment manoeuvres and high PEEP are often beneficial in life-threatening hypoxaemia, but increase the risk of barotrauma and impaired cardiac output.
Additional ventilatory strategies include inverse ratio ventilation (at I : E ratios of up to 4 : 1), airway pressure release ventilation and high-frequency ventilation. Extracorporeal membrane oxygenation has been used with varying success. Extracorporeal CO2 removal may be useful in life-threatening hypercapnia.
 prone ventilation improves oxygenation in some patients, although overall mortality is not significantly improved.
prone ventilation improves oxygenation in some patients, although overall mortality is not significantly improved.
 inhaled vasodilator drugs (e.g. prostacyclin, nitric oxide) have been used to decrease pulmonary vascular resistance in aerated areas of lung, thereby reducing
inhaled vasodilator drugs (e.g. prostacyclin, nitric oxide) have been used to decrease pulmonary vascular resistance in aerated areas of lung, thereby reducing  mismatch and improving oxygenation, but with no demonstrable improved survival.
mismatch and improving oxygenation, but with no demonstrable improved survival.
 corticosteroid therapy is controversial. There appears to be no benefit to their prophylactic administration, or in high-dose, short-term therapy at the onset of ALI/ARDS. However, corticosteroids may have a role in refractory ARDS.
corticosteroid therapy is controversial. There appears to be no benefit to their prophylactic administration, or in high-dose, short-term therapy at the onset of ALI/ARDS. However, corticosteroids may have a role in refractory ARDS.
 free radical scavengers, antiprostaglandins and antiproteases have been investigated.
free radical scavengers, antiprostaglandins and antiproteases have been investigated.
Diaz JV, Brower R, Calfee CS, Matthay MA (2010). Crit Care Med; 38: 1644–50
Acute-phase response. A reaction of the haemopoietic and hepatic systems to inflammation or tissue injury, assumed to be of benefit to the host. There is a rise in the number/activity of certain cells (neutrophils, platelets) and plasma proteins (e.g. fibrinogen, complement, C-reactive protein, plasminogen, haptoglobin) involved in host defence, whilst there is a reduction in proteins with transport and binding functions (e.g. albumin, haemoglobin, transferrin). Initiated by actions of cytokine mediators such as interleukins (IL-1α, IL-1β, IL-6 and IL-11), tumour necrosis factors (α and β) and leukaemia inhibitory factor.
Serum levels of acute-phase proteins (e.g. C-reactive protein) can be helpful in diagnosis, monitoring and prognosis of certain diseases. The rise in fibrinogen levels causes an elevation in ESR. The fall in albumin is due to redistribution and decreased hepatic synthesis.
Acute physiology, age, chronic health evaluation, see APACHE III scoring system
Acute physiology and chronic health evaluation, see APACHE/APACHE II scoring systems
Acute physiology score (APS). Physiological component of severity of illness scoring systems, such as APACHE II/III and Simplified APS. Weighted values (e.g. 0–4 in APACHE II) are assigned to each of a range of physiological variables (e.g. temperature, mean arterial blood pressure, serum creatinine) on the basis of its derangement from an established ‘normal’ range, as measured either upon ICU admission or within 24 h of entry. The sum of all assigned weighted values for the physiological variables that comprise a given scoring system constitutes the acute physiological score. The higher the acute physiology score, the sicker the patient.
See also, Mortality/survival prediction on intensive care unit; Simplified acute physiology score
Acute respiratory distress syndrome (ARDS), see Acute lung injury
Acute tubular necrosis, see Renal failure
Addiction, see Alcoholism; Substance abuse
Addison’s disease, see Adrenocortical insufficiency
Adenosine. Nucleoside, of importance in energy homeostasis at the cellular level. Reduces O2 consumption, increases coronary blood flow, causes vasodilatation and slows atrioventricular conduction (possibly via increased potassium conductance and reduced calcium conductance). Also an inhibitory CNS neurotransmitter.
The drug of choice for treatment of SVT (including that associated with Wolff–Parkinson–White syndrome), and diagnosis of other tachyarrhythmias by slowing atrioventricular conduction. Its short half-life (8–10 s) and lack of negative inotropism make it an attractive alternative to verapamil. Not included in the Vaughan Williams classification of antiarrhythmic drugs.
Has also been used as a directly acting vasodilator drug in hypotensive anaesthesia. Increases cardiac output, with stable heart rate. Its effects are rapidly reversible on stopping the infusion.
• Dosage:
 hypotensive anaesthesia: 50–300 µg/kg/min. ATP has also been used.
hypotensive anaesthesia: 50–300 µg/kg/min. ATP has also been used.
• Side effects are usually mild and include flushing, dyspnoea and nausea. Bronchoconstriction may occur in asthmatics. Bradycardia is resistant to atropine. Adenosine’s action is prolonged in dipyridamole therapy (because uptake of adenosine is inhibited) and reduced by theophylline and other xanthines (because of competitive antagonism). Transplanted hearts are particularly sensitive to adenosine’s effects.
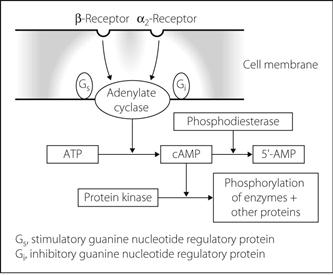
Adenosine monophosphate, cyclic (cAMP). Cyclic adenosine 3′,5′-monophosphate, formed from ATP by the enzyme adenylate cyclase. Activation of surface receptors may cause a guanine nucleotide regulatory protein (G protein) to interact with adenylate cyclase with resultant changes in intracellular cAMP levels (Fig. 5). Many substances act on surface receptors in this way, including catecholamines, vasopressin, ACTH, histamine, glucagon, parathyroid hormone and calcitonin.
Some substances inhibit adenylate cyclase via an inhibitory regulatory protein, e.g. noradrenaline at α2–adrenergic receptors (Fig. 5).
cAMP is termed a ‘second messenger’ as it causes phosphorylation of proteins, particularly enzymes, by activating protein kinases. Phosphorylation changes enzyme and thus cellular activity. cAMP is inactivated by phosphodiesterase to 5′-AMP. Phosphodiesterase inhibitors, e.g. aminophylline and enoximone, increase cAMP levels.
Adenosine triphosphate and diphosphate (ATP and ADP). ATP is the most important high-energy phosphate compound. When hydrolysed to form ADP, it releases energy that may be utilised in many cellular processes, e.g. active transport, muscle contraction. Its phosphate bonds are formed using energy from catabolism; aerobic respiration generates 38 moles of ATP per mole of glucose, while anaerobic respiration (i.e. simple glycolysis) yields 2 moles of ATP.
See also, Cytochrome oxidase system; Metabolism; Tricarboxylic acid cycle
Adenylate cyclase, see Adenosine monophosphate, cyclic
Adhesion molecules. Molecules normally sited on cell surfaces, involved in embryogenesis, cell growth and differentiation, and wound repair. Also mediate endothelial cell/leucocyte adhesion, transendothelial migration and cytotoxic T-cell-induced lysis. Four major families exist: integrins, cadherins, selectins (named after the tissues in which they were discovered: L-selectin [leucocytes], E-selectin [endothelial cells], P-selectin [platelets]) and members of the immunoglobulin superfamily.
In general, contact between an adhesion receptor and the extracellular milieu results in the transmission of information allowing the cell to interact with its environment. Defective interactions involving adhesion molecules are implicated in disease (e.g. certain skin diseases, metastasis of cancer cells). Many pathogens use adhesion receptors to penetrate tissue cells. Overexpression of intravascular adhesion molecules or receptors has been implicated in rheumatoid arthritis and rejection of transplanted organs. Control of vascular integrity and defence against invasive pathogens require regulation of adhesive interactions among blood cells and between blood cells and the vessel walls. Circulating leucocytes bind to the selectins of activated endothelial cells, become activated by chemoattractants and migrate through intracellular gaps to the site of inflammation. Thus adhesion molecules play a part in the inflammatory response in sepsis.
Adiabatic change. Volume change of a gas in which there is no transfer of heat to or from the system. Sudden compression of a gas without removal of resultant heat causes a rise in temperature. This may occur in the gas already present in the valves and pipes of an anaesthetic machine when a cylinder is turned on (hence the danger of explosion if oil or grease is present). Sudden adiabatic expansion of a gas results in cooling, as in the cryoprobe.
Modern valves, even when screwed fully down, will open at high pressures (60 cmH2O). Most are now encased in a hood for scavenging of waste gases.
[Jay A Heidbrink (1875–1957), US anaesthetist]
See also, Anaesthetic breathing systems; Non-rebreathing valves
Adrenal gland. Situated on the upper pole of the kidney, each gland is composed of an outer cortex and an inner medulla. The cortex consists of the outer zona glomerulosa (secreting aldosterone), the middle zona fasciculata (secreting glucocorticoids) and inner zona reticularis (secreting sex hormones). Hypersecretion may result in hyperaldosteronism, Cushing’s syndrome and virilisation/feminisation respectively. Hyposecretion causes adrenocortical insufficiency.
The adrenal medulla is thought to be derived from a sympathetic ganglion in which the postganglionic neurones have lost their axons, and secrete catecholamines into the bloodstream. Hypersecretion results in phaeochromocytoma. See also, Sympathetic nervous system
Adrenaline (Epinephrine). Catecholamine, acting as a hormone and neurotransmitter in the sympathetic nervous system and brainstem pathways. Synthesised and released from the adrenal gland medulla and central adrenergic neurones (for structure, synthesis and metabolism, see Catecholamines). Called epinephrine in the USA because the name adrenaline, used in other countries, was too similar to the US-registered trade name Adrenalin that referred to a specific product (both adrenaline (Latin) and epinephrine (Greek) referring to the location of the adrenal gland ‘on the kidney’).
Stimulates both α- and β-adrenergic receptors; displays predominantly β-effects at low doses, α- at higher doses. Low-dose infusion may lower BP by causing vasodilatation in muscle via β2-receptors, despite increased cardiac output via β1-receptors. Higher doses cause α1-mediated vasoconstriction and increased systolic BP, although diastolic pressure may still decrease.
 with local anaesthetic agents, as a vasoconstrictor.
with local anaesthetic agents, as a vasoconstrictor.
 in anaphylaxis, cardiac arrest, bronchospasm.
in anaphylaxis, cardiac arrest, bronchospasm.
Adrenaline may cause cardiac arrhythmias, especially in the presence of hypercapnia, hypoxia and certain drugs, e.g. halothane, cyclopropane and cocaine. During halothane anaesthesia, suggested maximal dosage of adrenaline is 10 ml 1 : 100 000 solution (100 µg) in 10 min, or 30 ml (300 µg) in 1 h. More dilute solutions should be used if possible. Adrenaline should not be used for ring blocks of digits or for penile nerve blocks, because of possible ischaemia to distal tissues.
α-Adrenergic receptor agonists. Naturally occurring agonists include adrenaline and noradrenaline, which stimulate both α1– and α2–adrenergic receptors.
Methoxamine and phenylephrine are synthetic α1-receptor agonists, used to cause vasoconstriction, e.g. to correct hypotension in spinal anaesthesia.
Clonidine acts on central α2-receptors. Clonidine and other α2-receptor agonists (e.g. dexmedetomidine) have been shown to reduce pain and anaesthetic requirements, and have also been used for sedation in ICU. Other α2-receptor agonists (e.g. xylazine, detomidine and medetomidine) have been used in veterinary practice as anaesthetic agents for many years.
β-Adrenergic receptor agonists. Include adrenaline, dobutamine and isoprenaline, which stimulate both β1– and β2–adrenergic receptors to varying degrees. Dopamine acts mainly at β1-receptors.
Salbutamol and terbutaline predominantly affect β2-receptors, and are used clinically to cause bronchodilatation in asthma, and as tocolytic drugs in labour. Formoterol and salmeterol are longer-acting agents given by inhalation for chronic asthma. Ritodrine is also used as a tocolytic drug. Some β1-receptor effects are seen at high doses, e.g. tachycardia. They have been used in the treatment of cardiac failure and cardiogenic shock; stimulation of vascular β2-receptors causes vasodilatation and reduces afterload.
α-Adrenergic receptor antagonists (α-Blockers). Usually refer to antagonists that act exclusively at α-adrenergic receptors.
– α1-receptors, e.g. prazosin, doxazosin, terazosin, indoramin, phenoxybenzamine. Tamsulosin acts specifically at α1A-receptors and is used in benign prostatic hypertrophy.
Labetalol and carvedilol (a drug with similar effects) are antagonists at both α- and β-receptors. Other drugs may also act at α-receptors as part of a range of effects, e.g. chlorpromazine, droperidol.
Used to lower BP and reduce afterload by causing vasodilatation. Compensatory tachycardia may occur.
β-Adrenergic receptor antagonists (β-Blockers). Competitive antagonists at β-adrenergic receptors.
• Actions:
 reduce heart rate, myocardial contractility and O2 consumption.
reduce heart rate, myocardial contractility and O2 consumption.
 increase coronary blood flow by increasing diastolic filling time.
increase coronary blood flow by increasing diastolic filling time.
 some have partial agonist activity (intrinsic sympathomimetic activity), e.g. pindolol, acebutolol, celiprolol and oxprenolol.
some have partial agonist activity (intrinsic sympathomimetic activity), e.g. pindolol, acebutolol, celiprolol and oxprenolol.
 practolol, atenolol, metoprolol, betaxolol, bisoprolol, nebivolol and acebutolol are relatively cardioselective, but all will block β2-receptors at high doses. Celiprolol has β1-receptor antagonist and β2-receptor agonist properties, thus causing peripheral vasodilatation in addition to cardiac effects.
practolol, atenolol, metoprolol, betaxolol, bisoprolol, nebivolol and acebutolol are relatively cardioselective, but all will block β2-receptors at high doses. Celiprolol has β1-receptor antagonist and β2-receptor agonist properties, thus causing peripheral vasodilatation in addition to cardiac effects.
 labetalol and carvedilol have α- and β-receptor blocking properties. The former is available for iv administration and is widely used for acute reduction in BP.
labetalol and carvedilol have α- and β-receptor blocking properties. The former is available for iv administration and is widely used for acute reduction in BP.
 esmolol: a few minutes; hydrolysed by red cell esterases.
esmolol: a few minutes; hydrolysed by red cell esterases.
 metoprolol, oxprenolol, pindolol, propranolol, timolol: 2–4 h.
metoprolol, oxprenolol, pindolol, propranolol, timolol: 2–4 h.
Most are readily absorbed enterally, and undergo extensive first-pass metabolism. Practolol, atenolol, celiprolol, nadolol and sotalol are water-soluble and largely excreted unchanged in the urine; propranolol and metoprolol are lipid-soluble and almost completely metabolised in the liver.
• Uses:
 hypertension, ischaemic heart disease, MI, arrhythmias, hyperthyroidism, anxiety, migraine prophylaxis. Have been shown to improve outcome in cardiac failure when added to ACE inhibitor therapy. Acute perioperative withdrawal of β-blockers from patients already receiving them is known to worsen outcomes; they should therefore be continued.
hypertension, ischaemic heart disease, MI, arrhythmias, hyperthyroidism, anxiety, migraine prophylaxis. Have been shown to improve outcome in cardiac failure when added to ACE inhibitor therapy. Acute perioperative withdrawal of β-blockers from patients already receiving them is known to worsen outcomes; they should therefore be continued.
 perioperatively: to reduce the hypertensive response to laryngoscopy; to treat perioperative hypertension, tachycardias and myocardial ischaemia; in hypotensive anaesthesia. The use of perioperative β-blockade to reduce perioperative cardiovascular morbidity and mortality in high-risk patients undergoing non-cardiac surgery is no longer advocated as the cardiovascular benefits are outweighed by the increased incidence of cerebrovascular accidents.
perioperatively: to reduce the hypertensive response to laryngoscopy; to treat perioperative hypertension, tachycardias and myocardial ischaemia; in hypotensive anaesthesia. The use of perioperative β-blockade to reduce perioperative cardiovascular morbidity and mortality in high-risk patients undergoing non-cardiac surgery is no longer advocated as the cardiovascular benefits are outweighed by the increased incidence of cerebrovascular accidents.
 cardiac failure, especially in combination with other negative inotropes.
cardiac failure, especially in combination with other negative inotropes.
 bronchospasm and peripheral arterial insufficiency, via blockade of β2-receptors.
bronchospasm and peripheral arterial insufficiency, via blockade of β2-receptors.
 increased risk of diabetes when used to treat hypertension and reduced cardiovascular (β1) and metabolic (β2) response to hypoglycaemia in diabetics.
increased risk of diabetes when used to treat hypertension and reduced cardiovascular (β1) and metabolic (β2) response to hypoglycaemia in diabetics.
 depression and sleep disturbance: less likely with water-soluble drugs.
depression and sleep disturbance: less likely with water-soluble drugs.
β-Adrenergic receptor antagonist poisoning. Uncommon, but overdose is hazardous because of these drugs’ low therapeutic ratio. General features include cardiac failure, bradycardia, cardiac conduction defects, hypotension, bronchospasm, coma and convulsions, the latter two especially with propranolol. Sotalol may cause ventricular tachyarrhythmias. Hypoglycaemia is rare.
 as for poisoning and overdoses generally.
as for poisoning and overdoses generally.
 CVS effects may require CPR, glucagon (2–20 mg [50–150 µg/kg in children] iv followed by 50 µg/kg/h) or iv infusion of isoprenaline. Atropine is often ineffective but should be tried in vagal-blocking doses (3 mg iv [40 µg/kg in children]). Cardiac pacing may be required.
CVS effects may require CPR, glucagon (2–20 mg [50–150 µg/kg in children] iv followed by 50 µg/kg/h) or iv infusion of isoprenaline. Atropine is often ineffective but should be tried in vagal-blocking doses (3 mg iv [40 µg/kg in children]). Cardiac pacing may be required.
Adrenergic receptors. G protein-coupled receptors, activated by adrenaline and other catecholamines, and divided into α- and β-receptors. Further subdivided into β1/β2/β3 and α1/α2 receptors (Table 3).
Their effects are mediated by ‘second messengers’: α1-receptor effects by increases in intracellular calcium ion concentration, α2-receptor effects by reducing intracellular cAMP, and β1– and β2-receptor effects by increasing cAMP. There is evidence for mixed receptor populations at both pre- and postsynaptic membranes. α2-receptors have been further subdivided into α2A (responsible for central regulation of BP, sympathetic activity, pain processing and alertness), α2B (causes vasoconstriction) and α2C (thought to be involved in behavioural responses).
See also, α-Adrenergic receptor agonists; β-Adrenergic receptor agonists; α-Adrenergic receptor antagonists; β-Adrenergic receptor antagonists; Sympathetic nervous system
Table 3 Classification and actions of adrenergic receptors
| Receptor type | Site | Effect of stimulation |
| α1 | Vascular smooth muscle | Contraction |
| Bladder smooth muscle (sphincter) | Contraction | |
| Radial muscle of iris | Contraction | |
| Intestinal smooth muscle | Relaxation, but contraction of sphincters | |
| Uterus | Variable | |
| Salivary glands | Viscous secretion | |
| Liver | Glycogenolysis | |
| Pancreas | Decreased secretion of enzymes, insulin and glucagon | |
| α2 | Presynaptic membranes of adrenergic synapses | Reduced release of noradrenaline |
| Postsynaptic membranes | Smooth muscle contraction | |
| Platelets | Aggregation | |
| β1 | Heart | Increased rate and force of contraction |
| Adipose tissue | Breakdown of stored triglycerides to fatty acids | |
| Juxtaglomerular apparatus | Increased renin secretion | |
| β2 | Vascular smooth muscle (muscle beds) | Relaxation |
| Bronchial smooth muscle | Relaxation | |
| Intestinal smooth muscle | Relaxation | |
| Bladder sphincter | Relaxation | |
| Uterus | Variable; relaxes the pregnant uterus | |
| Salivary glands | Watery secretion | |
| Liver | Glycogenolysis | |
| Pancreas | Increased insulin and glucagon secretion | |
| β3 | Adipose tissue | Lipolysis |
Adrenocortical insufficiency. May be due to:
 primary adrenal failure (Addison’s disease, high ACTH) due to:
primary adrenal failure (Addison’s disease, high ACTH) due to:
– autoimmune disease: the most common cause; may be associated with other autoimmune disease, e.g. diabetes, thyroid disease, pernicious anaemia, vitiligo.
– TB, amyloidosis, metastatic infiltration, haemorrhage, drugs or infarction, e.g. in shock. Waterhouse–Friderichsen syndrome comprises bilateral adrenal cortical haemorrhage associated with severe meningococcal disease.
 secondary adrenal failure (low ACTH) due to:
secondary adrenal failure (low ACTH) due to:
– corticosteroid therapy withdrawal.
– ACTH deficiency, e.g. due to surgery, head injury, disease of the pituitary or hypothalamus.
The term ‘Addisonian’ describes features of adrenal insufficiency irrespective of the cause.
 acute: hypotension and electrolyte abnormalities (hyponatraemia, hyperkalaemia, hypochloraemia, hypercalcaemia and hypoglycaemia), muscle weakness. In critically ill patients, treatment of occult adrenocortical insufficiency may be necessary for reversal of hypotension that is resistant to vasopressor drugs.
acute: hypotension and electrolyte abnormalities (hyponatraemia, hyperkalaemia, hypochloraemia, hypercalcaemia and hypoglycaemia), muscle weakness. In critically ill patients, treatment of occult adrenocortical insufficiency may be necessary for reversal of hypotension that is resistant to vasopressor drugs.
 acute: iv saline, hydrocortisone 100 mg iv qds (preferably as the sodium succinate).
acute: iv saline, hydrocortisone 100 mg iv qds (preferably as the sodium succinate).
 chronic: hydrocortisone 20–30 mg/day, fludrocortisone 50–300 µg/day, both orally. Typically, both are required in primary insufficiency but only hydrocortisone in secondary insufficiency, although this may not always hold true.
chronic: hydrocortisone 20–30 mg/day, fludrocortisone 50–300 µg/day, both orally. Typically, both are required in primary insufficiency but only hydrocortisone in secondary insufficiency, although this may not always hold true.
Adrenocorticotrophic hormone (ACTH). Polypeptide hormone (39 amino acids; mw 4500) secreted by corticotropic cells of the anterior pituitary gland in response to corticotropin releasing factor secreted by the hypothalamus. Release of ACTH is highest in the early morning and is increased by emotional and physical stress, including surgery. It increases corticosteroid synthesis in the adrenal glands, particularly glucocorticoids but also aldosterone. ACTH production is inhibited by glucocorticoids (i.e. negative feedback). ACTH or its synthetic analogue tetracosactide (Synacthen) has been used in place of corticosteroid therapy in an attempt to reduce adrenocortical suppression, and is used for diagnostic tests in endocrinology. They have also been given to treat or prevent post-dural puncture headache.
Adult respiratory distress syndrome, see Acute respiratory distress syndrome
Advance decision (Advance directive; ‘Living will’). Statement, usually written, that provides for a mentally competent person to refuse certain future medical treatments, usually involving life-saving therapies, if he/she were subsequently to become mentally or physically incompetent. The directive may be triggered by certain background conditions such as dementia, persistent vegetative state and terminal disease, or acute events including cardiorespiratory arrest, pneumonia, acute kidney injury, major CVA or spinal cord injury. Legal and ethical issues relate to competence (capacity) of the individual, the possibility of changing one’s mind, advances in medicine or techniques since the directive was written, the refusal of what might be considered basic care (e.g. feeding), difficulties anticipating the specific circumstances that may arise and therefore be covered, and the objections of doctors, other healthcare staff and relatives. Previously commonly referred to as advance directives, they were renamed ‘advance decisions’ in the Mental Capacity Act 2005, which enshrined them into statute for the first time. From April 2007, in order to be legally valid, an advance decision must:
Advanced (Cardiac) Life Support (ACLS/ALS). System of advanced management of cardiac arrest and its training to paramedics, doctors, nurses and other healthcare professionals. Also encompasses the recognition and management of periarrest arrhythmias and postresuscitation care. In the UK, ALS courses are run by the Resuscitation Council (UK). In the USA, the American Heart Association runs ACLS courses.
See also, Advanced life support, adult; Basic life support, adult; Cardiopulmonary resuscitation
Advanced life support, adult. Component of CPR involving specialised equipment, techniques (e.g. tracheal intubation), drugs, monitoring and 100% oxygen. Airway management may include use of tracheal intubation, LMA, Combitube or, rarely, tracheostomy.
• Recommendations of the European Resuscitation Council (2010):
 attention to ‘ABC’ of basic life support ± advanced airway management, if there is a delay in getting a defibrillator. Defibrillation should take place without delay for a witnessed or in-hospital cardiac arrest. A precordial thump should be considered for witnessed, monitored collapse when a defibrillator is not immediately available.
attention to ‘ABC’ of basic life support ± advanced airway management, if there is a delay in getting a defibrillator. Defibrillation should take place without delay for a witnessed or in-hospital cardiac arrest. A precordial thump should be considered for witnessed, monitored collapse when a defibrillator is not immediately available.
 actions then depend on the initial rhythm:
actions then depend on the initial rhythm:
– ‘shockable’, i.e. VF/pulseless VT:
– adrenaline 1 mg iv if VF/VT persists after the third shock, then 1 mg every 3–5 min if it still persists.
– consider and correct potentially reversible causes (see below); if not already done, secure the airway, administer oxygen, get iv access. Cardiac massage and ventilation at 30 : 2; compressions should be uninterrupted once the airway has been secured.
– in refractory VF despite three shocks, consider amiodarone 300 mg as an iv bolus; 150 mg as a second bolus followed by 900 mg/24 h. Lidocaine 1 mg/kg is an alternative if amiodarone is unavailable, but should not be used in addition (maximum 3 mg/kg in the first hour). Consider use of different pad positions/contacts, different defibrillator, buffers, if refractory. Magnesium sulphate 8 mmol may be indicated in hypomagnesaemia, torsades de pointes or digoxin toxicity.
– ‘non-shockable’, i.e. asystole or pulseless electrical activity (PEA):
– reassess after 2 min and repeat if necessary.
 potentially reversible causes (4 ‘H’s and 4 ‘T’s): hypoxaemia, hypovolaemia, electrolyte and metabolic disorders (especially hyper-/hypokalaemia), hypothermia, tension pneumothorax, cardiac tamponade, toxic/therapeutic disturbances (poisoning and overdoses), thromboembolic/mechanical obstruction (PE). A fifth ‘H’ (hydrogen ions, i.e. acidosis) and a fifth ‘T’ (coronary thrombosis) have also been suggested.
potentially reversible causes (4 ‘H’s and 4 ‘T’s): hypoxaemia, hypovolaemia, electrolyte and metabolic disorders (especially hyper-/hypokalaemia), hypothermia, tension pneumothorax, cardiac tamponade, toxic/therapeutic disturbances (poisoning and overdoses), thromboembolic/mechanical obstruction (PE). A fifth ‘H’ (hydrogen ions, i.e. acidosis) and a fifth ‘T’ (coronary thrombosis) have also been suggested.
 consider buffers, e.g. bicarbonate 50 ml 8.4% solution in severe acidosis (pH < 7.1 or base excess exceeding –10), or specific conditions, e.g. tricyclic antidepressant drug poisoning, hyperkalaemia.
consider buffers, e.g. bicarbonate 50 ml 8.4% solution in severe acidosis (pH < 7.1 or base excess exceeding –10), or specific conditions, e.g. tricyclic antidepressant drug poisoning, hyperkalaemia.
– checking of arterial blood gases, electrolytes and CXR.
– transfer to ICU and cardiorespiratory support as required. MODS may occur.
– trauma, choking, near-drowning, electrocution, anaphylaxis.
– paediatric and neonatal CPR (see Cardiopulmonary resuscitation, paediatric; Cardiopulmonary resuscitation, neonatal).
• Recent changes/controversies:
 atropine is no longer recommended for PEA/asystole.
atropine is no longer recommended for PEA/asystole.
 calcium is not recommended routinely because it reduces coronary and cerebral blood flow.
calcium is not recommended routinely because it reduces coronary and cerebral blood flow.
 hyperglycaemia is thought to worsen the effects of cerebral hypoxia. Avoidance of glucose solutions during CPR has been suggested.
hyperglycaemia is thought to worsen the effects of cerebral hypoxia. Avoidance of glucose solutions during CPR has been suggested.
 the decision to stop CPR may be difficult in certain circumstances, as may the decision not to start, e.g. terminally ill, elderly patients. Routine regular consideration of do not resuscitate orders has been suggested. Advance decisions may specify conditions under which patients do or do not wish to be resuscitated.
the decision to stop CPR may be difficult in certain circumstances, as may the decision not to start, e.g. terminally ill, elderly patients. Routine regular consideration of do not resuscitate orders has been suggested. Advance decisions may specify conditions under which patients do or do not wish to be resuscitated.
 manipulation of intrathoracic pressure has been suggested in order to improve cardiac output (see Cardiac massage).
manipulation of intrathoracic pressure has been suggested in order to improve cardiac output (see Cardiac massage).
European Resuscitation Council (2010). Resuscitation; 81: 1219–76
See also, Acute Cardiac Life Support; Brainstem death; Cough-CPR; Resuscitation Council (UK)
Advanced Life Support Group. UK charity established in 1993, aiming to promote and provide both the public and healthcare professionals with training and education in life-saving techniques. Develops and administers several courses such as APLS, MOET, STaR.
Advanced Life Support in Obstetrics (ALSO). Training system developed in the USA and administered by the American Association of Family Physicians; introduced in the UK as part of the Maternal and Neonatal Emergency Training (MANET) project. Aimed at training medical and midwifery staff in the practical management of maternal and neonatal emergencies and provision of life support for the mother and child. Similar to other CPR training systems (e.g. ACLS, ATLS) in its approach and structure.
Advanced Paediatric Life Support (APLS). System of management of the sick or injured child, devised by specialists in paediatrics, accident and emergency medicine, anaesthesia and paediatric surgery in the UK and administered by the Advanced Life Support Group. The system integrates basic and advanced resuscitation techniques, similar to those employed in ALS/ACLS and ATLS, to permit the assessment and treatment of paediatric emergencies (e.g. airway obstruction, cardiac arrest, hypothermia, convulsions, poisoning and trauma). Incorporates assessment of the airway, breathing and circulation with special emphasis on determining if the child has failure of respiration or circulation. Stresses the anatomical and physiological differences between adults and children whilst emphasising paediatric normal ranges (e.g. for weight, cardiorespiratory parameters). Underlines the usefulness of specific interventions (e.g. insertion of an intraosseous needle).
Advanced Trauma Life Support (ATLS). System of trauma management devised by physicians in Nebraska, USA in 1978 and adopted by the American College of Surgeons in 1979. Based on the concept of reducing morbidity and mortality in the first (‘golden’) hour following trauma during which patients may die from potentially survivable injuries (airway obstruction, hypovolaemia, pneumothorax, cardiac tamponade), by teaching all members of the trauma team the same algorithms and procedures which are then carried out by rote on every patient. Has been criticised (mainly by senior doctors) because of its very didactic nature, although this is one of its strengths since those most likely to be in the ‘front line’ of trauma care are traditionally junior doctors who lack the expertise and judgement of their seniors.
Consists of the following phases:
 primary survey: incorporates assessment of the integrity and function of the airway, cervical spine, breathing, circulation and neurological status. The patient is fully exposed to detect all life-threatening injuries, with resuscitation taking place in tandem with the primary survey. The need for radiographs of the chest, lateral cervical spine and pelvis should be considered during the primary survey but should not delay resuscitation (the cervical spine is assumed to require immobilisation for any injury above the clavicles until proven otherwise). This takes place simultaneously with the primary survey.
primary survey: incorporates assessment of the integrity and function of the airway, cervical spine, breathing, circulation and neurological status. The patient is fully exposed to detect all life-threatening injuries, with resuscitation taking place in tandem with the primary survey. The need for radiographs of the chest, lateral cervical spine and pelvis should be considered during the primary survey but should not delay resuscitation (the cervical spine is assumed to require immobilisation for any injury above the clavicles until proven otherwise). This takes place simultaneously with the primary survey.
 resuscitation of life-threatening conditions.
resuscitation of life-threatening conditions.
 secondary survey: the patient is fully examined from ‘head to toe’ to exclude injuries to scalp, cranium, face, neck, shoulders, chest, abdomen, perineum, long bones, spine and spinal cord. Special procedures (e.g. abdominal ultrasound, diagnostic peritoneal lavage, iv pyelogram, CT of brain) may be undertaken during this phase, before planning of definitive care.
secondary survey: the patient is fully examined from ‘head to toe’ to exclude injuries to scalp, cranium, face, neck, shoulders, chest, abdomen, perineum, long bones, spine and spinal cord. Special procedures (e.g. abdominal ultrasound, diagnostic peritoneal lavage, iv pyelogram, CT of brain) may be undertaken during this phase, before planning of definitive care.
 continued post-resuscitation monitoring and re-evaluation.
continued post-resuscitation monitoring and re-evaluation.
 definitive care: e.g. surgery, ICU admission or transfer to a specialist centre.
definitive care: e.g. surgery, ICU admission or transfer to a specialist centre.
Adverse drug reactions. Undesired drug effects, usually divided into:
 predictable (type A) dose-related side effects, e.g. hypotension following thiopental.
predictable (type A) dose-related side effects, e.g. hypotension following thiopental.
 unpredictable (type B; idiosyncratic) usually less common reactions. These are unrelated to known pharmacological properties of a drug (i.e. not due to common side effects or overdose), usually involving the immune system in some way (but not always, e.g. MH).
unpredictable (type B; idiosyncratic) usually less common reactions. These are unrelated to known pharmacological properties of a drug (i.e. not due to common side effects or overdose), usually involving the immune system in some way (but not always, e.g. MH).
Suspected adverse reactions are voluntarily reported to the Medicines and Healthcare products Regulatory Agency on yellow cards, introduced in 1964 and updated in 2000. The yellow card system was extended to nurse reporters in 2002. Specific yellow cards for reporting anaesthetic drug reactions were introduced in 1988, to encourage reporting of serious reactions to established drugs and any reaction to new drugs. Anaesthetists give many different drugs iv to large numbers of patients, and therefore reactions are often seen. Reactions may be more likely if drugs are given quickly and in combination.
– direct histamine release, e.g. tubocurarine, atracurium, thiopental, Althesin.
– alternative pathway complement activation, e.g. Althesin.
 apparently on first exposure: prior sensitisation by other (e.g. environmental) antigens may cause crossover sensitivity to subsequently administered drugs; e.g. reactions to dextrans may involve prior exposure to bacterial antigens.
apparently on first exposure: prior sensitisation by other (e.g. environmental) antigens may cause crossover sensitivity to subsequently administered drugs; e.g. reactions to dextrans may involve prior exposure to bacterial antigens.
– anaphylaxis, e.g. to thiopental.
– classical pathway complement activation, e.g. Althesin. Other drugs dissolved in Cremophor EL may crossreact.
 immediate medical management as for anaphylaxis.
immediate medical management as for anaphylaxis.
– a blood sample for mast cell tryptase levels should be taken as soon as possible and again at 1–2 h and 24 h after the reaction if anaphylaxis is suspected (99% of plasma tryptase arises from mast cells; normal plasma levels < 1 ng/ml). A significant rise is suggestive of anaphylaxis, but its absence does not exclude it; conversely it can occur in non-allergic reactions, albeit to a lesser extent. Tryptase is stable in solution and its half-life is 2.5 h, so back-calculation to the time of the reaction should still be possible if the samples’ actual times are accurately recorded. Histamine, complement and immunoglobulin levels may also be useful.
– skin testing is the gold standard for the detection of IgE-mediated reactions and includes:
– intradermal testing (injection of 0.02–0.05 ml dilute solution into the skin of the forearm or back) has a higher sensitivity but lower specificity and is more likely to trigger a systemic reaction. Results are read after 20–30 min.
– specific IgE antibodies may be assayed using fluorescence or (less commonly) radioactive detection systems and may be useful in reactions to suxamethonium, antibacterial drugs and latex; testing for other anaesthetic drugs is no longer considered specific enough. Less sensitive than skin testing.
– the role of other tests, e.g. in vitro basophil studies, is controversial.
Dewachter P, Mouton-Faivre C, Emala CW (2009). Anesthesiology; 111: 1141–50
Affinity. Extent to which a drug binds to a receptor. A drug with high affinity binds more avidly than one with lower affinity, whatever its intrinsic activity.
Afterload. Ventricular wall tension required to eject stroke volume during systole. For the left ventricle, afterload is increased by:
 an anatomical obstruction, e.g. aortic stenosis.
an anatomical obstruction, e.g. aortic stenosis.
 decreased elasticity of the aorta and large blood vessels.
decreased elasticity of the aorta and large blood vessels.
 increased ventricular wall thickness (greater tension is needed to produce the necessary pressure [Laplace’s law]).
increased ventricular wall thickness (greater tension is needed to produce the necessary pressure [Laplace’s law]).
Increased afterload results in increased myocardial work/O2 consumption, increased end-systolic ventricular volume and decreased stroke volume. Reduction of afterload may be achieved with vasodilator drugs.
Agonist. Substance that binds to a receptor to cause a response within the cell. It has high affinity for the receptor and high intrinsic activity.
A partial agonist binds to the receptor, but causes less response; it may have high affinity, but it has less intrinsic activity. It may therefore act as a competitive antagonist in the presence of a pure agonist.
An agonist–antagonist causes agonism at certain receptors and antagonism at others.
Agranulocytosis, see Leucocytes
Air. Air contains mostly nitrogen and oxygen, with various other constituents (Table 4). Density is approximately 1.2 kg/m3 (1.2 g/l).
‘Medical’ air may be supplied by a compressor or in cylinders. The latter (containing air at 137 bar) are grey with black and white shoulders. Piped air is normally at 4 bar.
Air embolism. Introduction of air bubbles into the circulation, usually into veins (although arterial air embolism has been caused by prolonged flushing of arterial lines in neonates). A potential risk when venous pressure is lower than atmospheric pressure. It may occur during any surgery when an open vein is raised above the heart; it is particularly likely in neurosurgery with the patient in the sitting position since the dural sinuses do not collapse. May also occur during insertion or removal of a CVP line (minimised by tilting the patient head-down during the former and using occlusive dressings as opposed to gauze in the latter). Procedures involving insufflation or injection of gas, e.g. laparoscopy, epidural anaesthesia using loss of resistance to air, laser surgery with gas-cooled probes, may also lead to embolism. N2O diffuses into bubbles, increasing their volume and exacerbating their effects. Morbidity and mortality are dependent on the volume of the air entrained and the rate of accumulation.
 tinkling sounds on auscultation, e.g. with an oesophageal/precordial stethoscope; large amounts of air may cause a ‘mill-wheel’ murmur. May be detected by a precordial Doppler probe or transoesophageal echocardiography, although the extreme sensitivity of these techniques may reveal many tiny bubbles of disputed clinical significance.
tinkling sounds on auscultation, e.g. with an oesophageal/precordial stethoscope; large amounts of air may cause a ‘mill-wheel’ murmur. May be detected by a precordial Doppler probe or transoesophageal echocardiography, although the extreme sensitivity of these techniques may reveal many tiny bubbles of disputed clinical significance.
 sudden reduction of end-tidal CO2 due to increased dead space and decreased cardiac output. End-tidal nitrogen monitoring has also been used to monitor air embolism.
sudden reduction of end-tidal CO2 due to increased dead space and decreased cardiac output. End-tidal nitrogen monitoring has also been used to monitor air embolism.
 raised pulmonary artery resistance and pressure.
raised pulmonary artery resistance and pressure.
 signs of right ventricular strain on the ECG; ventricular ectopics or fibrillation may occur.
signs of right ventricular strain on the ECG; ventricular ectopics or fibrillation may occur.
– increase venous pressure using head-down tilt, iv fluids, jugular venous compression, PEEP. The latter and the antigravity suit have been advocated for prevention of air embolism in neurosurgery in the traditional sitting position. The use of the modified sitting position with the legs placed horizontally may decrease the incidence.
 once air has entered the circulation:
once air has entered the circulation:
– CPR if required.
– hyperbaric O2 has been used to reduce the size of the embolism and improve oxygenation.
Mirski MA, Lele AJ, Fitzsimmons L, Toung TJK (2007). Anesthesiology; 106: 164–77
Airway. The upper airway includes the mouth, nose, pharynx and larynx. Maintenance of the airway in the unconscious or anaesthetised patient is achieved by combined flexion of the neck and extension at the atlanto-occipital joint (the ‘sniffing position’), and lifting the angles of the mandible forward. The tongue is lifted forward by the genioglossus muscle which is attached to the back of the point of the jaw; the hyoid bone and larynx are pulled forward by the hyoglossus, mylohyoid, geniohyoid and digastric muscles. Upward pressure on the soft tissues of the floor of the mouth may cause obstruction, particularly in children, and should be avoided. In the lateral (recovery) position, the tongue and jaw fall forward under gravity, improving the airway. Airway obstruction during anaesthesia has traditionally been attributed to the tongue falling back against the posterior pharyngeal wall. Radiological and electromyographic studies suggest that obstruction by the soft palate or epiglottis secondary to reduced local muscle activity may also be responsible.
Airway exchange catheter. Device placed into the trachea both to maintain oxygenation after tracheal extubation (i.e. inserted through the tracheal tube before the latter is removed), and to facilitate reintubation should it be required. Available devices share similar features: they are long, hollow catheters, able to be attached to an O2 supply at their proximal end (e.g. using a detachable 15-mm connector) and stiff enough to act as a guide for a tracheal tube passed over them. The distal end may be angulated, allowing the catheter also to be used as an introducer, e.g. during direct laryngoscopy. A specific type of exchange catheter (the Aintree catheter) has been designed for exchanging an LMA with a tracheal tube via a flexible fibrescope.
Airway obstruction. Obstruction may occur at the mouth, pharynx, larynx, trachea and large bronchi. It may be caused by the tongue, laryngospasm, strictures, tumours and soft tissue swellings, oedema, infection (e.g. diphtheria, epiglottitis) and foreign objects, including anaesthetic equipment. Hypotonia of the muscles involved in upper airway maintenance is common during anaesthesia. During IPPV, mechanical obstruction may occur at any point along the breathing system.
Airway obstruction results in hypoventilation and increased work of breathing. It may present as an acute medical emergency requiring immediate management.
– dyspnoea, noisy respiration, stridor.
– tachypnoea, tachycardia and other features of hypoxaemia, hypercapnia and respiratory failure.
– pulmonary oedema may occur if negative intrathoracic pressures are excessive.
– during anaesthesia, poor movement of the reservoir bag may occur.
– increased airway pressures with reduced chest movement; noisy respiration or wheeze.
– features of hypoxaemia and hypercapnia.
 O2 therapy (increased FIO2 during anaesthesia).
O2 therapy (increased FIO2 during anaesthesia).
– general measures in unconscious/anaesthetised patients:
– turn to the lateral position if practical.
– correct positioning of the head, elevation of the jaw, and use of oropharyngeal or nasopharyngeal airways. Tracheal intubation may be required as below.
– antibacterial drugs for infection.
– fresh frozen plasma or C1 esterase inhibitor for hereditary angio-oedema.
– nebulised adrenaline for croup.
– Heimlich manoeuvre for choking.
– increasing gas flow: helium–O2 mixtures.
– bypassing the obstruction: tracheal intubation, tracheostomy or cricothyrotomy. The last two may be difficult if the anatomy is distorted.
– causes of increased airway pressure and hypoventilation other than obstruction due to equipment (e.g. bronchospasm, pneumothorax, inadequate neuromuscular blockade and coughing) should be considered.
– while ventilating by hand, all tubing should be checked for kinks. A suction catheter will not pass down the tracheal tube if the latter is obstructed, e.g. by kinks, mucus, herniated cuff. If auscultation of the chest does not suggest bronchospasm or pneumothorax, the cuff should be deflated and the tracheal tube pulled back slightly. If ventilation does not improve, the tube should be removed and ventilation attempted by facemask.
• Anaesthesia for patients with airway obstruction:
– preoperative assessment for the above features and management as above.
– useful pre-induction investigations include:
– radiography, including tomograms, thoracic inlet views and CT scanning.
– flexible nasendoscopy images.
– arterial blood gas interpretation.
– premedication may aid smooth induction of anaesthesia but excessive sedation should be avoided.
– if general anaesthesia is deemed preferable and/or necessary it should be induced with full monitoring and facilities for resuscitation and tracheostomy/cricothyrotomy immediately available.
– patients with proximal airway obstruction usually adopt the optimal head and neck position for maximal air movement; muscle relaxation caused by iv induction may therefore lead to sudden complete obstruction. It may be impossible to ventilate the patient by facemask, and tracheal intubation may be difficult or impossible. IV anaesthetic agents and neuromuscular blocking drugs should therefore be used with extreme caution. Inhalational induction (e.g. sevoflurane in O2) is traditionally advocated, although induction may be slow and difficult. If obstruction worsens, anaesthesia is allowed to lighten. Tracheal intubation is performed without paralysis; lidocaine spray may be useful.
 postoperatively: as for difficult intubation (see Intubation, difficult).
postoperatively: as for difficult intubation (see Intubation, difficult).
In the recovery room or casualty department, all unconscious patients should be positioned in the recovery position, to protect them from aspiration and airway obstruction.
Airway pressure. Pressure within the breathing system and tracheobronchial tree. Useful as an indicator of mechanical obstruction to expiration during spontaneous ventilation. During IPPV, changes in airway pressure may indicate disconnection, dynamic hyperinflation, mechanical obstruction, decreased lung compliance or increased airway resistance, and the risk of barotrauma. The pressure measured at the mouth may considerably exceed alveolar pressure during positive pressure breaths, especially if airway resistance and gas flow rates are high. During expiration, mouth pressure falls to zero, but alveolar pressure may lag behind. In some ventilators airway pressure is measured in the expiratory limb of the breathing system.
Airway pressure release ventilation (APRV). CPAP with intermittent release to ambient pressure (or a lower level of CPAP) causing expiration. Spontaneous ventilation may continue between APRV breaths. Allows reduction of mean airway pressure, and has been used to support respiration in ARDS. Weaning is achieved by reducing the frequency of CPAP release until the patient is breathing spontaneously with CPAP maintained.
Esan A, Hess DR, Raoof S, George L, Sessler CN (2010). Chest; 137: 1203–16

Driving pressure is the difference between alveolar and mouth pressures, and may be measured using the body plethysmograph. Alternatively, gas flow may be halted repeatedly for a tenth of a second at a time with a shutter; during the brief period of no flow, alveolar pressure may be measured at the mouth. Gas flow can be measured with a pneumotachograph.
Airway resistance increases during anaesthesia; this may be caused by bronchospasm, reduction in FRC and lung volume, or by the tubes and connections of the breathing system.
Airways. Devices placed in the upper airway (but not into the larynx); used to:
Thus usually employed during anaesthesia and in unconscious patients.
• Types:
 oropharyngeal: Guedel airway is most commonly used. Modifications include a side port for attachment to a fresh gas source (Waters airway), 15 mm connectors for attachment to a breathing system, caps with side ports, and airways used for fibreoptic intubation (Fig. 6). A cuffed oropharyngeal airway (COPA) with a 15 mm connector was described in 1991 but is not widely used. Oropharyngeal airways are the commonest cause of damage to teeth in anaesthetised patients. They must be placed with care, particularly in children where soft tissue damage can easily occur.
oropharyngeal: Guedel airway is most commonly used. Modifications include a side port for attachment to a fresh gas source (Waters airway), 15 mm connectors for attachment to a breathing system, caps with side ports, and airways used for fibreoptic intubation (Fig. 6). A cuffed oropharyngeal airway (COPA) with a 15 mm connector was described in 1991 but is not widely used. Oropharyngeal airways are the commonest cause of damage to teeth in anaesthetised patients. They must be placed with care, particularly in children where soft tissue damage can easily occur.
 oral supraglottic: designed to be placed close to, but above, the larynx, resulting in a better seal and therefore the ability to control ventilation. Include (Fig. 7):
oral supraglottic: designed to be placed close to, but above, the larynx, resulting in a better seal and therefore the ability to control ventilation. Include (Fig. 7):
– LMA: the most widely used.
– laryngeal tube: requires less mouth opening. Has two cuffs, inflated by a single inflation tube. Available in seven sizes, suitable for babies up to adults. Performs better for IPPV in studies than for spontaneous ventilation. A new double-lumen version (laryngeal tube Sonda) is similar in function to the Combitube (see Oesophageal obturators and airways).
– others have been described but are not widely used:
– Cobra: single-use device similar in function to the LMA; available in eight sizes.
Albendazole. Agent used (off-licence) in conjunction with surgery to treat hydatid disease. May be used alone in inoperable cases.
Albumin. The most abundant plasma protein (mw 69 000): normal plasma levels: 35–50 g/l. Important in the maintenance of plasma oncotic pressure, as a buffer, and in the transport of various molecules such as bilirubin, hormones, fatty acids and drugs. It is synthesised by the liver and removed from the plasma into the interstitial fluid. It may then pass via lymphatics back to the plasma, or into cells to be metabolised. May have a role as a free radical scavenger. Depletion occurs in severe illness, infection and trauma. Available for transfusion as 4.5% and 20% solutions; currently the most expensive non-blood plasma substitute. Has been used as a colloid when providing iv fluid therapy for critically ill patients but hypoalbuminaemia in these patients usually results from increased metabolism of circulating albumin; administration does not generally result in maintained plasma albumin levels and no improvement in outcome has been found when compared with cheaper alternatives. The effect of albumin administration on mortality in critically ill patients remains controversial, although recent studies suggest mortality is not increased, except possibly in patients with traumatic brain injury. The lack of any clear advantage of using albumin solutions for resuscitation, and its expense, has led to a decline in its use.
Hartog CS, Bauer M, Reinhart K (2011). Anesth Analg; 112: 156–64
Albuterol, see Salbutamol
Alcohol poisoning. Problems, features and management depend on the alcohols ingested:
 ethanol: commonly complicates or precipitates acute illness or injury, especially trauma. Results in depressed consciousness (hindering assessment of head injury), potentiation of depressant drugs and disinhibition. Patients are often uncooperative. Other effects of acute intoxication include vasodilatation, tachycardia, arrhythmias, vomiting, reduced lower oesophageal sphincter pressure, gastric irritation, delayed gastric emptying, hypoglycaemia (typically 6–36 h after ingestion, especially in a starved or malnourished individual), metabolic acidosis, dehydration (due to diuretic effect), coma and convulsions. Intoxication occurs at blood levels ~100–150 mg/dl (22–33 mmol/l), loss of muscle coordination at ~150–200 mg/dl (33–43 mmol/l), decreased level of consciousness at ~200–300 mg/dl (43–65 mmol/l) and death at ~300–500 mg/dl (65–109 mmol/l). The fatal adult dose is about 300–500 ml absolute alcohol (500–1200 ml strong spirits) within 1 h.
ethanol: commonly complicates or precipitates acute illness or injury, especially trauma. Results in depressed consciousness (hindering assessment of head injury), potentiation of depressant drugs and disinhibition. Patients are often uncooperative. Other effects of acute intoxication include vasodilatation, tachycardia, arrhythmias, vomiting, reduced lower oesophageal sphincter pressure, gastric irritation, delayed gastric emptying, hypoglycaemia (typically 6–36 h after ingestion, especially in a starved or malnourished individual), metabolic acidosis, dehydration (due to diuretic effect), coma and convulsions. Intoxication occurs at blood levels ~100–150 mg/dl (22–33 mmol/l), loss of muscle coordination at ~150–200 mg/dl (33–43 mmol/l), decreased level of consciousness at ~200–300 mg/dl (43–65 mmol/l) and death at ~300–500 mg/dl (65–109 mmol/l). The fatal adult dose is about 300–500 ml absolute alcohol (500–1200 ml strong spirits) within 1 h.
Management is mainly supportive, as for poisoning and overdoses. Haemodialysis has been used in severe poisoning. Features of alcoholism require attention if present.
 methanol: toxicity results from hepatic metabolism of methanol to formaldehyde and thence formic acid, the latter being a potent inhibitor of mitochondrial cytochrome oxidase. Features occur after 8–36 h and include intoxication, vomiting, abdominal pain, coma, visual disturbances, severe metabolic acidosis with a large anion gap and osmolar gap, and hyperglycaemia. About 10 ml pure methanol may result in permanent blindness, with the fatal adult dose around 30 ml (methylated spirits contains 5% methanol and 95% ethanol, the latter causing the most toxicity). Blood levels greater than 500 mg/ml (15 mmol/l) indicate severe poisoning.
methanol: toxicity results from hepatic metabolism of methanol to formaldehyde and thence formic acid, the latter being a potent inhibitor of mitochondrial cytochrome oxidase. Features occur after 8–36 h and include intoxication, vomiting, abdominal pain, coma, visual disturbances, severe metabolic acidosis with a large anion gap and osmolar gap, and hyperglycaemia. About 10 ml pure methanol may result in permanent blindness, with the fatal adult dose around 30 ml (methylated spirits contains 5% methanol and 95% ethanol, the latter causing the most toxicity). Blood levels greater than 500 mg/ml (15 mmol/l) indicate severe poisoning.
Management includes gastric lavage if within 2 h of ingestion, administration of bicarbonate, inhibition of further methanol metabolism with ethanol (50 g orally/iv followed by 10–20 g/h iv to produce blood levels of 100–200 g/dl [20–30 mmol/l]). More recently, fomepizole has gained acceptance as an alternative to alcohol and may reduce the need for haemodialysis, which may still be required in severe cases. Folic acid or its metabolites have also been used (utilised in formate metabolism). Haemodialysis has been used in severe poisoning.
Management is with gastric lavage if within 2 h of ingestion and supportive thereafter.
 ethylene glycol: toxicity results from metabolism by alcohol dehydrogenase to glycoaldehyde and oxalic/formic acids. The former causes cerebral impairment, while the latter produce severe metabolic acidosis and acute tubular necrosis. The fatal adult dose is ~100 g, although patients have survived much larger doses. Features include intoxication, vomiting, haematemesis, coma, convulsions, depressed reflexes, myoclonus, nystagmus, papilloedema and ophthalmoplegia within the first 12 h; tachycardia, hypertension, pulmonary oedema and cardiac failure within the next 12 h; and renal failure over the subsequent 2–3 days. Investigations may reveal severe lactic acidosis, a large anion gap and osmolar gap, hypocalcaemia and hyperkalaemia. Blood levels exceeding ~200 mg/l (~35 mmol/l) indicate severe poisoning.
ethylene glycol: toxicity results from metabolism by alcohol dehydrogenase to glycoaldehyde and oxalic/formic acids. The former causes cerebral impairment, while the latter produce severe metabolic acidosis and acute tubular necrosis. The fatal adult dose is ~100 g, although patients have survived much larger doses. Features include intoxication, vomiting, haematemesis, coma, convulsions, depressed reflexes, myoclonus, nystagmus, papilloedema and ophthalmoplegia within the first 12 h; tachycardia, hypertension, pulmonary oedema and cardiac failure within the next 12 h; and renal failure over the subsequent 2–3 days. Investigations may reveal severe lactic acidosis, a large anion gap and osmolar gap, hypocalcaemia and hyperkalaemia. Blood levels exceeding ~200 mg/l (~35 mmol/l) indicate severe poisoning.
Alcohol withdrawal syndromes. May be precipitated by acute illness or surgery, even if regular intake of alcohol (ethanol) is apparently not excessive and without the other features of alcoholism.
 most commonly, tremulousness, agitation, nausea, vomiting: usually within 6–8 h of abstinence.
most commonly, tremulousness, agitation, nausea, vomiting: usually within 6–8 h of abstinence.
 hallucinations: usually visual; occur 10–30 h after abstinence and in under 10% of cases.
hallucinations: usually visual; occur 10–30 h after abstinence and in under 10% of cases.
 convulsions: occur up to 48 h after abstinence and in about 5% of cases.
convulsions: occur up to 48 h after abstinence and in about 5% of cases.
 delirium tremens: includes the above symptoms plus confusion, disorientation, sweating, tachycardia and hypertension. Occurs up to 2–3 days after abstinence and in about 5% of cases.
delirium tremens: includes the above symptoms plus confusion, disorientation, sweating, tachycardia and hypertension. Occurs up to 2–3 days after abstinence and in about 5% of cases.
Management includes sedative drugs (e.g. benzodiazepines, carbamazepine, clomethiaziole) and reassurance. More recently, clonidine has been used successfully to smooth withdrawal. Vitamin B6 supplements are usually administered, but if there is a risk of encephalopathy a mixture of vitamins is best prescribed.
McKeon A, Frye MA, Delanty N (2008). J Neurol Neurosurg Psychiatry; 79: 854–62
Alcoholism. Form of substance abuse in which there is narrowing of the drinking repertoire (loss of day-to-day variation in drinking habits), increased tolerance to alcohol (ethanol), symptoms of alcohol withdrawal syndromes when intake is stopped, a craving for alcohol and drinking in the early part of the day/middle of the night. May precipitate admission to hospital as a consequence of acute alcohol poisoning or intoxication, or impaired organ function resulting from chronic abuse; may also complicate the management of incidental disease or surgery.
• Effects of chronic alcohol intake:
 cerebral/cerebellar degeneration, peripheral neuropathy, psychiatric disturbances (Wernicke’s encephalopathy, Korsakoff’s psychosis).
cerebral/cerebellar degeneration, peripheral neuropathy, psychiatric disturbances (Wernicke’s encephalopathy, Korsakoff’s psychosis).
 withdrawal may lead to tremor, anxiety, hallucinations, convulsions, delirium tremens.
withdrawal may lead to tremor, anxiety, hallucinations, convulsions, delirium tremens.
 gastritis, peptic ulcer disease, oesophageal varices; may lead to gastrointestinal haemorrhage.
gastritis, peptic ulcer disease, oesophageal varices; may lead to gastrointestinal haemorrhage.
 fatty liver, hepatic enzyme induction, cirrhosis causing hepatic failure, coagulopathy.
fatty liver, hepatic enzyme induction, cirrhosis causing hepatic failure, coagulopathy.
 ethanol (ethyl alcohol; C2H5OH): commonly referred to as ‘alcohol’; widely drunk throughout the world and often abused, resulting in acute toxicity or alcoholism. Used in the past as an anaesthetic agent and as a tocolytic drug. May be added to irrigant solution to monitor the latter’s uptake during TURP. Also used for destructive injection, e.g. into neural tissue in chronic pain management. In the ICU, iv alcohol may be used to treat methanol poisoning; it may also be useful for sedating alcoholics (1–10 g/h of a 5–10% solution).
ethanol (ethyl alcohol; C2H5OH): commonly referred to as ‘alcohol’; widely drunk throughout the world and often abused, resulting in acute toxicity or alcoholism. Used in the past as an anaesthetic agent and as a tocolytic drug. May be added to irrigant solution to monitor the latter’s uptake during TURP. Also used for destructive injection, e.g. into neural tissue in chronic pain management. In the ICU, iv alcohol may be used to treat methanol poisoning; it may also be useful for sedating alcoholics (1–10 g/h of a 5–10% solution).
Metabolised mostly to acetaldehyde, then acetate, acetyl coenzyme A and finally CO2 and water via the tricarboxylic acid cycle. Five per cent is excreted unchanged, mostly in the urine but also in the breath. The rate of metabolism is 10 ml/h at concentrations above 100 mg/dl (22 mmol/l); below this level it is subject to first-order kinetics with a half-life of about 1 h (see Michaelis–Menten kinetics). Produces 30 kJ/g (7 Cal/g); it has been used as an iv energy source.
 ethylene glycol (glycol; (CH2OH)2): used as an antifreeze.
ethylene glycol (glycol; (CH2OH)2): used as an antifreeze.
 propylene glycol (CH3.CHOH.CH2OH): used as a solvent in drugs, e.g. etomidate, GTN.
propylene glycol (CH3.CHOH.CH2OH): used as a solvent in drugs, e.g. etomidate, GTN.
Alcuronium chloride. Non-depolarising neuromuscular blocking drug, introduced in 1961 and withdrawn in the UK in 1994. Caused mild histamine release and hypotension, but anaphylaxis, when it occurred, was often severe.