CASE 9
Mark is 2 years of age and has already suffered from more than his share of childhood infections and prolonged episodes of diarrhea. He has been hospitalized twice for bacterial pneumonia, has had almost constant (according to his mother) viral infections, and twice has had oral thrush (Candida albicans). He is under the care of an infectious disease specialist at a tertiary care center, who now believes he understands the etiology of the problem. Further exploration of the family tree revealed evidence of a distant great-grandparent who was perpetually “sick” and two cousins, Sally and Joe, who have recurrent infectious illnesses. Simple blood tests performed on many occasions have never revealed defects in the total number of B cells and T cells or neutrophils/monocytes. However, T cell subset analysis by flow cytometry indicated severe CD4+ lymphopenia and serum immunoglobulin levels, although present, were low. Antibody titers to childhood immunizations were negligible. Nitroblue tetrazolium tests indicated a normal respiratory burst after phagocytosis (see Case 6).
QUESTIONS FOR GROUP DISCUSSION
RECOMMENDED APPROACH
Implications/Analysis of Clinical History
A defect in phagocytes, leukocyte trafficking, or complement could account for the bacterial infections, but they would not, alone, account for other types of infection. A clinical history of fungal or viral infections strongly suggested a defect in T cell function. Therefore, a defect that affects both B cells and T cells should be considered.
Additional Laboratory Tests
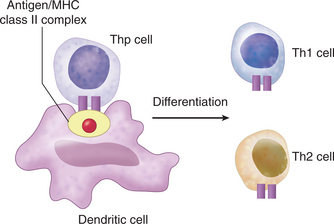
Serologic testing using an ELISA ruled out HIV as a cause of the low CD4+ T cell count. Flow cytometric analysis of various cell surface markers on T cells did not reveal any deficiencies. Analysis of B cell markers indicated a deficiency in expression of cell surface class II MHC proteins. Class II MHC heterodimers are constitutively expressed on antigen-presenting cells, where they display antigen fragments to CD4+ T cells (Fig. 9-1; see Antigen Recognition, later). Further analysis indicated that all antigen-presenting cells lacked expression of class II MHC. Because CD4+ T cells are only activated when they recognize the class II antigen complexes and because B cell activation requires CD4+ T cell help, a deficiency of class II on antigen-presenting cells alone could explain the reduction in B cell responses. Nevertheless, this does not explain the paucity of CD4+ T cells.
THERAPY
Bone Marrow Transplant
Patients with BLS have normal numbers of circulating T cells, but most of them are CD8+. The fact that there are a few patients with low numbers of circulating CD4+ T cells may be explained from studies with mice in which mice with defects in one of the trimeric complex proteins (RFX5, see later) express very low levels of MHC class II in the thymic cortex. Because fewer than 100 patients have been identified with BLS, it is difficult to investigate these issues in patient populations.
ETIOLOGY: BARE LYMPHOCYTE SYNDROME
CIITA and Nucleotide Oligomerization Domain Protein
CIITA is now known to belong to a family of molecules having in common a nucleotide oligomerization domain (NOD) responsible for self-self protein interactions, which are apparently essential for signaling function from these interacting molecules. The most important proteins bound by CIITA are not well characterized, although a number of nuclear factors have been shown to bind to it (including cyclic adenosine monophosphate response element binding protein [CREB]). The mutations in CIITA that affect its role may, in fact, reflect defective NOD functioning.