Bispectral Index Monitoring
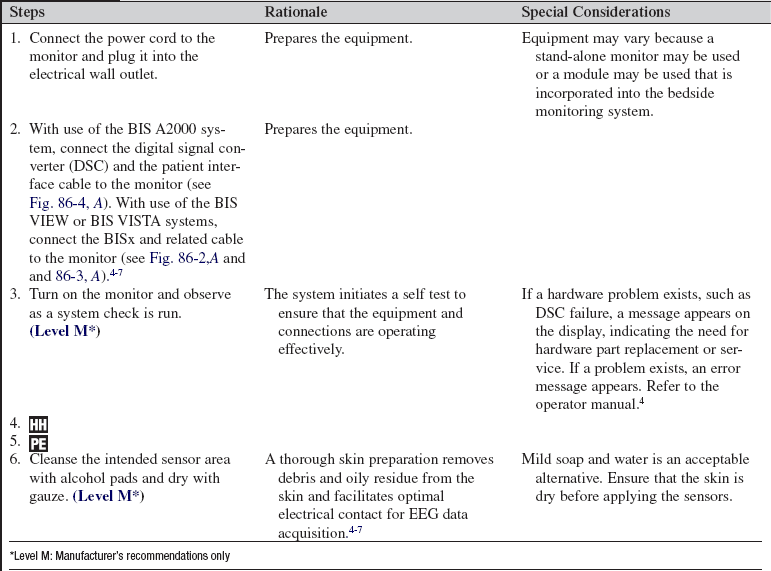
The bispectral index is a processed electroencephalogram-based parameter used in critically ill adults for assessment of level of consciousness and response to sedative, hypnotic, and anesthetic agents.1–4,10,13,16 The bispectral index also may indicate an arousal response to painful stimulation.2 Information derived from bispectral index monitoring may be used to guide sedative, hypnotic, and analgesic therapy.2,4,10,16
PREREQUISITE NURSING KNOWLEDGE
• Understanding of cerebral physiology is needed.
• Sedative, hypnotic, anesthetic, and analgesic agents produce clinical effects as a result of binding, in a dose-related manner, with specific receptors in the brain modulating cerebral physiology.24,34
• Understanding of the interrelationship between the electrical activity of the brain and cerebral metabolism is necessary.
• Electroencephalogram (EEG) tracings are obtained and recorded through the application of scalp electrodes and detect electrical activity in the brain.33
• Examination of EEG waveforms provides a complement to central nervous system (CNS) evaluation obtained through clinical neurologic assessment.
• On its basic level, EEG activity requires multiple energy-using steps, which need to occur in succession. These steps include electrical impulse discharge at the thalamus and impulse conduction to the cerebral cortex with associated presynaptic release of neurotransmitters.
• Any clinical state or therapy that affects cerebral metabolism may also affect the EEG.12,18,22,30
• See Table 86-1 for terminology associated with bispectral index (BIS) technology.
Table 86-1
Bispectral Index Monitoring Terminology
| Bispectral Index (BIS) | Processed EEG that assesses level of consciousness and response to sedative, hypnotic, and analgesic therapy. |
| Digital Signal Converter (DSC) | Amplifies, filters, and digitizes the patient’s EEG signals. |
| Electroencephalogram (EEG) | Measures electrical activity of the brain. |
| Electromyelograph (EMG) | Measures the presence of muscle activity or detects high frequency artifact from patient care devices. |
| Signal Quality Index (SQI) | A measure of the signal quality for the EEG channel source and is calculated based on impedance to electronic signal, electrode contact artifact and other variables. |
| Suppression Ratio (SR) | Percentage of EEG suppression (isoelectric EEG) over the past 63 seconds of collected data. |
• The close relationship between BIS and EEG activity should be understood.25,33,35,36
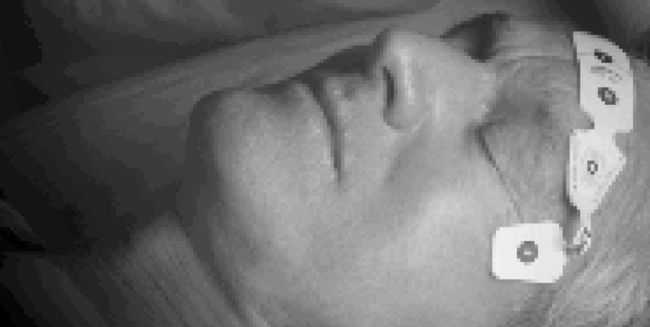
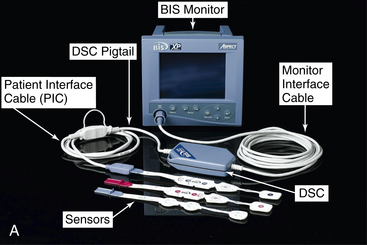
• When BIS monitoring is initiated, a sensor is placed across the patient’s forehead per manufacturer recommendations to detect one channel of EEG activity (Fig. 86-1).
• EEG activity is then subjected to multiple processing steps.
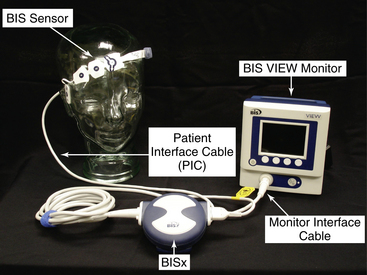
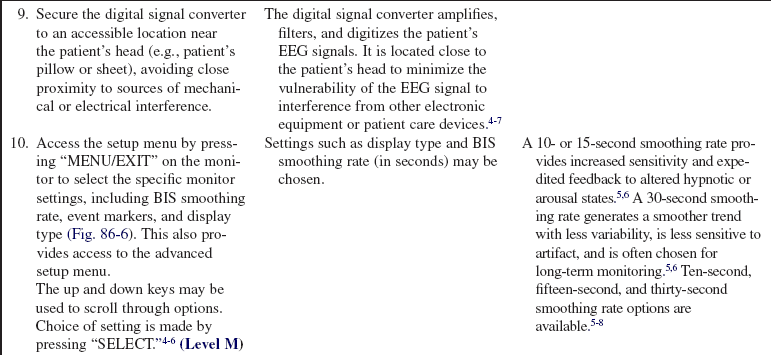
 The EEG signal is filtered and digitized within the amplifier head box (digital signal converter [DSC])2–4 (Fig. 86-4, A) or BISx5–7 near the patient’s head (Fig. 86-2, A and 86-3, A).
The EEG signal is filtered and digitized within the amplifier head box (digital signal converter [DSC])2–4 (Fig. 86-4, A) or BISx5–7 near the patient’s head (Fig. 86-2, A and 86-3, A).
 Artifacts (low-frequency and high-frequency) are eliminated.
Artifacts (low-frequency and high-frequency) are eliminated.
 Multiple processing steps are applied for calculation of a specific EEG state (frequency and amplitude) associated with the level of sedation, arousal, or anesthesia.
Multiple processing steps are applied for calculation of a specific EEG state (frequency and amplitude) associated with the level of sedation, arousal, or anesthesia.
 The level of EEG suppression and near suppression is determined.
The level of EEG suppression and near suppression is determined.
 The EEG features are combined to form the BIS, a single value that correlates with the level of consciousness and the specific EEG state.3,10,33,36
The EEG features are combined to form the BIS, a single value that correlates with the level of consciousness and the specific EEG state.3,10,33,36
 The BIS value is a single number based on the previous 10 to 30 seconds of EEG data (depending on the smoothing rate setting on the monitoring system) and is updated frequently; thus, changes in BIS value may lag behind clinical changes.5,6
The BIS value is a single number based on the previous 10 to 30 seconds of EEG data (depending on the smoothing rate setting on the monitoring system) and is updated frequently; thus, changes in BIS value may lag behind clinical changes.5,6
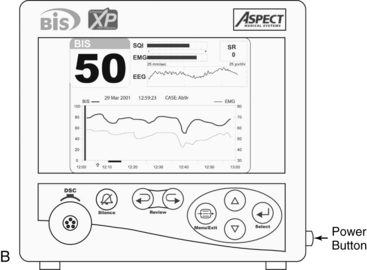
 The BIS monitor provides a single channel of an EEG tracing from the right or left frontal-temporal montage electrode placement (BIS A2000™, BIS VISTA™, BIS VIEW™; Fig. 86-4B).3–6
The BIS monitor provides a single channel of an EEG tracing from the right or left frontal-temporal montage electrode placement (BIS A2000™, BIS VISTA™, BIS VIEW™; Fig. 86-4B).3–6
• The BIS monitor may also provide bilateral EEG data acquisition from right and left frontal-temporal electrode placement (BIS VISTA bilateral monitoring system).7
• Understanding of factors that affect cerebral metabolism and EEG activity is needed.
 Sedation (dose-related): Related to the modulation of the EEG state and level of consciousness from medication administration.1–3,34
Sedation (dose-related): Related to the modulation of the EEG state and level of consciousness from medication administration.1–3,34
 Analgesic agents (dose-related): Related to attenuation of the arousal response or sedation as a side effect of opioid analgesia in higher doses.1,2,16,34
Analgesic agents (dose-related): Related to attenuation of the arousal response or sedation as a side effect of opioid analgesia in higher doses.1,2,16,34
 Anesthetic agents (dose-related).25,36,37
Anesthetic agents (dose-related).25,36,37
 Cerebral injury or hypoperfusion (hemodynamic stability, global neurologic injury, severe hypoxemia): Related to direct alterations in cerebral metabolic stability.9,18,26,29
Cerebral injury or hypoperfusion (hemodynamic stability, global neurologic injury, severe hypoxemia): Related to direct alterations in cerebral metabolic stability.9,18,26,29
• Potential indications for BIS monitoring include:
 Use of neuromuscular blockade: BIS monitoring may help in identification of patients at risk for awareness, recall, and pain during paralysis.2,23,39,40
Use of neuromuscular blockade: BIS monitoring may help in identification of patients at risk for awareness, recall, and pain during paralysis.2,23,39,40
 Use of BIS values to guide sedation and analgesia.2
Use of BIS values to guide sedation and analgesia.2
 Titration of sedation or analgesia in patients receiving controlled ventilation.5,7,15,23
Titration of sedation or analgesia in patients receiving controlled ventilation.5,7,15,23
 Avoidance of extremes of undersedation and oversedation.1,37
Avoidance of extremes of undersedation and oversedation.1,37
 Titration of medications for medication-induced coma.10,32,35
Titration of medications for medication-induced coma.10,32,35
 Determination of the dosage of sedation or analgesia during end-of-life care.12,20
Determination of the dosage of sedation or analgesia during end-of-life care.12,20
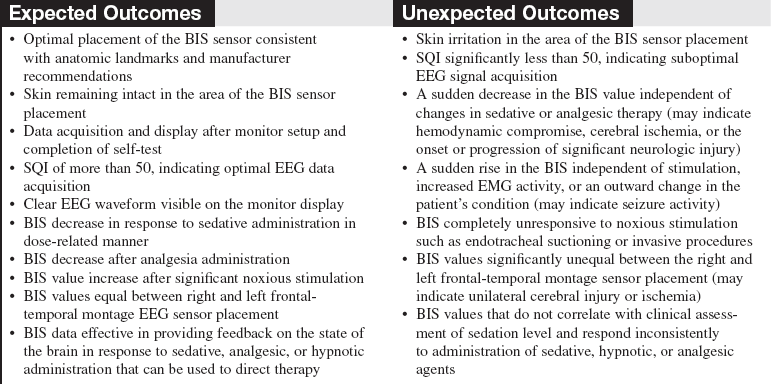
• Table 86-2 provides BIS values and correlation with clinical endpoints and level of sedation.
Table 86-2
BIS Values, Corresponding Level of Sedation, and EEG State3,13,17,20
| BIS Value | Corresponding Level of Sedation | Descriptors |
| 100 | Awake state; patient able to respond appropriately to verbal stimulation | Baseline state before sedation Anxiolysis |
| 80 | Patient able to respond to loud verbal, limited tactile stimulation, such as mild prodding/shaking | High-frequency EEG activity (Beta augmentation) Moderate sedation |
| 60 | Low probability of explicit recall; patient unresponsive to verbal stimulation | Low-frequency EEG activity Deep sedation |
| 40 | Patient unresponsive to verbal stimulation, less responsive to physical stimulation | Deep hypnotic state Drug-induced coma; burst-suppression EEG pattern |
| 20 | Minimal responsiveness | |
| 0 | No responsiveness mediated by brain function; spinal reflexes may be present | Isoelectric or completely suppressed EEG |
(Adapted from Arbour R: Continuous nervous system monitoring: EEG, the bispectral index and neuromuscular transmission, AACN Clin Iss 14(2):192, 2003.)
• See Fig. 86-1 for placement of the BIS sensor.
• Knowledge of factors that affect the BIS value is necessary.
 Sedation: Decrease in BIS value.11,14,15,21
Sedation: Decrease in BIS value.11,14,15,21
 Analgesia: Decrease in BIS value from attenuation of cerebral arousal or sedation occurring as a side effect of high-dose opioid analgesia.1,2
Analgesia: Decrease in BIS value from attenuation of cerebral arousal or sedation occurring as a side effect of high-dose opioid analgesia.1,2
 Neuromuscular blocking agents: Decrease in BIS value related to attenuation of high-frequency muscle activity across the patient’s forehead.8,17,23,40
Neuromuscular blocking agents: Decrease in BIS value related to attenuation of high-frequency muscle activity across the patient’s forehead.8,17,23,40
 Painful (noxious) stimulation: If analgesia is inadequate, arousal response may be produced within the cerebral cortex.1,2,19
Painful (noxious) stimulation: If analgesia is inadequate, arousal response may be produced within the cerebral cortex.1,2,19
 Sleep: BIS range is lower (20 to 70) during deep sleep, and BIS range is higher (75 to 92) during rapid eye movement (REM) sleep.21
Sleep: BIS range is lower (20 to 70) during deep sleep, and BIS range is higher (75 to 92) during rapid eye movement (REM) sleep.21
 Hypothermia: Decrease in BIS value.3,22,27
Hypothermia: Decrease in BIS value.3,22,27
 Cerebral ischemia: Decrease in BIS value.21,26,28,38
Cerebral ischemia: Decrease in BIS value.21,26,28,38
 Neurologic injury: Decrease in BIS value3,8,16 depending on location of injury and degree to which overall cerebral metabolism is affected.1,3,18
Neurologic injury: Decrease in BIS value3,8,16 depending on location of injury and degree to which overall cerebral metabolism is affected.1,3,18
 Encephalopathic states: Severe anoxic or ischemic encephalopathy (decrease in BIS value).1,3
Encephalopathic states: Severe anoxic or ischemic encephalopathy (decrease in BIS value).1,3
 Electromyographic (EMG) activity (high-frequency activity from muscle activity across forehead)3,6 may cause increase in BIS value independent of hypnotic state.8,17,19,40
Electromyographic (EMG) activity (high-frequency activity from muscle activity across forehead)3,6 may cause increase in BIS value independent of hypnotic state.8,17,19,40
 High-frequency electrical artifact from patient care equipment, such as pacemaker, or muscle activity, such as rapid head or eye movement (increase in BIS value).
High-frequency electrical artifact from patient care equipment, such as pacemaker, or muscle activity, such as rapid head or eye movement (increase in BIS value).
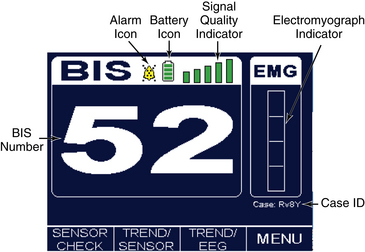
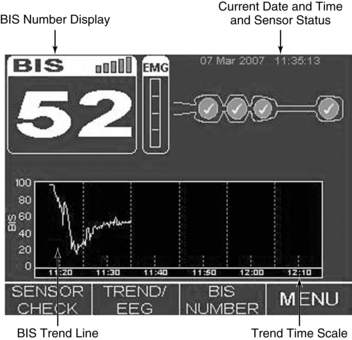
• Knowledge is needed of BIS display screen, monitor controls, and information array available on BIS monitor (Figs. 86-2, 86-3 and 86-4).
• Knowledge of data obtained from BIS monitoring is necessary.
 The BIS value is a single number on a linear (0 to 100) scale that reflects the level of sedation or cerebral arousal. BIS values correspond with specific clinical endpoints, indicating arousal and consciousness. A BIS value at or near 100 typically corresponds with an awake state. A BIS value at or near 0 corresponds with an isoelectric or near-isoelectric EEG reading and a deeply comatose patient.12,19,25,33
The BIS value is a single number on a linear (0 to 100) scale that reflects the level of sedation or cerebral arousal. BIS values correspond with specific clinical endpoints, indicating arousal and consciousness. A BIS value at or near 100 typically corresponds with an awake state. A BIS value at or near 0 corresponds with an isoelectric or near-isoelectric EEG reading and a deeply comatose patient.12,19,25,33
 The suppression ratio (SR) represents the percentage of suppressed EEG over the last 63 seconds of collected data within the EEG data sample. This parameter may be elevated in patients receiving high-dose propofol or barbiturates. The SR may also be elevated in a patient with severe cerebral injury, such as encephalopathy or catastrophic brain trauma. An SR of 15 indicates that the EEG signal was isoelectric over an interval of 15% of the previous 63 seconds of collected data.3,4,10,25
The suppression ratio (SR) represents the percentage of suppressed EEG over the last 63 seconds of collected data within the EEG data sample. This parameter may be elevated in patients receiving high-dose propofol or barbiturates. The SR may also be elevated in a patient with severe cerebral injury, such as encephalopathy or catastrophic brain trauma. An SR of 15 indicates that the EEG signal was isoelectric over an interval of 15% of the previous 63 seconds of collected data.3,4,10,25
 The EMG displays the power (in decibels) within the range of 70 to 110 Hz (cycles per second). This frequency range includes electrical activity from muscle artifact and patient care devices.3,4,10,25
The EMG displays the power (in decibels) within the range of 70 to 110 Hz (cycles per second). This frequency range includes electrical activity from muscle artifact and patient care devices.3,4,10,25
• Interpretation of BIS value:
 BIS is interpreted over time, in response to stimulation and within the context of whether therapeutic endpoints and overall goals of therapy are met.1,2,4,25
BIS is interpreted over time, in response to stimulation and within the context of whether therapeutic endpoints and overall goals of therapy are met.1,2,4,25
 Decisions to increase or decrease titration of sedative or analgesic therapy should be based on clinical assessment and judgment, goals of therapy, and the BIS value.1,2,25
Decisions to increase or decrease titration of sedative or analgesic therapy should be based on clinical assessment and judgment, goals of therapy, and the BIS value.1,2,25
 Relying on the BIS alone for sedation and analgesia management is not recommended.1,3
Relying on the BIS alone for sedation and analgesia management is not recommended.1,3
 Movement such as in response to painful stimulation may occur with low BIS values.
Movement such as in response to painful stimulation may occur with low BIS values.
 BIS values should be interpreted with caution in patients with brain injury or disease and in those receiving psychoactive medications.
BIS values should be interpreted with caution in patients with brain injury or disease and in those receiving psychoactive medications.
 BIS monitoring is not intended for regional cerebral ischemia monitoring. With use of BIS in the presence of known CNS injury, a baseline BIS value is recommended before administration of sedative, analgesic, or anesthetic agents.4,25
BIS monitoring is not intended for regional cerebral ischemia monitoring. With use of BIS in the presence of known CNS injury, a baseline BIS value is recommended before administration of sedative, analgesic, or anesthetic agents.4,25
 Elevation in BIS value may result from:
Elevation in BIS value may result from:
 Sources of noxious stimuli (arousal response and potential increase in EMG activity).17
Sources of noxious stimuli (arousal response and potential increase in EMG activity).17
 Decrease in level of neuromuscular blockade (affecting EMG activity).1–3,38,40
Decrease in level of neuromuscular blockade (affecting EMG activity).1–3,38,40
 Interruption in sedative therapy, development of tolerance.
Interruption in sedative therapy, development of tolerance.
 Interruption in analgesic therapy, development of tolerance.
Interruption in analgesic therapy, development of tolerance.
 Seizure activity (potentially).
Seizure activity (potentially).
 Shivering (particularly in combination with EMG activity).
Shivering (particularly in combination with EMG activity).
 Environmental noise: Cerebral arousal from excessive auditory stimulation.
Environmental noise: Cerebral arousal from excessive auditory stimulation.
 Decrease in BIS value may result from:
Decrease in BIS value may result from:
 Attenuation of arousal response or EMG activity after opioid administration.1,2,4,25
Attenuation of arousal response or EMG activity after opioid administration.1,2,4,25
 Administration of a neuromuscular blocking agent.2,3,39,40
Administration of a neuromuscular blocking agent.2,3,39,40
 Attenuation of EMG activity.3,8,25
Attenuation of EMG activity.3,8,25
 Hypothermia (patient cooling).3,22,27
Hypothermia (patient cooling).3,22,27
 Progression to deeper stages of sleep.
Progression to deeper stages of sleep.
 Hemodynamic instability.21,26,28,31
Hemodynamic instability.21,26,28,31
• Knowledge of sedative and analgesic therapy is needed.
• Specific medication therapies (e.g., opioids, benzodiazepines, propofol) should be known.13,32,34,35
• Indications and contraindications of specific medication classes should be understood.
• Goals of care should be known.
• Clinical assessment for establishing goals and end points of therapy is necessary.2,3,24,25
• Knowledge of medication effects on BIS value is needed.
 Opioids: May decrease in a dose-related manner (with the side effect of sedation at higher doses) and decrease BIS value related to attenuation of the arousal response from pain.2,3,15,25
Opioids: May decrease in a dose-related manner (with the side effect of sedation at higher doses) and decrease BIS value related to attenuation of the arousal response from pain.2,3,15,25
 Benzodiazepines: Decrease BIS value in a dose-related manner.
Benzodiazepines: Decrease BIS value in a dose-related manner.
 Propofol: Decrease in BIS value in dose-related manner.
Propofol: Decrease in BIS value in dose-related manner.
 Single-agent therapy with ketamine may not result in a dose-related decrease in BIS value.25 Ketamine results in increased cerebral blood flow and activation of EEG, specifically in higher frequencies.13 Higher EEG frequencies are associated with lighter levels of sedation.1,3 The BIS value may remain elevated in the presence of deeper sedation, as determined with clinical assessment.25
Single-agent therapy with ketamine may not result in a dose-related decrease in BIS value.25 Ketamine results in increased cerebral blood flow and activation of EEG, specifically in higher frequencies.13 Higher EEG frequencies are associated with lighter levels of sedation.1,3 The BIS value may remain elevated in the presence of deeper sedation, as determined with clinical assessment.25
• Knowledge of neuromuscular blockade and monitoring issues is necessary.
 Differentiation between monitoring level of sedation and cortical arousal and monitoring level of neuromuscular blockade.
Differentiation between monitoring level of sedation and cortical arousal and monitoring level of neuromuscular blockade.
 Monitoring level of sedation and cortical arousal is a phenomenon mediated by the CNS and is evaluated with clinical assessment of level of consciousness and arousal and with a processed EEG parameter such as the BIS. Medication effects evaluated include CNS depressants, such as propofol, barbiturates, benzodiazepines, and opioids at higher doses.
Monitoring level of sedation and cortical arousal is a phenomenon mediated by the CNS and is evaluated with clinical assessment of level of consciousness and arousal and with a processed EEG parameter such as the BIS. Medication effects evaluated include CNS depressants, such as propofol, barbiturates, benzodiazepines, and opioids at higher doses.
 Monitoring level of neuromuscular blockade is a phenomenon mediated by the peripheral nervous system (PNS) and measures the effects of neuromuscular blocking agents at producing varying degrees of skeletal muscle relaxation. The degree of neuromuscular blockade is evaluated two ways. First is clinical assessment of ventilator synchrony, resolution of life-threatening agitation, and the degree to which clinical goals and end points are met. Second is peripheral nerve stimulation and assessment of the evoked response. Peripheral nerve stimulation is commonly performed at the ulnar nerve in the wrist. After nerve localization and electrode placement, an electrical stimulus is applied and the localized response of the target muscle is assessed (see Procedure 38).
Monitoring level of neuromuscular blockade is a phenomenon mediated by the peripheral nervous system (PNS) and measures the effects of neuromuscular blocking agents at producing varying degrees of skeletal muscle relaxation. The degree of neuromuscular blockade is evaluated two ways. First is clinical assessment of ventilator synchrony, resolution of life-threatening agitation, and the degree to which clinical goals and end points are met. Second is peripheral nerve stimulation and assessment of the evoked response. Peripheral nerve stimulation is commonly performed at the ulnar nerve in the wrist. After nerve localization and electrode placement, an electrical stimulus is applied and the localized response of the target muscle is assessed (see Procedure 38).
 Risk of awareness and pain during paralysis should be understood.
Risk of awareness and pain during paralysis should be understood.
 Clinical goals for aggressive sedation and analgesia during paralysis should be known.
Clinical goals for aggressive sedation and analgesia during paralysis should be known.
 Knowledge of monitoring parameters is needed.
Knowledge of monitoring parameters is needed.
 Hemodynamic changes (marginal value and affected by multiple factors) should be understood.
Hemodynamic changes (marginal value and affected by multiple factors) should be understood.
 Diaphoresis (affected by multiple factors) should be understood.
Diaphoresis (affected by multiple factors) should be understood.
• Initial monitoring and setup of the sensor and equipment includes:
 Signal quality index (SQI) is displayed on the monitor screen. The SQI bar that extends to the right side of the SQI bar graph display indicates optimal (100%) EEG signal quality. The BIS value on the numeric region of the monitor display is shown as a solid number. SQI less than 50% (SQI less than middle range of display) is indicated by a BIS value shown as an outlined number. If SQI is inadequate for calculation of a BIS value, no data are displayed.3,4,25
Signal quality index (SQI) is displayed on the monitor screen. The SQI bar that extends to the right side of the SQI bar graph display indicates optimal (100%) EEG signal quality. The BIS value on the numeric region of the monitor display is shown as a solid number. SQI less than 50% (SQI less than middle range of display) is indicated by a BIS value shown as an outlined number. If SQI is inadequate for calculation of a BIS value, no data are displayed.3,4,25
PATIENT AND FAMILY EDUCATION
• Assess factors that affect the patient (if still awake) and family readiness to learn.  Rationale: Teaching is individualized to specific patient and family needs.
Rationale: Teaching is individualized to specific patient and family needs.
• Explain the purpose of BIS monitoring, including content regarding specific information obtained, how it may be used, and an explanation of the equipment.  Rationale: The patient (if still awake) and family may experience less anxiety and have increased understanding of the patient equipment at the bedside.
Rationale: The patient (if still awake) and family may experience less anxiety and have increased understanding of the patient equipment at the bedside.
• Explain to the patient (if appropriate) and family what will happen with the initiation of BIS monitoring (skin preparation, placement of electrodes, moderate pressure for electrode contact).  Rationale: The explanation prepares the patient and family for events associated with initiation of BIS monitoring and also provides an opportunity to reinforce preprocedural teaching and assess level of understanding.
Rationale: The explanation prepares the patient and family for events associated with initiation of BIS monitoring and also provides an opportunity to reinforce preprocedural teaching and assess level of understanding.
• Although rare, some patients may have mild skin irritation develop in the area in contact with the sensor. This irritation typically resolves within 1 hour after sensor removal.  Rationale: The patient and family are prepared for the possible minor issue with sensor application and the possible need for removal or repositioning of the sensor.
Rationale: The patient and family are prepared for the possible minor issue with sensor application and the possible need for removal or repositioning of the sensor.
• Explain that BIS monitoring and electrode placement pose no risk to the patient beyond that of mild skin irritation (in rare instances) and that the patient experiences no discomfort as part of monitoring procedure.  Rationale: Anxiety may be decreased.
Rationale: Anxiety may be decreased.
PATIENT ASSESSMENT AND PREPARATION
Patient Assessment
• Assess the patient’s level of sedation, responsiveness, and arousal.  Rationale: Baseline data are provided.
Rationale: Baseline data are provided.
• In collaboration with other healthcare providers, establish overall goals and endpoints of sedative and analgesic therapy.  Rationale: A coordinated plan is established with integration of the BIS data into decision making regarding sedation and analgesia.
Rationale: A coordinated plan is established with integration of the BIS data into decision making regarding sedation and analgesia.
• Assess the skin at the intended sites for sensor placement.  Rationale: Provides baseline information regarding the patient’s skin.
Rationale: Provides baseline information regarding the patient’s skin.
• Assess the patient’s neurologic status.  Rationale: Baseline data are provided. BIS values may be decreased with significant neurologic injury, which needs to be determined before initiation of BIS monitoring. If possible, obtain a baseline BIS value before initiating therapy with sedative, analgesic, or anesthetic agents.
Rationale: Baseline data are provided. BIS values may be decreased with significant neurologic injury, which needs to be determined before initiation of BIS monitoring. If possible, obtain a baseline BIS value before initiating therapy with sedative, analgesic, or anesthetic agents.
Patient Preparation
• Verify correct patient with two identifiers.  Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
• Determine anatomic landmarks for the BIS sensor placement.  Rationale:
Rationale:
Landmarks provide for accurate placement of the sensor.
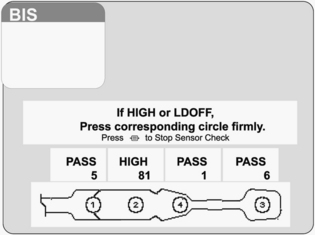
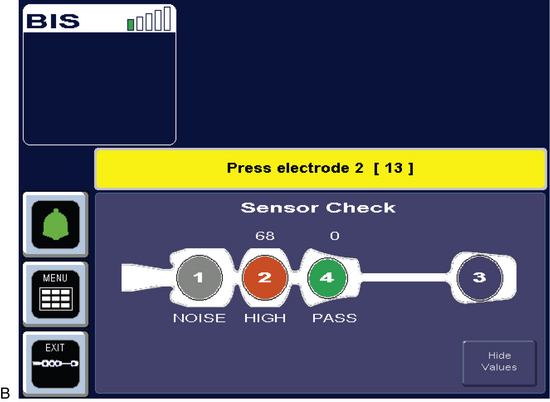
Figure 86-5 BIS sensor check at start of monitoring.4 Circle 1 is positioned at center of forehead approximately 2 inches (5 cm) above nose. Circle 4 is placed directly above and parallel to the eyebrow. Circle 3 is placed on the temple area between the hairline and the outer canthus of the eye. Circle 2 is placed between circles 1 and 4 on the patient’s forehead. (Courtesy Aspect Medical Systems, Norwood, MA.)
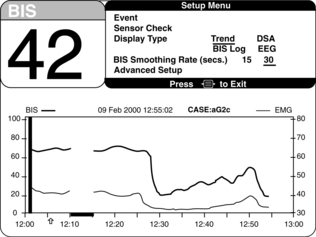
Figure 86-6 Setup menu for selection of monitor settings optimal to specific patient needs.4 Display of specific monitoring parameters such as BIS trend and smoothing rate may be adjusted on this display. (Courtesy Aspect Medical Systems, Norwood, MA.)
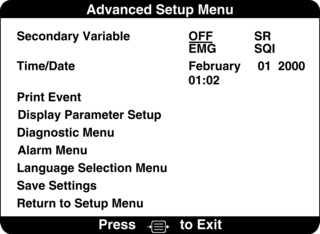
Figure 86-7 Advanced setup menu enabling the operator to select secondary monitoring parameters to be displayed with BIS trend and other, less frequently changed settings. Secondary parameters that may be selected include electromyography (EMG), suppression ratio (SR), and signal quality index (SQI).4 (Courtesy Aspect Medical Systems, Norwood, MA.)
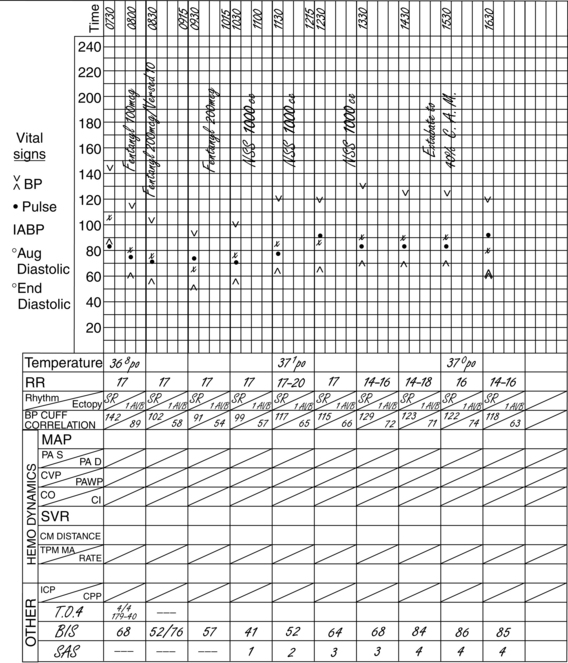
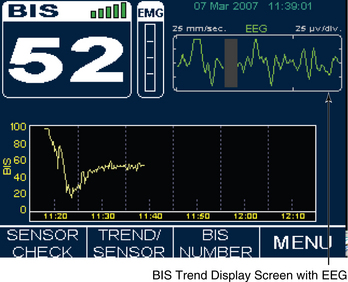
Figure 86-8 Documentation of BIS values over time and after medication administration in critically ill patient. At 7:30 AM, the patient was still receiving neuromuscular blockade with a BIS value of 68. Additional sedation/analgesia was administered (fentanyl and midazolam), with resulting BIS decline to 52. After stimulation, BIS value elevated to 76, necessitating supplemental dosing with analgesics (fentanyl). BIS value remained at less than 60, and neuromuscular blockade was discontinued. The upward trend in the BIS value tracked recovery of consciousness to baseline mental status. (Courtesy Aspect Medical Systems, Norwood, MA.)
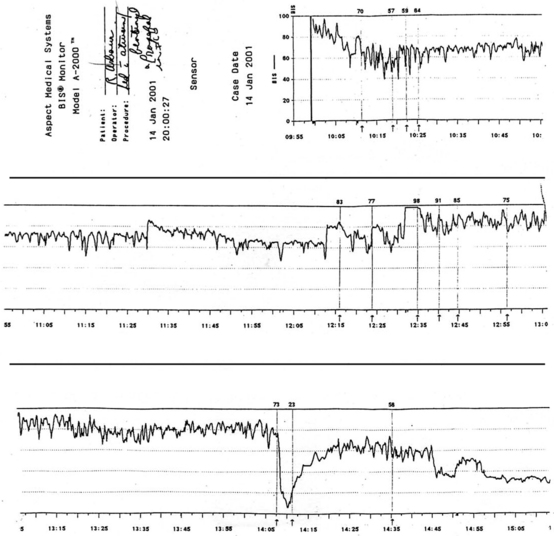
Figure 86-9 An illustration of BIS trend recording of sedation/analgesia management in critically ill patient. BIS monitoring was initiated at approximately 10:00 AM. Sedation was managed initially with fentanyl and lorazepam. Decline in BIS from between 90 and 97 to between 55 and 65 occurred over 25 to 30 minutes in response to therapy. Decline in BIS value matched clinical assessment of increased level of sedation. The patient had periods of breakthrough agitation and ventilator dyssynchrony beginning at 12:15 PM. Agitation was refractory to current therapy despite upward titration of sedation and analgesia. The sedation management was changed to propofol at approximately 14:05 PM. The precipitous drop in BIS value indicated increased sedation. Propofol was titrated back in a controlled, incremental manner. Sedation was more closely and optimally managed with application of BIS monitoring and avoiding, in a controlled manner, extremes of excessive sedation and inadequate sedation/risk of agitation and ventilator dyssychrony. This tracing has been used in electronic format beginning in June 2003 for educational purposes on behalf of Aspect Medical Systems. (Courtesy Aspect Medical Systems, Norwood, MA.)
References
1. Arbour, R, Impact of bispectral index monitoring on sedation and outcomes in critically ill patients. a case series. Crit Care Nurs Clin North Am 2006; 18:227–241.
![]() 2. Arbour, R, Using bispectral index monitoring to detect -potential breakthrough awareness and limit duration of neuromuscular blockade. case report and discussion. Am J Crit Care 2004; 13:66–73.
2. Arbour, R, Using bispectral index monitoring to detect -potential breakthrough awareness and limit duration of neuromuscular blockade. case report and discussion. Am J Crit Care 2004; 13:66–73.
![]() 3. Arbour, R. Continuous nervous system monitoring, EEG, the bispectral index and neuromuscular transmission. AACN Clin Issues. 2003; 14:185–207.
3. Arbour, R. Continuous nervous system monitoring, EEG, the bispectral index and neuromuscular transmission. AACN Clin Issues. 2003; 14:185–207.
4. Aspect Medical Systems. A-2000™ Bispectral Index™ (BIS) monitoring system operating manual,. Norwood, MA: Aspect Medical Systems, Inc; 2006.
5. Aspect Medical Systems. BIS VIEW™ monitoring system operating manual,. Norwood, MA: Aspect Medical Systems, Inc; 2007.
6. Aspect Medical Systems. BIS VISTA monitoring system operating manual. Norwood, MA: Aspect Medical Systems, Inc; 2008.
7. Aspect Medical Systems. BIS VISTA monitoring -system bilateral monitoring addendum operating -manual,. Norwood, MA: Aspect Medical -Systems Inc; 2008.
![]() 8. Aspect Medical Systems, Overview. the effects of electromyography (EMG) and other high-frequency signals on the bispectral index (BIS),. Aspect Medical Systems, Inc, Norwood, MA, 2000.
8. Aspect Medical Systems, Overview. the effects of electromyography (EMG) and other high-frequency signals on the bispectral index (BIS),. Aspect Medical Systems, Inc, Norwood, MA, 2000.
![]() 9. Azim, N, Wang, CY, Case report. the use of bispectral index during a cardiopulmonary arresta potential indicator of cerebral perfusion. Anesthesiology 2004; 59:610–612.
9. Azim, N, Wang, CY, Case report. the use of bispectral index during a cardiopulmonary arresta potential indicator of cerebral perfusion. Anesthesiology 2004; 59:610–612.
![]() 10. Bader, MK, Arbour, R, Palmer, S, Refractory increased intracranial pressure in severe traumatic brain injury . barbiturate coma and bispectral index monitoring. AACN Clin Issues 2005; 16:526–541.
10. Bader, MK, Arbour, R, Palmer, S, Refractory increased intracranial pressure in severe traumatic brain injury . barbiturate coma and bispectral index monitoring. AACN Clin Issues 2005; 16:526–541.
11. Brunh, J, Myles, PS, Sneyd, R, et al, Depth of anesthesia monitoring. what’s available, what’s validated and what’s next. Br J Anaesth 2006; 97:85–94.
12. Chisholm, CJ, Zurica, J, Mironov, D, et al. Comparison of electrophysiologic monitors with clinical assessment of sedation. Mayo Clin Proc. 2006; 81:46–52.
13. Chiu, CL, Ong, G, Majid, AA. Impact of bispectral index monitoring on propofol administration in patients undergoing cardiopulmonary bypass. Anaesth Intensive Care. 2007; 35:342–347.
14. Consales, G, Chelazzi, C, Rinaldi, S, et al. Bispectral index compared to Ramsay score for sedation monitoring in intensive care units. Minerva Anestesiol. 2006; 72:329–336.
![]() 15. Courtman, SP, Wardurgh, A, Petros, AJ. Comparison of the bispectral index monitor with the Comfort score in assessing level of sedation of critically ill children. Intens Care Med. 2003; 29:2239–2246.
15. Courtman, SP, Wardurgh, A, Petros, AJ. Comparison of the bispectral index monitor with the Comfort score in assessing level of sedation of critically ill children. Intens Care Med. 2003; 29:2239–2246.
16. Dahaba, AA, Lischnig, U, Kronthaler, R, et al, Bispectral-index-guided versus clinically guided remifentanyl/propofol analgesia/sedation during interventional radiological procedures. an observer-blinded randomized study. Anesth Analg 2006; 103:378–384.
![]() 17. Dahaba, AA. Different conditions that could result in the bispectral index indicating an incorrect hypnotic state. Anesth Analg. 2005; 101:765–773.
17. Dahaba, AA. Different conditions that could result in the bispectral index indicating an incorrect hypnotic state. Anesth Analg. 2005; 101:765–773.
![]() 18. Escudero, D, Otero, J, Muniz, G, et al, The bispectral index scale. its use in the detection of brain death. Transplant Proc 2005; 37:3661–3663.
18. Escudero, D, Otero, J, Muniz, G, et al, The bispectral index scale. its use in the detection of brain death. Transplant Proc 2005; 37:3661–3663.
![]() 19. Fraser, GL, Riker, RR. Bispectral index monitoring in the intensive care unit provides more signal than noise. Pharmacotherapy. 2005; 25(5 Pt 2):19S–27S.
19. Fraser, GL, Riker, RR. Bispectral index monitoring in the intensive care unit provides more signal than noise. Pharmacotherapy. 2005; 25(5 Pt 2):19S–27S.
![]() 20. Gambrell, M, Using the BIS monitor in palliative care. a case study. J Neurosci Nurs 2005; 37:140–143.
20. Gambrell, M, Using the BIS monitor in palliative care. a case study. J Neurosci Nurs 2005; 37:140–143.
21. Hashimoto, H, Nakamura, H, Hirota, K. Marked reduction in bispectral index with severe bradycardia without hypotension in a diabetic patient undergoing ophthalmic surgery. J Anesth. 2008; 22:300–303.
22. Honan, D, Doherty, D, Frizelle, H. A comparison of the effects on bispectral index of mild vs. moderate hypothermia during cardiopulmonary bypass. Eur J Anaesthesiol. 2006; 23:385–390.
23. Inoue, S, Kawaguchi, M, Sasaoka, N, et al. Effects of neuromuscular block on systemic and cerebral hemodynamics and bispectral index during moderate or deep sedation in critically ill patients. Intens Care Med. 2006; 32:391–397.
![]() 24. Jacobi, J, Fraser, GL, Coursin, DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002; 30:119–141.
24. Jacobi, J, Fraser, GL, Coursin, DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002; 30:119–141.
![]() 25. Kelly, SD, Monitoring level of consciousness during anesthesia and sedation. a clinician’s guide to the bispectral indexAspect Medical Systems. http://www.aspectmedical.com/resources/handbook/default.mspx, June 4, 2004 [2003, retrieved].
25. Kelly, SD, Monitoring level of consciousness during anesthesia and sedation. a clinician’s guide to the bispectral indexAspect Medical Systems. http://www.aspectmedical.com/resources/handbook/default.mspx, June 4, 2004 [2003, retrieved].
![]() 26. Kin, N, Konstadt, SN, Sato, K, et al, Reduction of bispectral index value associated with clinically significant cerebral air embolism. J Cardiothorac Vasc Anesth 2004; 18 :82–84.
26. Kin, N, Konstadt, SN, Sato, K, et al, Reduction of bispectral index value associated with clinically significant cerebral air embolism. J Cardiothorac Vasc Anesth 2004; 18 :82–84.
27. Kosik, TM, Induced hypothermia for patients with cardiac arrest. role of the clinical nurse specialist. Crit Care Nurs 2007; 27:36–42.
28. Lauwick, S, English, M, Hemmerling, TM, An unusual case of cerebral hypoperfusion detected by bispectral index monitoring. Can J Anaesth. 2007; 54:680–681.
29. LeBlanc, JM, Dasta, JF, Kane-Gill SL. Role of the bispectral index in sedation monitoring in the ICU. Ann Pharmacother. 2006; 40:490–500.
30. Misis, M, Raxach, JG, Molto, HP, et al. Bispectral index monitoring for early detection of brain death. Transplant Proc. 2008; 40:1279–1281.
![]() 31. Morimoto, Y, Monden, Y, Ohtake, K, et al. The detection of cerebral hypoperfusion with bispectral index monitoring during general anesthesia. Anesth Analg. 2005; 100:158–161.
31. Morimoto, Y, Monden, Y, Ohtake, K, et al. The detection of cerebral hypoperfusion with bispectral index monitoring during general anesthesia. Anesth Analg. 2005; 100:158–161.
32. Prins, SA, de Hoog, M, Blok, JH, et al. Continuous noninvasive monitoring of barbiturate coma in critically ill children using the bispectral index monitor. Crit Care. 2007; 11:R108.
![]() 33. Rampil, IJ. A primer for EEG signal processing in anesthesia. Anesthesiology. 1998; 89:980–1002.
33. Rampil, IJ. A primer for EEG signal processing in anesthesia. Anesthesiology. 1998; 89:980–1002.
![]() 34. Rhoney, DH, Parker, D, Use of sedative and analgesic agents in neurotrauma patients. effects on cerebral physiology. Neurol Res 2001; 23:237–259.
34. Rhoney, DH, Parker, D, Use of sedative and analgesic agents in neurotrauma patients. effects on cerebral physiology. Neurol Res 2001; 23:237–259.
![]() 35. Riker, RR, Fraser, GL, Wilkins, ML. Comparing the bispectral index and suppression ratio with burst suppression of the electroencephalogram during pentobarbital infusions in adult intensive care patients,. Pharmacotherapy. 2003; 23:1087–1093.
35. Riker, RR, Fraser, GL, Wilkins, ML. Comparing the bispectral index and suppression ratio with burst suppression of the electroencephalogram during pentobarbital infusions in adult intensive care patients,. Pharmacotherapy. 2003; 23:1087–1093.
![]() 36. Rosow, C, Manberg, PJ. Bispectral index monitoring. Anesth Clin North Am. 2001; 19:946–966.
36. Rosow, C, Manberg, PJ. Bispectral index monitoring. Anesth Clin North Am. 2001; 19:946–966.
37. Sackey, PV, Radell, PJ, Granath, F, et al. Bispectral index as a predictor of sedation depth during isoflurane or midazolam sedation in ICU patients. Anaesth Intens Care. 2007; 35:348–356.
![]() 38. Sen, I, Puri, GD, Bapuraj, JR. Early detection of cerebral vasospasm during a neurointerventional procedure using the BIS. Anaesth Intens Care. 2005; 33:691–692.
38. Sen, I, Puri, GD, Bapuraj, JR. Early detection of cerebral vasospasm during a neurointerventional procedure using the BIS. Anaesth Intens Care. 2005; 33:691–692.
![]() 39. Tobias, JD, Grindstaff, R. Bispectral index monitoring during the administration of neuromuscular blocking agents in a pediatric intensive care unit patient. J Intens Care Med. 2005; 20:233–237.
39. Tobias, JD, Grindstaff, R. Bispectral index monitoring during the administration of neuromuscular blocking agents in a pediatric intensive care unit patient. J Intens Care Med. 2005; 20:233–237.
![]() 40. Vivien, B, Di Maria, S, Quattera, A, et al. Overestimation of bispectral index in sedated intensive care unit patients revealed by administration of muscle relaxant. Anesthesiology. 2003; 99:9–17.
40. Vivien, B, Di Maria, S, Quattera, A, et al. Overestimation of bispectral index in sedated intensive care unit patients revealed by administration of muscle relaxant. Anesthesiology. 2003; 99:9–17.
41. Vretzakis, G, Draguomanis, C, Argiriadou, H, et al. Inaccuracy of BIS values produced by the cardiopulmonary bypass machine during operative repair of an aortic dissection. J Cardiothorac Vasc Anesth. 2006; 20:68–70.
42. Zanner, R, Schneider, G, Kochs, EF. Falsely increased bispectral index values caused by the use of a forced-air-warming device. Eur J Anaesthesiol. 2006; 23:618–619.
Arbour, R, Electroencephalograph-derived monitoringInAACN-AANN protocols for practice. monitoring technologies in critically ill patients. Littlejohns, LR. Bader, MK. Jones and Bartlett, Sudbury, MA, 2009:175–197.
Claassen, J, Mayer, SA, Kowalski, RG, et al. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004; 62:1743–1748.
Deogaonkar, A, Gupta, R, DeGeorgia, M, et al. Bispectral index monitoring correlates with sedation scales in brain-injured patients. Crit Care Med. 2004; 32:2403–2406.
March, K, Wellwood, J, Arbour, R. Technology. In: Littlejohns LR, Bader MK, eds. AANN core curriculum for neuroscience nursing. St Louis: Saunders; 2004:199–204.
Markand, ON. Pearls, perils and pitfalls in the use of the electroencephalogram. Semin Neurol. 2003; 23:7–46.
Nasraway, SA, The bispectral index. expanded performance for everyday use in the intensive care unit. Crit Care Med 2005; 33:685–687.
Parker, BM, Anesthetics and anesthesia techniques. impacts on perioperative management and postoperative outcomes. Clev Clin J Med. 2006; 73(Suppl 1):S13–S17.
Tonner, PH, Wei, C, Bein, B, et al. Comparison of two bispectral index algorithms in monitoring sedation in postoperative intensive care patients. Crit Care Med. 2005; 33:580–584.
Watson, BD, Kane-Gill, SL, Sedation assessment in critically ill adults. 2001-2004 update. Ann Pharmacother 2004; 38:1898–1906.
Welsby, IJ, Ryan, M, Booth, JV, et al, The bispectral index in the diagnosis of perioperative stroke. a case report and discussion. Anesth Analg 2003; 96:435–437.