CASE 81
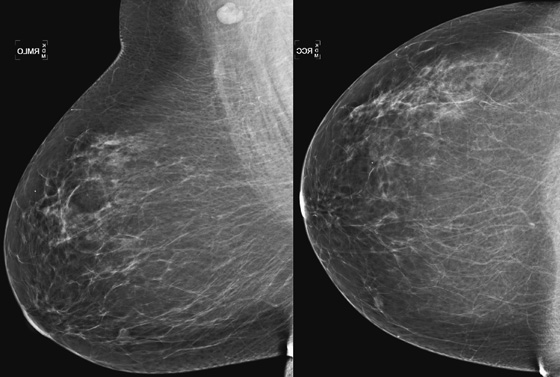
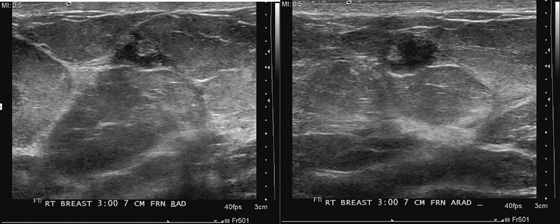
History: A 63-year-old woman for routine mammogram. Two years previously, she was diagnosed with ductal carcinoma in situ in the left breast, which was treated with breast conservation therapy. A new mass was noted to develop in the right breast.
1. What should be included in the differential diagnosis, based on the right mammogram and ultrasound images shown? (Choose all that apply.)
A. Simple cyst in the right breast
C. Papilloma in the right breast
D. Complex cystic mass in the right breast
2. Which of the following statements is true about color Doppler imaging?
A. If there is no flow, you can be confident there is no malignancy.
B. Flow seen at the edge of the mass excludes a benign cyst.
C. Flow seen within an intracystic mass raises the suspicion for malignancy.
D. If the lesion is round and anechoic, color Doppler is not needed.
3. What is the next step in management?
A. Short-interval follow-up for this likely benign mass
B. Return to routine mammogram for this likely benign lesion
C. Biopsy using ultrasound guidance
D. MRI to check for abnormal enhancement
4. If the tissue diagnosis from the core needle biopsy is “benign breast tissue,” what is the next step?
A. Consider the biopsy nondiagnostic and repeat.
B. Consider the biopsy result definitive, and recommend mammogram in 1 year.
D. Consider the biopsy diagnostic, and recommend that she be followed by her surgeon.
ANSWERS
CASE 81
Cystic-Appearing Cancer
1. B, C, and D
2. C
3. C
4. A
References
Berg WA, Campassi C, Ioffe OB. Cystic lesions of the breast: sonographic-pathologic correlation. Radiology. 2003;227(1):183–191.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:159.
Comment
A complex cystic mass is a mass that contains fluid but also has a thick wall, intracystic masses, thick internal septations, or mural projections and requires biopsy. These masses may represent malignancy, intracystic papilloma, necrotic tumor, hematoma, or abscess.
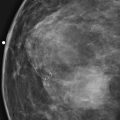
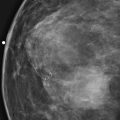
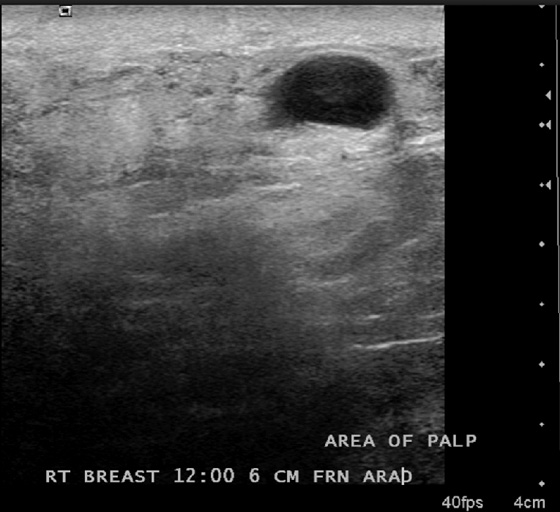
This patient had a new mass develop in her right breast, seen on mammography (see the figures). On the mammogram the mass has an appearance more suggestive of a benign process, such as a cyst. It has low density and relatively sharp margins on the standard views. However, this postmenopausal woman who has survived breast cancer in the contralateral breast is at increased risk for a new cancer. Spot compression views and ultrasound are needed for further evaluation of the developing mass. On ultrasound, the mass appears cystic, with internal masses. The walls are not thick, but the shape of the mass is irregular, not round or oval (see the figures). These are suspicious characteristics, and biopsy is recommended.
It is important to differentiate a complex cystic mass from a complicated cyst. A complicated cyst is oval or round, has a thin wall, and has diffuse low-level internal echoes or internal debris. A complex cystic mass has a thick wall or an internal mass, or both, such as in this patient (see the figures). In one series, 35% of cystic masses with a thick wall or thick septations were malignant. High-grade infiltrating ductal carcinoma may have circumscribed borders. This patient had infiltrating ductal carcinoma, high grade on core biopsy.
Core biopsy of the complex cystic mass is preferred rather than aspiration. The cyst fluid may be necrotic or acellular, which may result in a false-negative result. If a nonspecific benign result is received after needle biopsy of a suspicious lesion, the biopsy should be repeated. The nonspecific benign diagnosis is nonconcordant and does not explain the reason for the intracystic masses or thick wall of the complex cystic mass. Lesions with a specific benign diagnosis such as papilloma or atypical hyperplasia should be excised. Clips should be placed at the time of needle biopsy because the lesion may be poorly seen after biopsy, particularly if small.
CASE 82
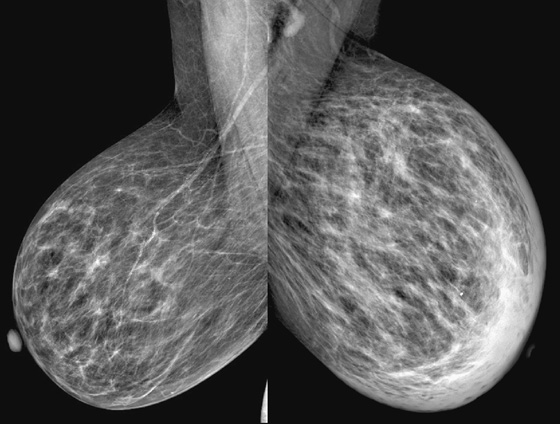
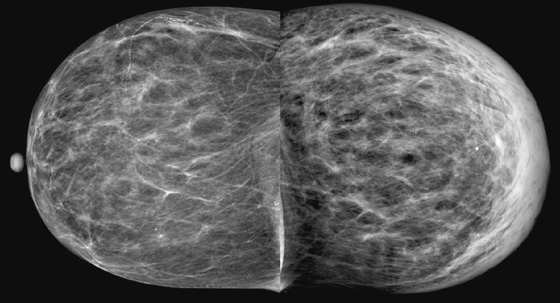
History: A 73-year-old woman presents with recent left breast enlargement. Her medical history includes long-term hypertension and multiple previous hospitalizations for shortness of breath and edema of the legs.
1. What should be included in the differential diagnosis? (Choose all that apply.)
D. Congestive heart failure (CHF)
2. Considering the differential diagnosis, which of the following does not constitute a reasonable alternative in management?
B. Treatment with antibiotics and short-term follow-up
D. Treatment with diuretics and cardiotherapeutic agents and short-term follow-up
3. Other conditions associated with unilateral breast edema include all of the following except:
A. Changes after lumpectomy or radiation
C. Arteriovenous hemodialysis complications
4. Which of the following statements regarding breast edema secondary to CHF is true?
A. Unilateral breast edema is a common manifestation of CHF.
B. The edema is secondary to decreased hydrostatic pressure.
C. Pitting edema can help distinguish between CHF and malignancy.
D. A known clinical history of CHF excludes other etiologies of unilateral breast edema.
ANSWERS
CASE 82
Unilateral Breast Edema
1. B, C, and D
2. C
3. D
4. C
References
Cao MM, Hoyt AC, Bassett LW. Mammographic signs of systemic disease. Radiographics. 2011;31(4):1085–1100.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:389.
Comment
Unilateral breast edema raises concern for inflammatory breast carcinoma. However, edema can have different etiologies, including benign and malignant conditions. Conditions associated with unilateral breast edema include inflammatory breast carcinoma, mastitis, changes following surgical and radiation treatments, lymphatic obstruction, central venous obstruction, arteriovenous hemodialysis complications, granulomatous diseases, nephrotic syndrome, scleroderma, CHF, some skin conditions, lymphoma, leukemia, and metastasis.
Knowledge of these entities, in conjunction with a thorough clinical history including preexisting medical conditions and previous procedures, is important in developing an accurate diagnosis, affecting the patient’s management. If inflammatory breast cancer is suspected, biopsy should be performed to obtain a definitive diagnosis; biopsy specimens of the skin and of any identifiable discrete mass should be obtained.
Clinically, breast edema, thickening, and associated enlargement are common to all of the above-mentioned etiologies. Nevertheless, additional distinctive features may be helpful in distinguishing some of these conditions. Inflammatory breast cancer also manifests with erythema that may be associated with sensation of heat in the affected breast; mastitis is accompanied by erythema, breast pain, and fever that regress after antibiotic treatment; CHF has pitting edema on clinical examination, with absence of a discrete palpable mass, and signs and symptoms resolve after standard treatment. Other entities can be suspected based on the clinical history (e.g., previous lumpectomy with axillary dissection or sentinel node biopsy, radiation to the breast, hemodialysis with upper extremity arteriovenous fistula).
The appearance of breast edema on mammography is characterized by skin thickening, increased parenchymal density, and prominent interstitial markings (trabecular thickening), regardless of the etiology (see the figures). The presence of a discrete mass or microcalcifications, or both, may help to diagnose breast malignancy.
On ultrasound, there is skin thickening, with associated lymphatic engorgement. The presence of a discrete mass may be helpful in diagnosing breast malignancy and can be targeted for biopsy.
On MRI, breast edema manifests as skin thickening and prominent interstitial markings, which appear hyperintense on T2-weighted images. If breast edema is the only finding, no enhancement is seen after injection of contrast material.
In the presented case, the edema of the left breast was secondary to CHF, which is a common clinical condition in the general population. When CHF causes breast edema, usually both breasts are affected because of increased hydrostatic pressure and subsequent lymphatic obstruction. Unilateral breast edema secondary to CHF is rare, and it may be due to lateralization to the dependent breast if the patient lies on one side for extended periods.
CASE 83
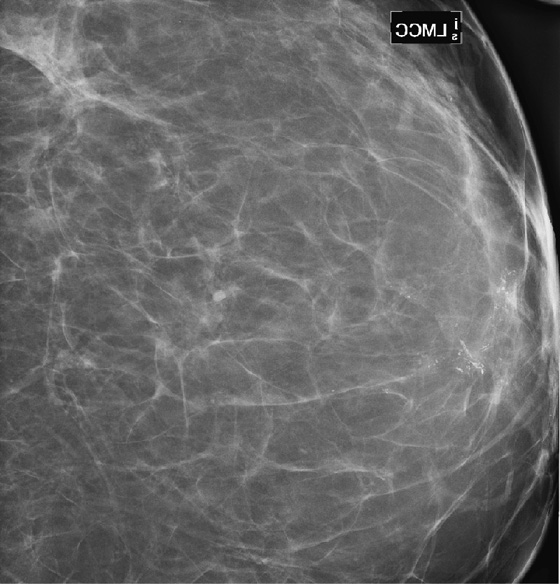
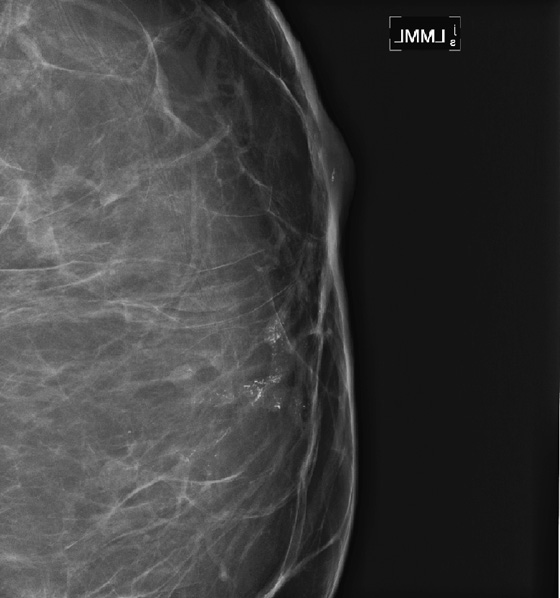
History: A 68-year-old woman presents with a chronic scaly red patch on her left nipple. Magnification views of the left subareolar area are shown.
1. What is the differential diagnosis and Breast Imaging Reporting and Data System (BI-RADS) category for these special views? (Choose all that apply.)
A. Benign-appearing calcifications in the subareolar breast, BI-RADS 2
B. Indeterminate calcifications in subareolar ducts, BI-RADS 3
2. What significance is the clinical finding of the scaly red patch on the nipple?
A. This is not related to the mammographic concern.
B. The area of concern is likely eczema, not related to breast disease.
C. This might represent Paget’s disease and may be related to the calcifications on the mammogram.
3. What is the next step in diagnosis?
A. Send the patient to a surgeon for a biopsy of the nipple abnormality.
B. Schedule a 6-month follow-up of indeterminate calcifications.
C. Perform an image-guided biopsy of the microcalcifications.
D. The radiologist should perform a skin biopsy.
4. How often is there a mammographic abnormality in the setting of red, scaly nipple abnormality?
ANSWERS
CASE 83
Paget’s Disease
1. C
2. C
3. C
4. C
References
Ikeda DM, Helvie MA, Frank TS, et al. Paget disease of the nipple: radiologic-pathologic correlation. Radiology. 1993;189(1):89–94.
Valdes EK, Feldman SM. Paget’s disease of the breast. Breast J. 2006;12(1):83.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:397.
Comment
This patient presents with a suspicious clinical finding. When a red scaly patch is seen on or adjacent to the nipple-areolar complex, Paget’s disease should be suspected. Skin biopsy will be needed, but the first step is to evaluate the mammogram for any evidence of intraductal or invasive malignancy. In this case (see the figures), there are casting-type calcifications filling the subareolar ducts, highly suspicious for ductal carcinoma in situ (DCIS). Note the calcifications within the nipple in the second figure.
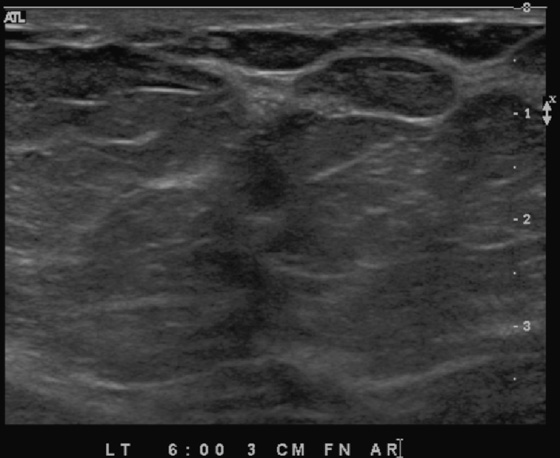
The extent of disease should be established before the patient goes to surgery. Abnormal mammographic findings require a biopsy. Calcifications may be targeted for stereotactic biopsy; however, stereotactic biopsy can be difficult in the immediate subareolar area, and ultrasound can be used to try to image the calcifications in the distended ducts (see the figures). In this patient, ultrasound-guided biopsy was performed. The biopsy result was DCIS, grade III. On skin biopsy performed by the surgeon, the nipple area contained the same cells as were present in the breast biopsy, which is typical for Paget’s disease.
Paget’s disease is an uncommon form of breast cancer, accounting for less than 5% of all breast cancers. More than 97% of patients with pagetoid changes of the nipple have underlying breast cancer. The mammogram might not demonstrate the underlying malignancy, however. Only about 50% have a mammographic abnormality, including nipple, areolar, or subareolar distortion, nipple retraction, masses, or calcifications. The breast abnormality may be subareolar, as in this case (see the figures), or may be distant from the nipple. The disease may be multicentric or multifocal. MRI can be used to evaluate extent of disease, prior to definitive treatment.
CASE 84
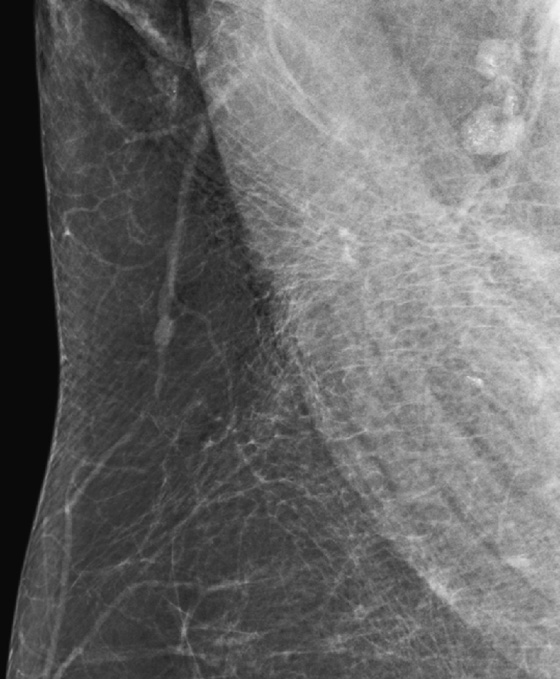
History: Two different patients present for routine screening mammograms. Portions of the view showing axillary nodes are provided.
1. What should be included in the differential diagnosis for this mammographic appearance? (Choose all that apply.)
2. If the patient has a history of gold therapy for rheumatoid arthritis, what is the Breast Imaging Reporting and Data System (BI-RADS) category for this finding?
A. BI-RADS 0—incomplete; further evaluation with magnification views is needed
B. BI-RADS 4—biopsy of a node to confirm gold deposits
C. BI-RADS 3—short-interval follow-up
3. If this is a new finding in a patient with a history of ipsilateral breast cancer, what is the BI-RADS category for this finding?
A. BI-RADS 4—biopsy should be considered
C. BI-RADS 3—short-interval follow-up
4. If you elected to perform a biopsy of an axillary lymph node, can this procedure be accomplished with needle biopsy with image guidance?
A. No, the node must be excised surgically.
B. No, calcifications require stereotactic biopsy, which is not an option in the axilla.
C. Yes, ultrasound-guided core needle biopsy is an option.
ANSWERS
CASE 84
Calcifications in Axillary Lymph Nodes
1. B, C, D, and E
2. D
3. A
4. C
References
Bruwer A, Nelson GW, Spark RP. Punctate intranodal gold deposits simulating microcalcifications on mammograms. Radiology. 1987;163(1):87–88.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:396.
Comment
Calcifications or dense particulate matter may be seen in axillary lymph nodes, often in the setting of an asymptomatic woman undergoing a screening mammogram. If the material is calcium density, the differential diagnosis includes granulomatous disease, such as sarcoidosis and metastatic disease from breast cancer or ovarian cancer (psammomatous calcifications). In cases of metastatic disease, the lymph nodes have other features of malignancy in addition to calcifications, including loss of hilum and increased density. Axillary lymph node calcifications are identified in 3% of patients with breast cancer.
If the particulate matter in the nodes is of metal density, gold therapy (chrysotherapy) is often the reason. Other possibilities are silicone material in the nodes in patients who have had silicone implants or injections and tattoo pigment.
In the cases presented here, the woman in the first figure had a history of gold therapy for rheumatoid arthritis. The patient in the second figure underwent an ultrasound-guided core needle biopsy of an axillary node because of a concern that it might represent metastatic disease from ovarian cancer. The histology of the core biopsy was consistent with sarcoid, a granulomatous disease.
CASE 85
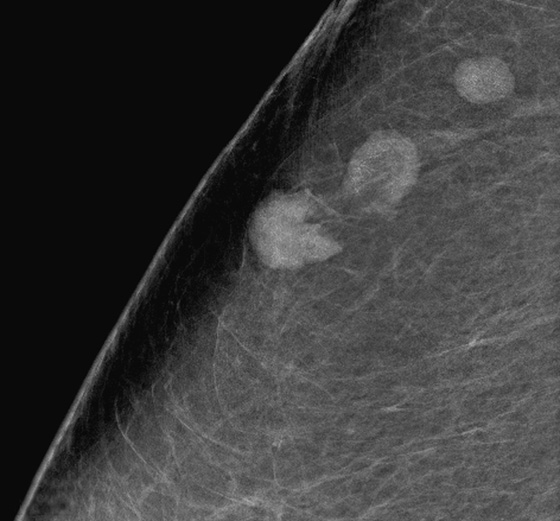
History: A woman with a personal history of left breast cancer presents for routine mammogram. New calcifications were seen in the right breast.
1. What should be included in the differential diagnosis for the magnification views shown? (Choose all that apply.)
B. Suspicious microcalcifications
D. Indeterminate calcifications
2. Why would ultrasound be utilized to evaluate calcifications?
A. It is not useful; mammography is the only way to evaluate calcifications.
B. Ultrasound is used to evaluate for a mass at the site of calcifications.
C. It may be used to identify a mass but would not be used to target the biopsy.
D. It is not helpful; MRI is better than ultrasound in evaluating extent of DCIS.
3. If ultrasound is performed and shows a mass associated with calcifications, what is the next step in management?
A. Referral of the patient to a surgeon for excision
C. Ultrasound-guided core biopsy
D. Short-interval follow-up in 6 months
4. Which of the following is not an advantage of ultrasound-guided biopsy compared with stereotactic biopsy?
A. Real-time imaging during the sampling
D. Improved samples from a larger bore needle
ANSWERS
CASE 85
Use of Ultrasound to Evaluate Suspicious Microcalcifications
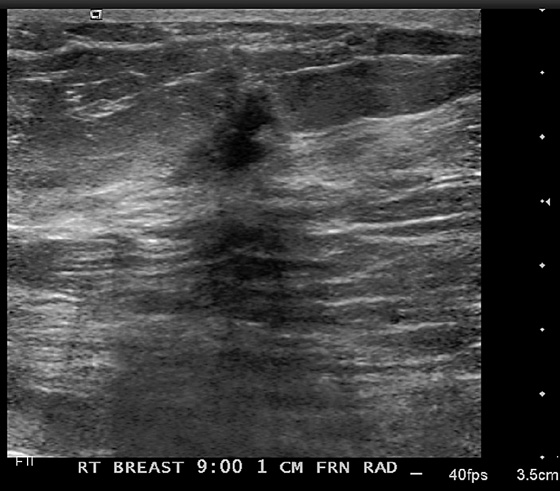
1. B and D
2. B
3. C
4. D
References
Cho N, Moon WK, Cha JH, et al. Ultrasound-guided vacuum-assisted biopsy of microcalcifications detected at screening mammography. Acta Radiol. 2009;50(6):602–609.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:212.
Comment
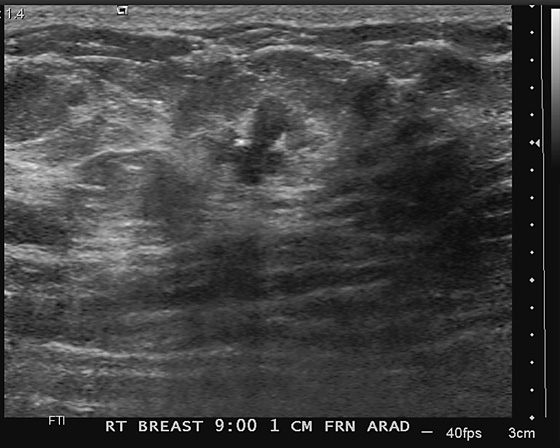
Newly developing microcalcifications detected on routine mammography may indicate malignant disease. The likelihood of calcifications representing malignancy is greater in elderly patients and in women with increased risk (see the figures). Biopsy is necessary unless the developing calcifications are characteristic of a benign type, such as milk of calcium, or lucent-centered calcifications. Stereotactic biopsy is often the method of choice for biopsy of calcifications. However, there are advantages to using ultrasound guidance, and if the finding can be seen on ultrasound, the biopsy can be performed using ultrasound guidance. Advantages of ultrasound-guided biopsy over stereotactic biopsy include real-time assessment of needle location during the sampling, patient comfort, quicker biopsy time, and lack of radiation exposure.
Several studies have shown that calcifications may not be seen on ultrasound, but if they are identified, there is a higher likelihood of malignancy. The calcifications may be in a mass, as in the patient in this case (see the figures) or in a duct. The presence of a hypoechoic mass heightens the visibility of tiny hyperechoic calcifications. Calcifications associated with a mass seen on ultrasound are more likely to be malignant (69% positive predictive value in one series).
Seeing a mass associated with microcalcifications on ultrasound and using ultrasound guidance to sample the mass, rather than just the calcifications on mammogram, increases the chances of sampling the invasive component of the disease. It is helpful to perform a specimen radiograph of the biopsied cores of tissue to check for calcifications.
In one study of 75 patients with microcalcifications seen on mammogram, 71% had calcifications retrieved on ultrasound-guided biopsy. The retrieval chance was higher when there was a mass or dilated duct associated with the calcifications (85% vs. 41% retrieval of calcifications not associated with a mass or dilated duct).
CASE 86
History: A 43-year-old woman presents with intermittent bloody discharge from the left nipple. The mammogram was negative (not shown). Ductography, ultrasound, and MRI were performed for further evaluation.
1. What should be included in the differential diagnosis? (Choose all that apply.)
B. Ductal carcinoma in situ (DCIS)
2. Which of the following statements regarding nipple discharge is true?
A. Bilateral nipple discharge and discharge from multiple openings should be evaluated with imaging.
B. It is more common in postmenopausal women.
C. Spontaneous bloody nipple discharge may be a sign of malignancy.
D. Nipple discharge is a manifestation of breast pathology only.
3. Regarding imaging appearance of papillary lesions, which of the following statements is false?
A. They may appear on the mammogram as microcalcifications.
B. Ductogram usually shows involvement of multiple ductal systems.
D. On MRI, papillomas appear as enhancing masses with or without a dilated duct.
4. Which of the following statements is false?
A. Intraductal papillomas are considered high-risk lesions.
B. Management of intraductal papillomas is controversial.
C. A benign papilloma may evolve to a papillary carcinoma.
D. Distinction between benign papillary lesions and papillary carcinoma is important.
ANSWERS
CASE 86
Intraductal Papilloma
1. B, C, and D
2. C
3. B
4. C
References
Brookes MJ, Bourke AG. Radiological appearances of papillary breast lesions. Clin Radiol. 2008;63(11):1265–1273.
Ibarra JA. Papillary lesions of the breast. Breast J. 2006;12(3):237–251.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:383.
Comment
Papillary lesions are rare tumors, accounting for 0.7% to 4% of solid breast lesions. They include various pathologic processes, ranging from benign papillomas to papillary carcinomas. Benign papillary lesions are the most common intraductal mass. Histologically, they are benign ductal neoplasms with a papillary fibrovascular core covered with ductal epithelium and myoepithelial cells (the presence of myoepithelial cells differentiates benign papillomas from papillary carcinoma). Papillomas can be solitary or multiple, often arising in contiguous areas of a duct system. Solitary papillomas usually arise in a large duct near the nipple (central), whereas multiple papillomas most commonly occur in smaller, more peripheral ducts.
Many patients with solitary central papillomas present with nipple discharge. Bloody nipple discharge may occur if the papilloma twists on its fibrovascular stalk and becomes ischemic and necrotic, whereas clear (serous) nipple discharge may occur owing to secretions produced by the papilloma. Unilateral, bloody or serous nipple discharge from a single duct raises the level of concern because it may also be a manifestation of carcinoma. Bilateral or multiple duct, milky or green to brown discharges are not clinically significant, and they are usually associated with other conditions, such as fibrocystic change, hormonal imbalance, pregnancy and lactation, prolactinoma, and medication. In these cases, further evaluation with imaging is unnecessary. Nipple discharge generally may be present at any age.
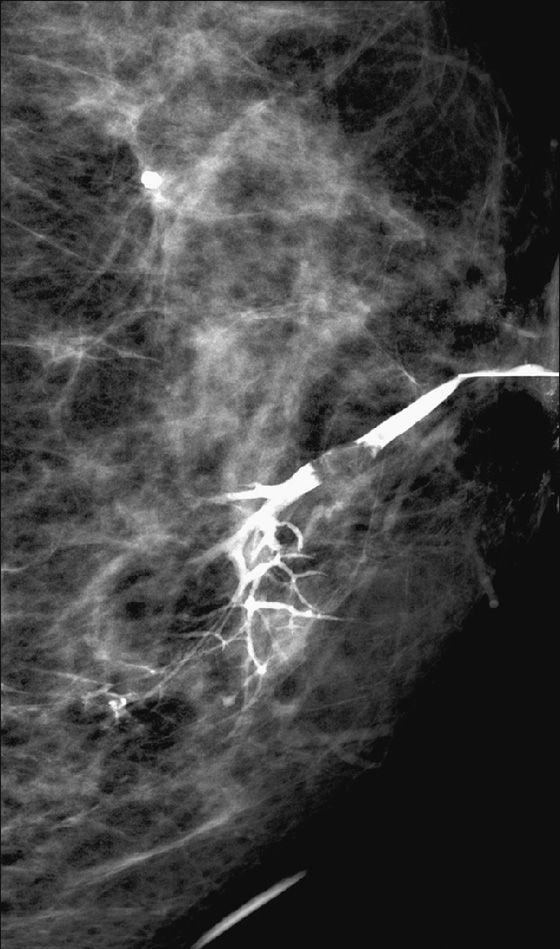
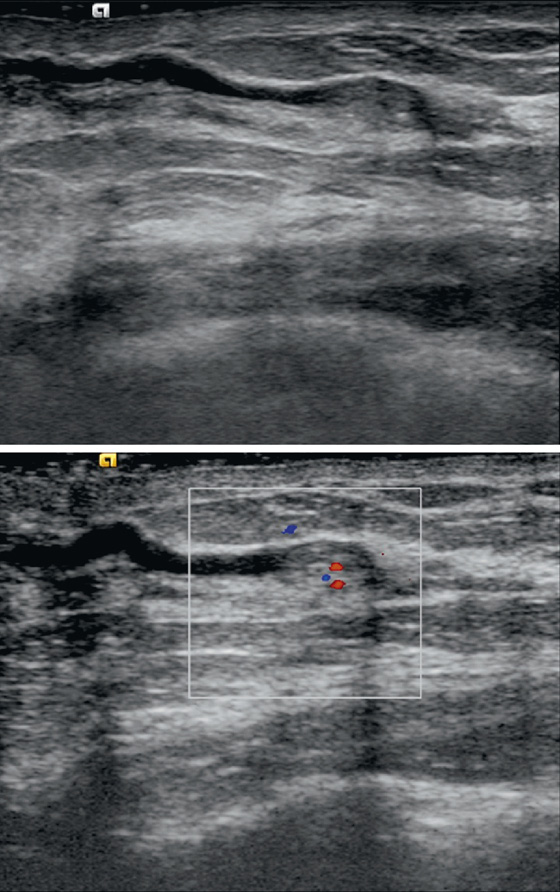
Intraductal papillomas may be difficult to recognize with mammography. When visible, they may appear as round, often well-circumscribed subareolar masses with or without calcifications or as a small cluster of calcifications without a mammographically evident mass. Ductography can be helpful in localizing a papilloma within the discharging duct, which appears as an intraductal filling defect, sometimes with irregular borders (see the figures). On ultrasound, intraductal papillomas appear as a solid mass or masses with internal vascularity inside a duct, which may be focally dilated (see the figures).
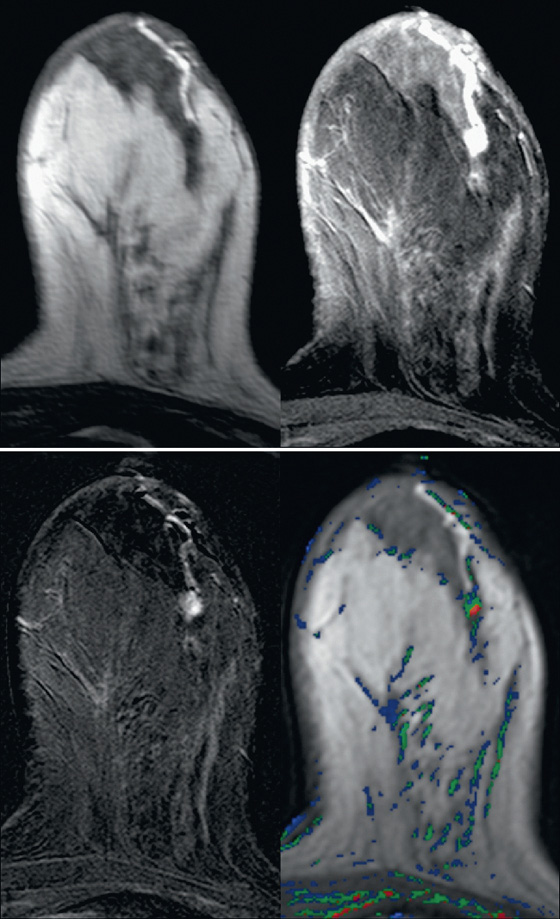
MRI usually gives a more global picture and may reveal additional lesions in women with nipple discharge. On MRI, papillomas appear as enhancing masses with or without an accompanying dilated duct. The duct contents may have a different appearance depending on the fluid composition; serous content appears hypointense on T1-weighted images and hyperintense on T2-weighted images, whereas hemorrhagic content appears hyperintense on both T1-weighted and T2-weighted images.
Papillary lesions are considered high-risk lesions because some of them may be malignant, and many of them may contain atypical ductal hyperplasia or DCIS. In addition, DCIS and infiltrating ductal carcinoma may manifest as an intraductal mass. A benign papilloma does not evolve to a papillary carcinoma.
Histologic diagnosis is needed to exclude intraductal cancer. Recommendations on management vary. Although some clinicians advocate needle biopsy for initial diagnosis followed by excision, others recommend surgical excision only. If needle biopsy has been performed, some institutions excise all papillomas, whereas others excise only the ones with atypia. The patient in this case had a vacuum-assisted, ultrasound-guided core biopsy, which revealed an intraductal papilloma, with no atypia.
CASE 87
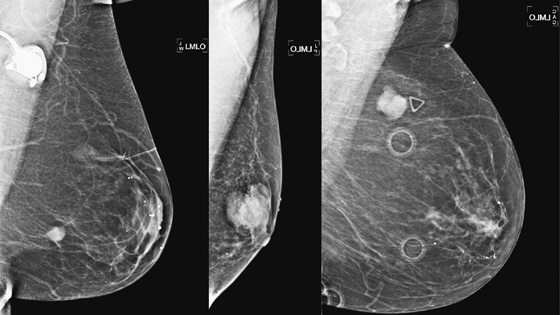
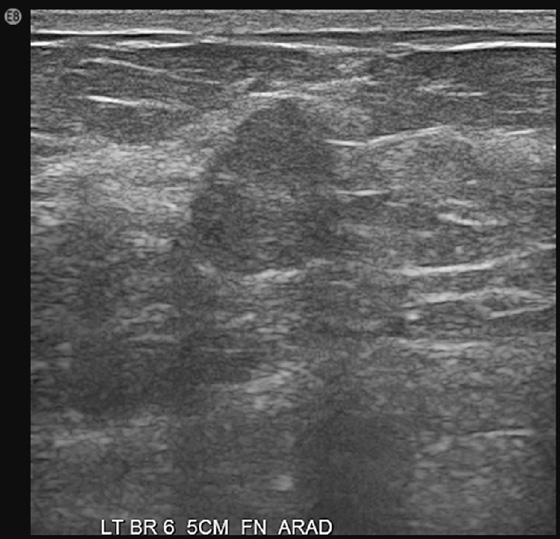
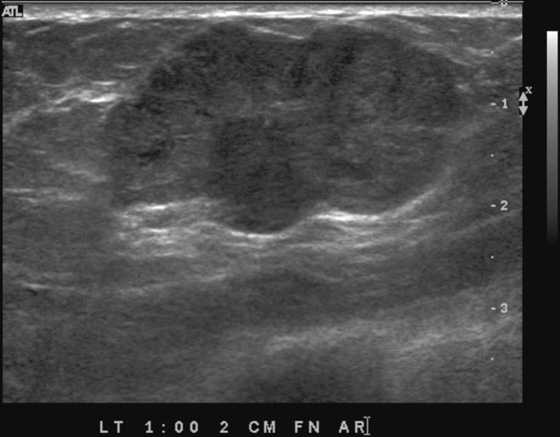
History: Three different patients present with a left breast palpable mass. The left mediolateral oblique (MLO) view and ultrasound images have been provided.
1. What should be included in the differential diagnosis of the palpable finding? (Choose all that apply.)
C. Invasive ductal carcinoma (IDC), mucinous type
D. Invasive ductal carcinoma, medullary type
2. What is the next step in management?
A. MRI to differentiate benign from malignant
B. Ultrasound-guided needle biopsy
C. Fine-needle aspiration biopsy
D. Short-interval follow-up ultrasound
3. What is mucinous carcinoma?
A. A special type of invasive lobular carcinoma
B. A tumor characterized by extracellular mucin
C. Another name for medullary carcinoma
4. What imaging characteristics are not seen with mucinous carcinoma?
A. A dense mass on mammogram and isoechoic mass on ultrasound
B. High signal intensity on T2-weighted MRI
C. Markedly spiculated mass on mammography and dense shadowing on ultrasound
D. Well-defined mass on mammography and cystic spaces seen on ultrasound
ANSWERS
CASE 87
Mucinous Carcinoma
1. B, C, and D
2. B
3. B
4. C
References
Bode MK, Rissanen T. Imaging findings and accuracy of core needle biopsy in mucinous carcinoma of the breast. Acta Radiol. 2011;52(2):128–133.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:114.
Comment
Mucinous carcinoma is a special type of IDC. The other special types are medullary, invasive papillary, and tubular carcinoma. The special types, as differentiated from IDC not otherwise specified (NOS), indicate a more differentiated cell type, with a better prognosis. Mucinous carcinoma manifests as a pure or mixed form. In the pure form, there are larger amounts of mucin (50% to 75%), and the lesion appears more well defined on mammography. The density of the tumor on mammography has been reported as denser than water, as in the patients in this case (see the figures), but also reported to be lower in density. In the pure form, the borders are smooth and lobulated. With the mixed form, the borders become more ill defined.
The pure form of mucinous carcinoma occurs more commonly in older women, average age 63, and is less likely to be palpable or, if palpable, is relatively soft. The pure form has positive axillary nodes in only 6%, and prognosis is better than in IDC NOS. However, in the mixed form, the tumor behavior is closer to IDC NOS, with a higher percentage of patients presenting with a hard palpable mass, positive nodes in 36%, and a worse prognosis.
The ultrasound appearance varies. Smaller masses (see the figures) are often isoechoic to fat and difficult to identify, and their appearance overlaps with fibroadenoma. However, in contrast to fibroadenomas, the tumors tend to be taller than wide, with a microlobulated surface (see the figures). They do not cause shadowing and may have enhanced through-transmission (see the figures). They may also contain cystic spaces.
On MRI, the mucinous carcinoma is bright on T2-weighted images and may have nonenhancing internal septa. These septa should be thicker than in fibroadenoma, but differentiating mucinous carcinoma from fibroadenoma on MRI may be difficult, particularly with the pure form of mucinous carcinoma. After contrast agent administration, the initial enhancement curve may be slow, intermediate, or rapid and then may be persistent or plateau, also overlapping with benign tumors.
CASE 88
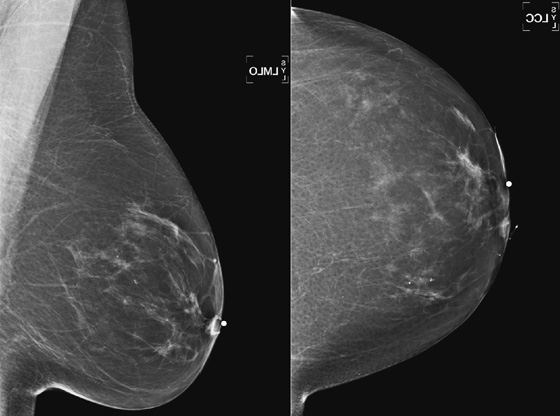
History: A 62-year-old woman presents with a palpable mass near the left nipple.
1. What should be included in the differential diagnosis based on the mammogram and ultrasound images shown? (Choose all that apply.)
D. Infiltrating ductal carcinoma
2. Is the ultrasound appearance diagnostic of a benign mass?
A. No, because there is irregular solid material within the mass
B. Yes, because the mass is oval and wider than tall
C. Yes, because the location suggests it is in the dermal layer
D. Yes, because the borders are smooth and well defined
3. What is the next step in management?
B. Fine-needle aspiration biopsy
C. Short-interval follow-up because this may be a sebaceous cyst
D. MRI of both breasts to evaluate for abnormal enhancement
4. If pathology is benign papilloma on core biopsy, what is the next step?
C. Surgical consultation for excision
D. MRI to evaluate enhancement of the mass
ANSWERS
CASE 88
Palpable Subcutaneous Mass
1. A, B, and D
2. A
3. A
4. C
References
Berg WA, Campassi CI, Ioffe OB. Cystic lesions of the breast: sonographic-pathologic correlation. Radiology. 2003;227(1):183–191.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:120. 383
Comment
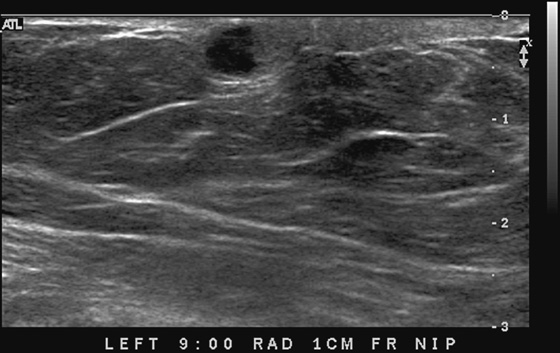
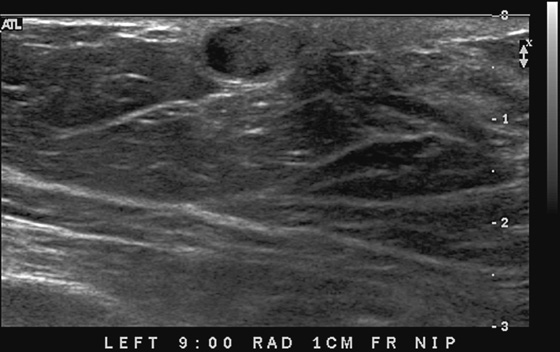
This 62-year-old woman presented with a palpable mass near the nipple. Mammography was performed first (see the figures). Ultrasound was then performed, and the images showed an oval, sharply circumscribed, complex cystic mass, located in the superficial breast adjacent to the nipple. All of the ultrasound features are benign except for the echogenic, irregular material within the mass (see the figures).
The BI-RADS (Breast Imaging Reporting and Data System) code and the decision to perform a biopsy of a mass should be based on the worst characteristic, which is the irregular material within the cystic mass in this patient. This mass is termed a complex cystic mass, which has an increased chance of being malignant compared with a complicated cyst or simple cyst. (A complicated cyst contains internal echoes on ultrasound.) Ultrasound features of a complex cystic mass are thick, irregular septations; thick, irregular wall; indistinct or angular margins; intracystic mass; and associated solid component. In one series, 18 of 79 complex cystic lesions were malignant.
Core biopsy of this mass was performed, and histology showed intraductal papilloma. The mass was excised, and the surgical histopathology was infiltrating ductal carcinoma, grade II/III, micropapillary type, with ductal carcinoma in situ at the surgical margin.
This case illustrates that biopsy should be performed on cystic lesions that contain intracystic masses. If a papilloma is diagnosed at core biopsy, surgical excision should be considered. There is a 12% to 14% risk of “upgrading” the benign papillary lesion to carcinoma on excision.
This case also illustrates that palpable masses that are negative on mammography should be evaluated further with ultrasound. This is true particularly for a mass felt near the nipple, where a mass may be overlooked or obscured by the nipple and subareolar ducts.
CASE 89
History: A 70-year-old man presents with a tender palpable right breast mass.
1. What is the differential diagnosis in this patient? (Choose all that apply.)
2. What imaging studies are needed to evaluate a palpable mass in a male?
A. Unilateral mammogram and ultrasound of the area of concern
B. Bilateral mammogram; add ultrasound if needed
D. Ultrasound only is sufficient.
3. What is the etiology of this gynecomastia?
A. Altered hormone levels, liver disease, renal disease, hyperthyroidism, medication
B. Family history of breast cancer
D. Multiple lipomas on the trunk, arms, and legs
4. What is the usual presentation?
A. Tender mass behind the nipple
B. Bilateral masses in the upper outer quadrant of the breast
C. Axillary adenopathy and a palpable breast mass
ANSWERS
CASE 89
Male Gynecomastia
1. B and C
2. B
3. A
4. A
References
Appelbaum AH, Evans GFF, Levy KR, et al. Mammographic appearances of male breast disease. Radiographics. 1999;19:559–568.
Braunstein GD. Gynecomastia. N Engl J Med. 1993;328(7):490–495.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:370. 374
Comment
This patient is a 70-year-old man being treated for prostate cancer. Gynecomastia is a common condition in men, and it is the most common reason for men to present for a mammogram. It is an overgrowth of ductal epithelium and stromal elements; it may be tender on exam and may be detected by the patient or the referring physician. The differential diagnosis for a palpable mass in a man includes breast cancer, lipoma, epithelial inclusion cyst, fat necrosis, lymph node, and hematoma.
Gynecomastia develops from high exogenous or endogenous estrogen levels or a decrease in testosterone. It is also related to endocrine disorders; liver, kidney, or lung disease; and many medications. Men who present with a mass should initially have a bilateral mammogram.
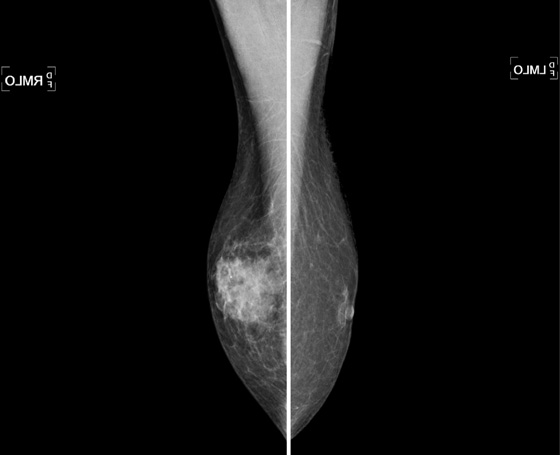
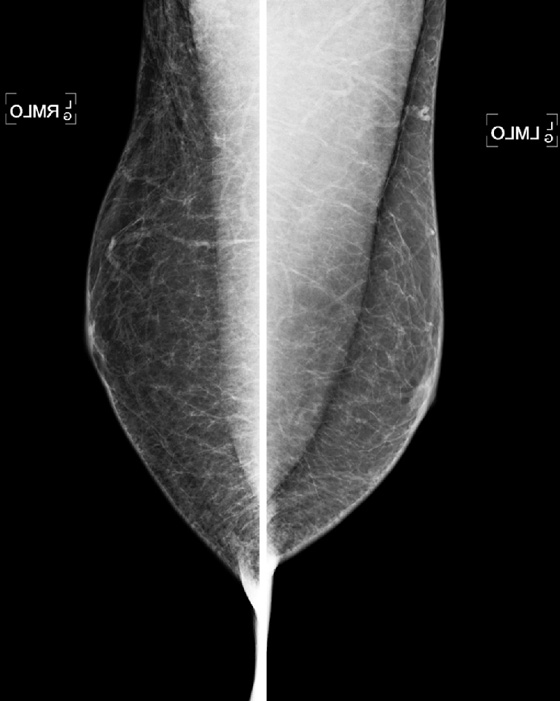
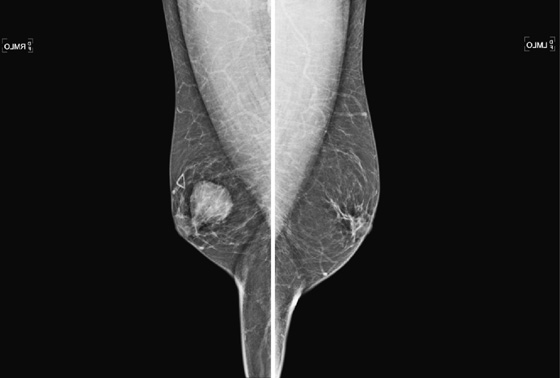
The mammogram in gynecomastia classically shows a fan-shaped area of density behind the nipple. The edges of the mass fade into the surrounding fat (see the figures). There is also a nodular form in which the edges may be well circumscribed. The focal asymmetry seen can extend into the upper outer quadrant of the breast and is often asymmetric. It can also occur at some distance from the nipple. It can be unilateral or bilateral. Note in the first figure, the left breast also has a density behind the nipple, although smaller than on the right. Compare to the second figure, of a normal male breast.
Ultrasound of the palpable finding may be important because gynecomastia can obscure a small cancer on the mammogram. On ultrasound, the entire area of gynecomastia should be evaluated for a hypoechoic mass. Gynecomastia should not contain a mass. If the density is well evaluated by mammography alone, and there is no suggestion of mass within the glandular density, ultrasound is not necessary.
Solid masses or complex cysts seen on ultrasound are suspicious for malignancy. Hyperechoic masses contiguous with the skin are typical for epidermal inclusion cysts. Fat necrosis has a varying appearance but is usually hyperechoic and can shadow. Lipomas are typically hyperechoic, located in the subdermal layer, oval, and wider than tall. This is an example of bilateral, asymmetric gynecomastia. This concern is managed clinically. Surgery is not necessary.
In the second figure, a mammogram is shown of a symptomatic 45-year-old patient who felt palpable fullness on both sides. This is the mammogram of a normal male breast, with no ductal proliferation or masses. This is referred to as pseudogynecomastia.
CASE 90
History: A 60-year-old man presents with a palpable mass in his right breast.
1. What is the differential diagnosis in this patient, based on the mammogram? (Choose all that apply.)
2. What is the next step in the work-up?
A. MRI is performed to differentiate benign from malignant.
C. Recommend a short-interval follow-up because this is possibly an infectious mass.
D. No work-up is needed for this benign mass.
3. How common is male breast cancer?
A. Men comprise approximately 10% of the breast cancer population.
B. Men comprise approximately 25% of the breast cancer population.
C. Men comprise approximately 5% of the breast cancer population.
D. Men comprise approximately 1% of the breast cancer population.
4. What masses do not occur in the male breast?
ANSWERS
CASE 90
Male Breast Cancer
1. C and D
2. B
3. D
4. D
References
Appelbaum AH, Evans GFF, Levy KR, et al. Mammographic appearances of male breast disease. Radiographics. 1999;19:559–568.
Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet. 2006;367(9510):595–604.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:370. 376
Comment
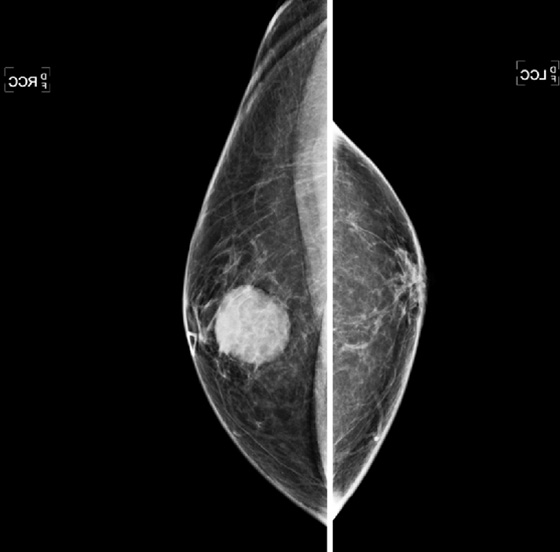
A mass in the male breast is most commonly benign. Gynecomastia is the most common finding and is typically a fan-shaped density centered on the nipple, although it can be remote from the nipple. Other masses include sebaceous cyst, lipoma, and abscess. Malignancy is relatively uncommon, but, as with palpable masses in women, it deserves serious consideration whenever a man presents with a palpable mass.
The work-up begins with a bilateral, four-view standard mammogram, with a marker placed over the area of clinical concern (see the figures). Ultrasound is a very useful imaging tool in the male patient, because it can help determine if the mass is in the skin, as in sebaceous cyst, an echogenic lipoma (typically the mammogram is normal, and the lipoma is not seen against the background of normal fatty breast), or an abscess (usually seen in the setting of an inflamed, tender breast). Breast cancer in men has an appearance similar to ductal carcinoma in women: a circumscribed mass that can rarely include the presence of calcifications (see the figures). Ductal carcinoma in situ (DCIS) can also occur; it accounts for approximately 10% of male breast cancer, with calcifications seen on the mammogram. Most tumors are of ductal origin. Presentation is usually more advanced than the presentation of breast cancer in women, with more than 40% presenting in stage III or IV. This late presentation is related to the lack of screening. Men are not screened with imaging for breast cancer, so detection is delayed until a mass is felt.
Risk factors include increased estrogen levels (Klinefelter’s syndrome, gonadal dysfunction, cirrhosis, obesity), radiation exposure, and family history, particularly with BRCA2 mutation. Male breast cancer is not related to gynecomastia. Peak age of incidence is 71 years, and in the majority of men, breast cancer is diagnosed after age 60 years.
CASE 91
History: A 70-year-old woman presents with a palpable concern in her right breast. She reveals a recent history of a motor vehicle accident in which she was in the passenger seat.
1. What is the differential diagnosis for the mammographic findings in the right breast? (Choose all that apply.)
A. Band of increased density, highly suspicious for malignancy
B. Band of increased density, consistent with trauma from seat belt in motor vehicle accident
C. Increased density in segmental distribution, suggesting malignant spread along ducts
D. Band of increased density, consistent with surgical scar
2. What is the next step in the work-up?
A. Nothing, the mammogram is diagnostic.
B. MRI should be performed to differentiate malignancy from trauma.
C. Ultrasound of the palpable finding should be performed.
3. What is the etiology of this finding on the mammogram?
B. The breast hits the steering wheel, causing trauma.
C. There is increased density caused by fibrocystic change.
D. Increased density is a result of malignancy in a regional distribution.
4. What follow-up should be recommended for traumatic injury to the breast?
A. The patient should be followed every 6 months with mammograms and ultrasound.
B. Biopsy is needed for the palpable mass.
C. The patient should be referred to a surgeon.
D. Annual mammogram is sufficient.
ANSWERS
CASE 91
Seat Belt Trauma
1. B and D
2. C
3. A
4. D
References
DiPiro P, Meyer J, Frenna T, Denison C. Seat belt injuries of the breast: findings on mammography and sonography. AJR Am J Roentgenol. 1995;164:317–320.
Majeski J. Shoulder restraint injury of the female breast. Int Surg. 2007;92:99–102.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:75.
Comment
The three-point seat belt is commonly used in automobiles. When a collision occurs, the breast can be forcibly compressed between the belt and the rib cage. The diagonal shoulder portion of the seat belt can cause a bandlike injury to the breast, usually to the left breast in the driver and to the right breast in the passenger. Fractures of the clavicle, ribs, and sternum can also occur, depending on the severity of the collision.
Mild injury results in bruising, skin blistering, breast swelling, tenderness, and friction burns over the contact area. More severe crush injury includes hematoma formation, fat necrosis, and skin laceration. Even more severe injury can result in laceration or even transection of the breast, with severe bleeding.
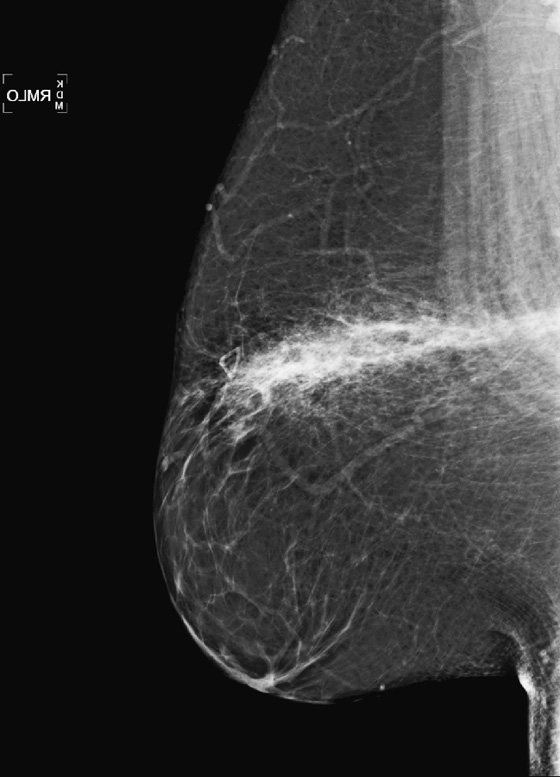
The mammographic appearance of the injury is typically a band of increased density that follows the path of the seat belt, diagonally across the breast. The increased density reflects edema and hematoma formation, and it is easier to see the injury if the breast tissue is predominantly fatty, as in this patient (see the figures). Fat necrosis can develop, typically seen as lucent round masses (oil cysts), often with rim-type calcification. The hematoma and fat necrosis can be palpable and tender.
In this patient (see the figures), the breast is predominantly fatty, and there is a discrete linear band of increased density in the upper breast. The palpable finding on the patient’s self-exam has been marked with a marker, and ultrasound of the area (see the figures) demonstrates an anechoic mass, consistent with oil cyst, and surrounding echogenic edema. These mammographic and ultrasound findings indicate seat belt trauma. No further follow-up is necessary in this patient. Routine annual mammograms can be recommended.
CASE 92
History: A 48-year-old woman whose mother was recently diagnosed with breast cancer at age 72 undergoes a routine screening mammogram. Microcalcifications are seen in the left breast.
1. What should be included in the differential diagnosis of the microcalcifications seen? (Choose all that apply.)
A. Ductal carcinoma in situ (DCIS)
B. Atypical ductal hyperplasia (ADH)
D. Dermal calcifications in the lower left breast
2. What is the next step in management?
A. MRI to check for abnormal enhancement
B. Follow-up magnification views in 6 months
3. When is a biopsy result discordant?
A. When ADH is upgraded to DCIS on surgical excision
B. When there are no calcifications on the specimen radiograph
C. When the biopsy results match the expected outcome based on imaging
D. When the patient has an infection as a result of the biopsy
4. Which of the following is a high-risk lesion?
D. Extensive intraductal component
ANSWERS
CASE 92
Atypical Ductal Hyperplasia
1. A, B, and C
2. C
3. B
4. B
References
Eby PR, Ochsner JE, DeMartini WB, et al. Is surgical excision necessary for focal atypical ductal hyperplasia found at stereotactic vacuum-assisted breast biopsy?. Ann Surg Oncol. 2008;15(11):3232–3238.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:230.
Comment
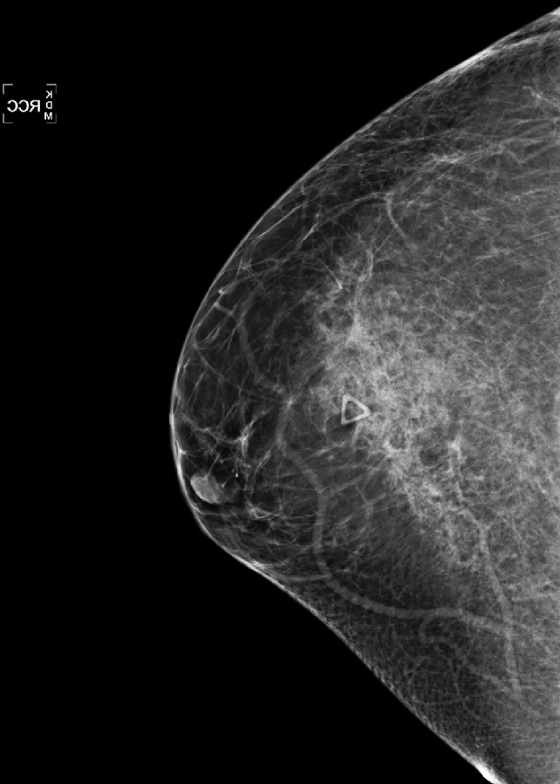
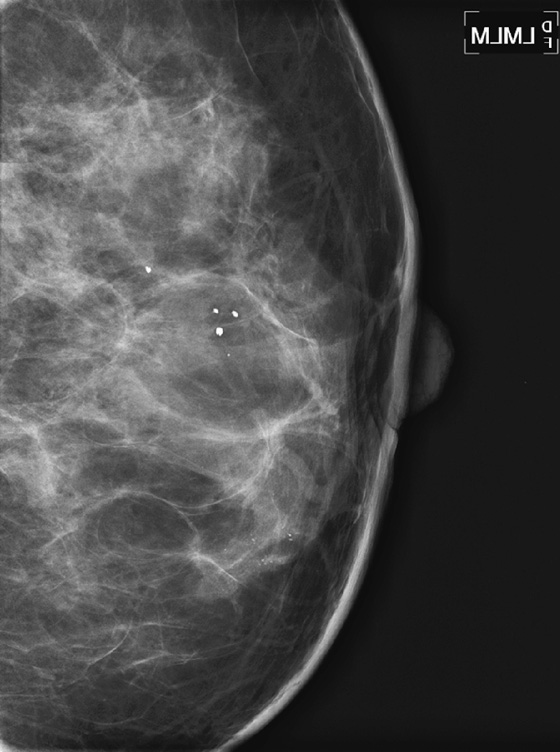
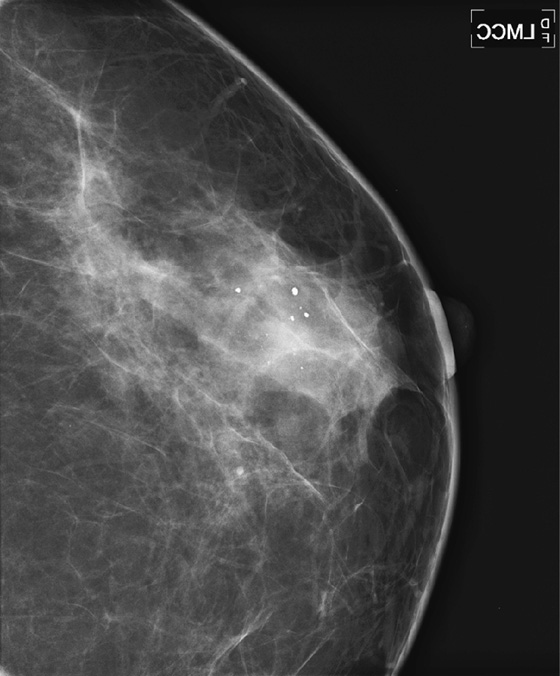
ADH is a condition in the spectrum of epithelial proliferative disease that leads to DCIS and invasive ductal carcinoma. The presence of ADH increases a woman’s risk of breast cancer to four to five times that of the general population. Histologically, ADH may contain all of the features of low-grade DCIS but to a limited degree. ADH is found on mammography by calcifications (see the figures). A focal density may be seen, and rarely ADH develops at the edge of a radial scar or focal area of sclerosing adenosis, seen on mammogram as spiculation. ADH may differ from DCIS only by degree of abnormality.
A core biopsy is a sampling procedure only. When ADH is found on core biopsy, as in the present patient, excision should be performed. The potential exists for inadequate sampling with core needle technique, and DCIS or invasive carcinoma may be adjacent to the ADH sampled. When ADH is excised, and the excision specimen shows malignancy, this is termed upgrading. The rate of upgrade from core biopsy to excision of ADH to DCIS is approximately 20%. When the degree of ADH is greater in the tissue specimens, the upgrade rate to DCIS is higher. In one recent study, if at least three foci of ADH were present in the needle biopsy samples, the rate of upgrade to DCIS was 28%.
ADH cannot be a Breast Imaging Reporting and Data System (BI-RADS) 3 lesion because this requires a 2% or less chance of malignancy. It is a BI-RADS 4 lesion, and excision is recommended. This patient had ADH on 10-gauge vacuum-assisted biopsy, and excision showed DCIS grade III.
CASE 93
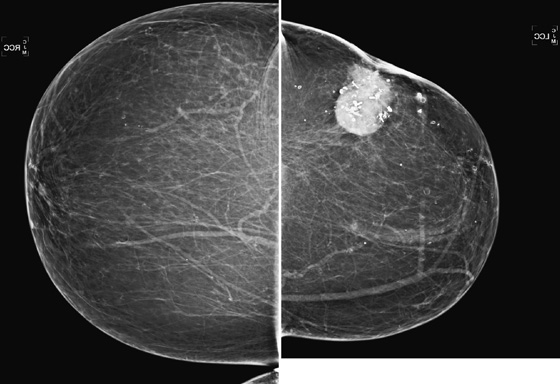
History: A 71-year-old woman returns for routine imaging 9 years after breast conservation therapy for infiltrating ductal carcinoma in the left breast.
1. What is the differential diagnosis for this mammogram? (Choose all that apply.)
B. Calcified mass in the left breast, highly suspicious for recurrent cancer at the lumpectomy site
C. Calcified mass in the left breast; cannot exclude recurrent cancer at the lumpectomy site
2. What is the next step in the management of this patient?
A. Perform MRI to evaluate for neovascularity in the mass.
3. When prior mammograms are compared, the mass is stable, and the coarse calcifications have increased in and around the mass. What is the Breast Imaging Reporting and Data System (BI-RADS) category and next step?
B. BI-RADS 2—1-year follow-up mammogram
C. BI-RADS 0—need additional magnification views
D. BI-RADS 3—short-interval follow-up
4. If the mass is palpable, does that change your management?
A. Yes, a palpable mass at the lumpectomy site should be biopsied.
B. Yes, any palpable mass in a cancer patient should be biopsied.
D. No, but ultrasound should be performed to evaluate a palpable mass at the lumpectomy site.
ANSWERS
CASE 93
Postoperative Hematoma
1. A and C
2. B
3. B
4. C
References
Bloomer WD, Berenberg AL, Weissman BN. Mammography of the definitively irradiated breast. Radiology. 1976;118:425–428.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:307. 318
Comment
Breast conservation therapy is an alternative to mastectomy. Surgical excision of the tumor (lumpectomy) and radiation therapy to the whole breast, and sometimes to the chest wall and axilla, are performed. Some patients are treated with localized, rather than whole-breast, radiation. Expected changes that are seen in the treated breast include:
▪ Increased interstitial markings throughout the breast and skin thickening due to edema
▪ Architectural distortion at the lumpectomy site
▪ Hematoma or seroma at the lumpectomy site
▪ Calcifications within the surgical bed and radiation field due to fat necrosis
These findings may be seen in the first follow-up mammogram after radiation therapy is completed (usually 6 months after completion). All findings usually decrease in prominence over time during the next 5 years, except for calcifications of fat necrosis, which commonly persist.
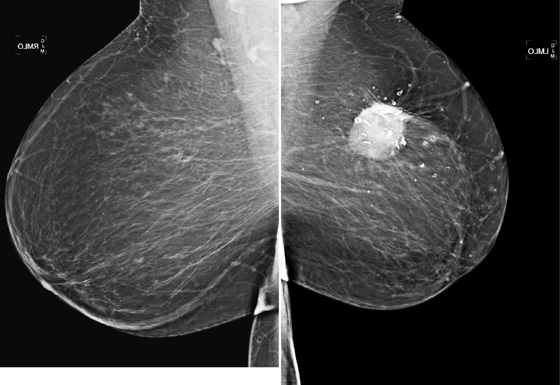
In this patient (see the figures), the surgical seroma/hematoma did not resolve over time. Nine years after surgery and radiation, the hematoma is the same size. This is a relatively uncommon result after breast conservation therapy. The majority of hematomas resolve spontaneously. In this patient, calcifications consistent with fat necrosis developed at the hematoma site, which is commonly seen.
Hematomas that develop at the lumpectomy site need not be aspirated. They may be quite difficult to aspirate owing to organization of the clot with fibrin. If they are symptomatic, painful, or inflamed, surgical drainage may be indicated.
Ultrasound of the lumpectomy site can be performed if there is concern that the developing mass at the site may be tumor rather than fluid collection. However, the complex appearance of the hematoma and developing fat necrosis can be confused with malignancy. Extensive calcification in the wall of the hematoma/seroma, as in this case (see the figures), can cause dense shadowing on ultrasound, limiting interpretation. MRI can be used to evaluate fat necrosis, but this can be tricky because fat necrosis can enhance. The mammogram is the single best tool for evaluating and managing the patient with a postoperative hematoma and fat necrosis.
CASE 94
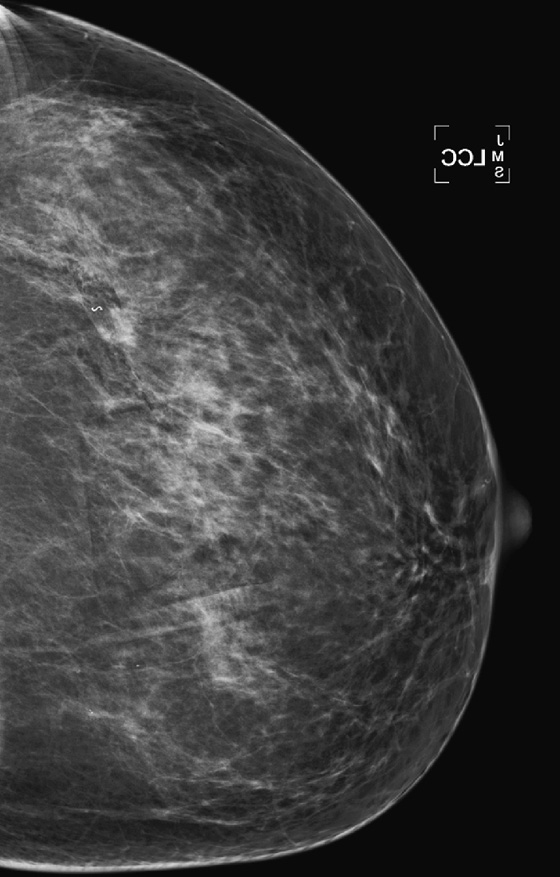
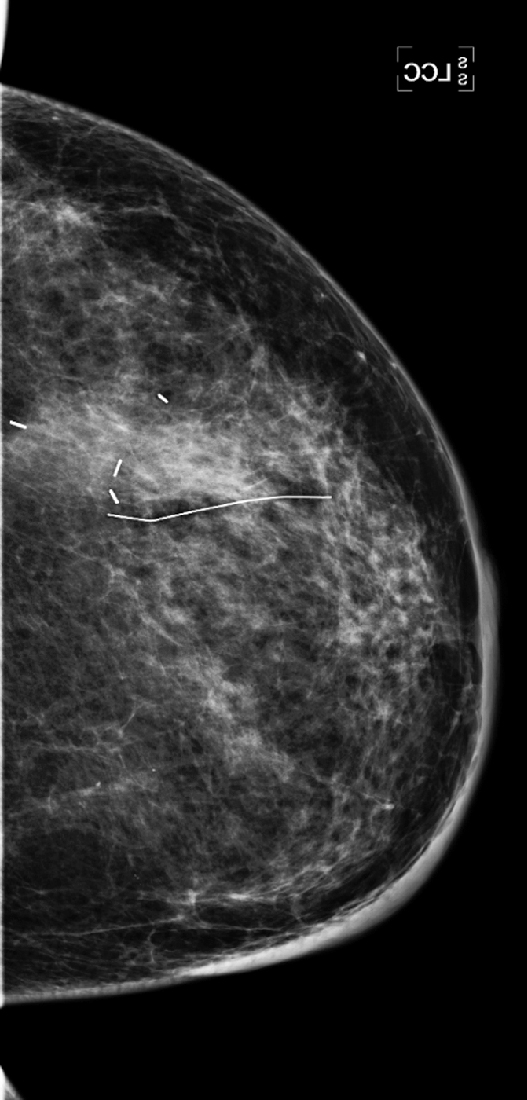
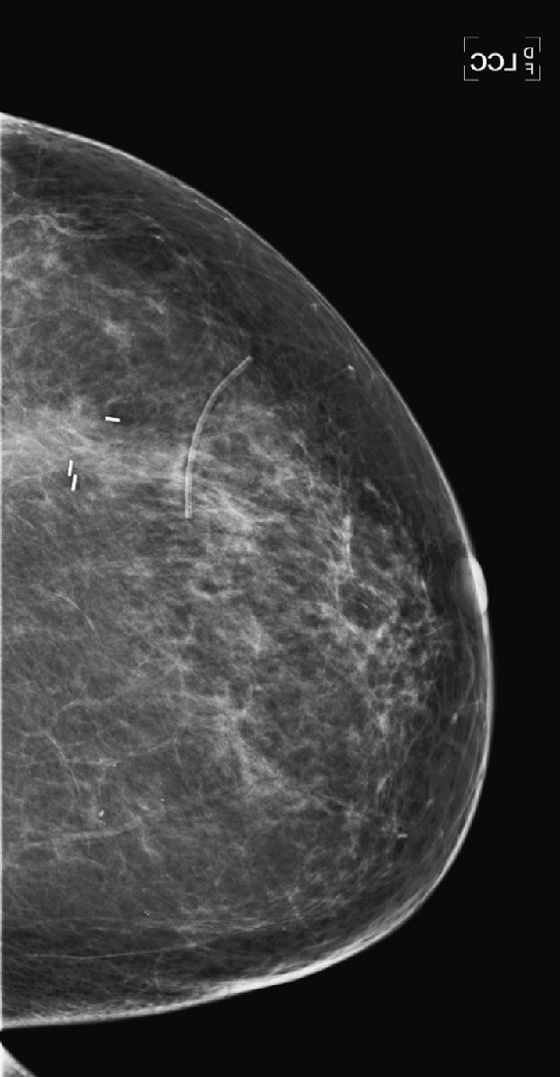
History: A 47-year-old woman developed a malignant mass in her left breast and was treated with breast conservation therapy (surgery and whole-breast irradiation). Images are shown on the day of needle biopsy, immediately after treatment, and 2 years after treatment.
1. What is the differential diagnosis for the appearance of the breast in the second figure?
B. Infection in the breast (mastitis)
C. Expected changes after breast conservation therapy
2. What are the findings expected on mammography after breast conservation therapy?
C. Calcifications indicating recurrent cancer
D. Malignancy developing in the contralateral breast
3. What does the mammogram 2 years after completion of therapy demonstrate?
B. Enlargement of the biopsy cavity
D. Increase in fat necrosis calcifications
4. Why are magnification views performed after breast conservation therapy?
A. To evaluate the seroma at the lumpectomy site
B. To evaluate for residual and recurrent malignancy
C. To evaluate punctate calcifications seen after radiation therapy
D. To evaluate typical fat necrosis calcifications
ANSWERS
CASE 94
Breast Conservation Therapy
1. B, C, and D
2. B
3. C
4. B
References
Brenner RJ, Pfaff JM. Mammographic features after conservation therapy for malignant breast disease: serial findings standardized by regression analysis. AJR Am J Roentgenol. 1996;167:171–178.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:318.
Comment
To conserve the breast after breast cancer has been diagnosed, local therapy is performed. This includes surgical excision of the malignancy usually followed by radiation therapy. (In some cases of very early disease, radiation therapy might not be given.) Radiation can be given as whole-breast irradiation or as partial-breast irradiation to the tumor site.
This patient had an 8-mm infiltrating ductal carcinoma, initially seen on screening mammogram. Ultrasound exam detected the small mass in the upper outer quadrant of the left breast. Biopsy was performed with ultrasound guidance, with a clip placed on completion of the biopsy (see the figures). She then had surgical excision followed by whole-breast irradiation. The mammogram in the second figure is a view of her left breast after surgery and radiation (breast conservation therapy).
After therapy there are expected changes in the breast that are important to recognize. A mass is usually present at the lumpectomy site. This represents the biopsy cavity filled with serous fluid (seroma) and blood (hematoma). There may be surgical clips at the site, placed at the time of surgery. There are often architectural distortion at the surgical site and skin retraction from the scar. Clips and distortion might also be seen in the axilla if axillary node surgery was performed.
The breast will also show changes from irradiation, which initially is predominantly the result of edema. In the skin this appears as skin thickening, and in the interstitial tissues of the breast this is seen as thickening of the white trabecular markings in the breast. About 25% of patients develop calcifications at the lumpectomy site, which may be benign punctate types, rim-type calcifications indicating fat necrosis, or suture calcifications. Fat necrosis can develop, seen as lucent mass, spiculated density, and calcifications. In this patient, shown in the second figure immediately after treatment, skin and trabecular thickening is present, as well as a mass and distortion at the lumpectomy site.
With time, generally up to 3 years, in most patients the changes resolve, as is seen in the last figure. Calcifications of fat necrosis can increase as time progresses. Edema and cavity size should not increase over time, and they usually resolve, as in this patient. However, these findings can remain stable over time in a small proportion of patients.
CASE 95
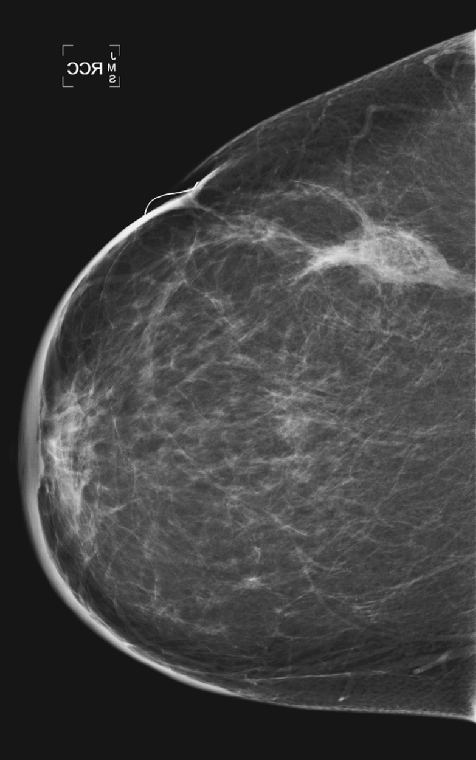
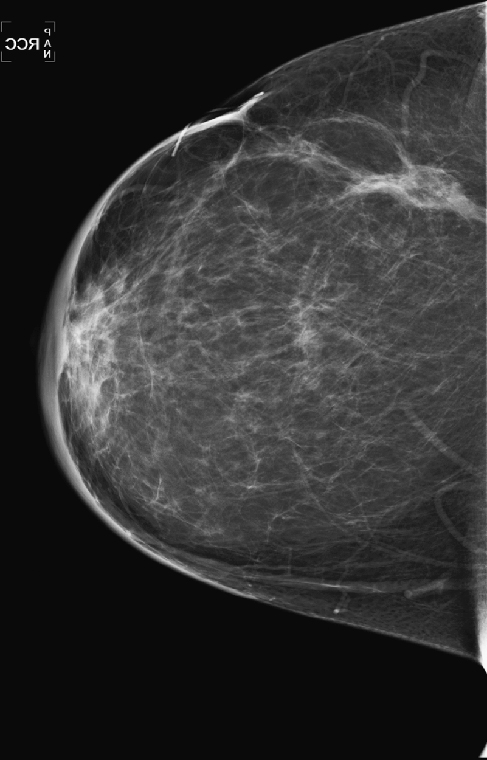
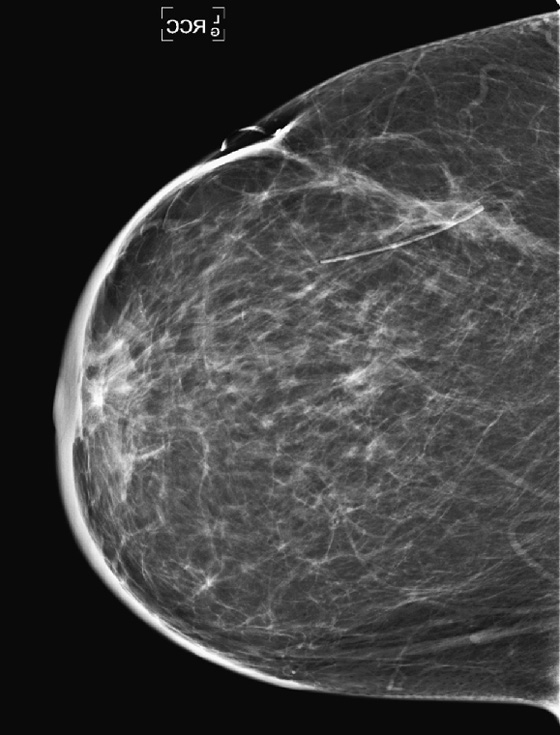
History: A 51-year-old woman was diagnosed with mucinous carcinoma in the outer right breast. She was treated with breast conservation therapy including lumpectomy and external-beam radiation therapy. Annual follow-up mammograms are shown, beginning with the third year after treatment.
1. What should be included in the differential diagnosis based on the images shown? (Choose all that apply.)
A. Recurrent cancer in the right breast at the lumpectomy site
B. Fat necrosis at the lumpectomy site
C. Skin thickening suggestive of lymphovascular invasion
D. Normal postlumpectomy and radiation changes
2. Which of the following is not an expected appearance of the breast 2 years after lumpectomy and radiation?
A. Mass at the lumpectomy site
D. Increased density at the surgical site compared with previous mammogram
3. What is the benefit of radiation given to women being treated for breast cancer?
A. Radiation reduces the recurrence rate at the surgical site.
B. Radiation reduces recurrences away from the surgical site.
D. The reduced recurrence rate is mitigated by the complications of slower healing and edema.
4. What is the MRI appearance of the lumpectomy site after conservation therapy?
A. Seroma or hematoma that is hyperintense on T2, thin-rim enhancement after contrast
B. Focal areas of irregular enhancement due to fat necrosis, increasing over time
C. Increased enhancement throughout the breast caused by chemotherapy
D. Enhancement of the skin caused by radiation therapy
ANSWERS
CASE 95
Postoperative Breast
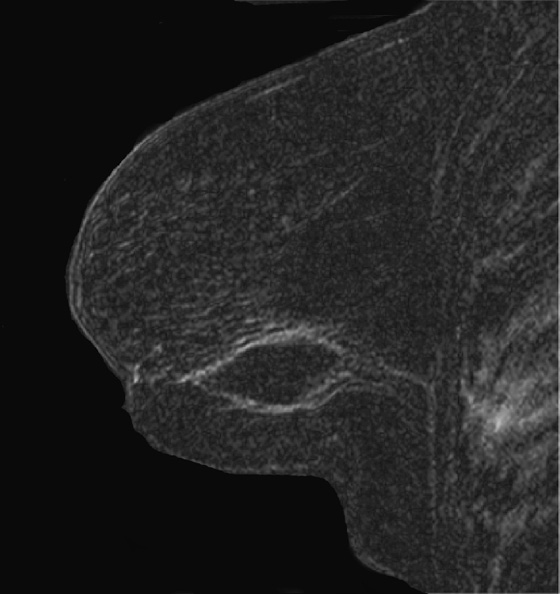
1. B and D
2. D
3. A
4. A
References
Brennan S, Liberman L, Dershaw DD, et al. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol. 2010;195(2):510–516.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:307.
Comment
Breast conservation therapy is commonly used as a treatment of breast cancer. The term breast conservation therapy includes lumpectomy and radiation therapy, often external-beam, whole-breast radiation therapy. Lumpectomy is wide excision of the tumor, with the excisional cavity left to fill in with fluid. Radiation therapy begins several weeks after surgery and continues for about 6 weeks.
This treatment causes changes in the breast, and it is important for the radiologist to understand the changes that are seen in routine healing and to differentiate those changes from the findings seen with recurrent cancer. Evidence of recurrence is rare before 18 months. Mammography has been the imaging method of choice for postoperative surveillance, but ultrasound and MRI are also useful to supplement the mammogram.
Mammogram findings after surgery and radiation therapy include masses, fluid collections, increased breast density, skin thickening, architectural distortion, and calcifications. Radiation therapy exacerbates these findings and delays their resolution. The masses seen may represent hematoma, seroma, abscess, fat necrosis, or fibrosis. These findings evolve over time, with decrease in the size of the mass until stable. Any changes after stabilization, in particular, increased masslike density and pleomorphic calcifications, should be viewed with suspicion.
This patient underwent breast conservation therapy 6 years ago for a 7-mm mucinous carcinoma. The first mammogram view shown is from her follow-up mammogram 3 years after treatment and shows a focal oval mass at the lumpectomy site, with increased interstitial markings and skin thickening (see the figures). On the next mammogram, 1 year later, the mass is smaller and less dense (see the figures). These changes are continual, as shown over 4 years (see the figures). There is no evidence of developing mass or microcalcifications. The patient has skin thickening, particularly apparent in the anterior breast; this is common after radiation therapy and may persist. Normal skin thickness is 2 mm; after radiation therapy, the skin thickness may measure 10 mm. This skin thickening is initially due to small vessel damage and later is due to fibrotic change.
This patient does not have calcifications, but they are commonly seen after therapy; 28% of patients develop calcifications at the surgical site within 12 months of treatment. These are benign dystrophic calcifications, or fat necrosis, and rarely suture calcifications. If the calcifications are few and punctate, they can usually be followed. If pleomorphic calcifications are seen, this may be due to fat necrosis, but biopsy may be needed to exclude recurrence. The ability of mammography to detect recurrence has been estimated at 25% to 45%. Ultrasound and MRI are also used to increase the detection rate. MRI is sensitive for the differentiation between scar and recurrence.
MRI can be used to evaluate for residual disease and to screen high-risk women. The American Cancer Society guidelines for screening breast MRI found no convincing evidence to support screening women with a personal history of breast cancer if they had no other risk factors. However, a recent study found detection of recurrent cancer in 12% of biopsy specimens of developing MRI abnormalities in patients with previous breast conservation therapy. The fourth figure is an MRI scan of a different patient who had breast conservation therapy. The seroma cavity is bordered by a rim of uniformly thin enhancement, consistent with healing. There is no evidence of recurrence. MRI evidence of residual or recurrent disease includes thick, irregular enhancement around the seroma, or an enhancing mass.
CASE 96
History: Asymptomatic 45-year-old woman, with two sets of screening mammograms 2 years apart.
1. What is the differential diagnosis for this set of mammograms? (Choose all that apply.)
A. The patient had cancer and had bilateral lumpectomies.
B. The patient has had bilateral reduction mammaplasty.
C. The patient has lost weight.
D. The patient has undergone pregnancy and lactational changes.
2. What are the expected changes on the mammogram for this circumstance?
3. Is there an increased risk of malignancy with this surgery?
A. Yes, any surgery increases the risk of cancer.
B. Yes, the scar tissue can mask malignancy.
C. No, there is no increased risk.
D. Yes, women with larger breasts have inherently higher risk than women with smaller breasts.
4. What is a common problem after reduction mammaplasty?
A. Palpable lump along the scars
B. Increased cyclic pain before menses
D. Increased “heaviness” of the breasts
ANSWERS
CASE 96
Reduction Mammaplasty
1. B and C
2. D
3. C
4. A
References
Douglas-Jones AG, Varma M. Screening at breast reduction: more than a little extra work. BMJ. 2009;338:b2342.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:360. 346
Comment
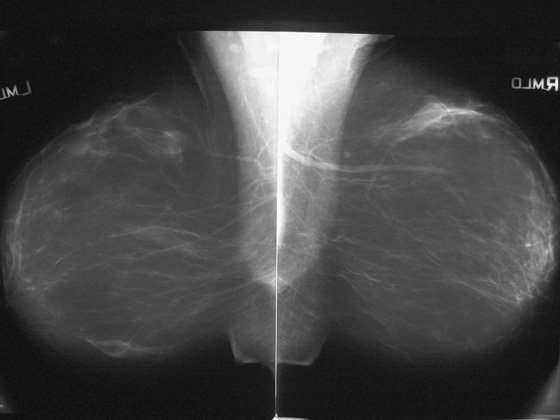
Reduction mammaplasty is a relatively common surgery performed on the breast for cosmetic reasons. Usually, this is done to reduce the size and weight of the breast, but it may also be performed to “lift” the breast. The surgeon makes incisions in the breast, removes breast tissue, and then may replace the nipple higher on the mound of the breast. The incisions most commonly used are a vertical scar from the nipple to the inframammary fold, a transverse incision along the inframammary fold, and a circumareolar incision if the nipple is moved up.
The incisions made in the breast will lead to scar formation and not uncommonly to fat necrosis. The scar tissue can develop calcifications. The most common mammography findings after reduction surgery are architectural distortion, punctate calcifications, focal fat lucency, and lines of density along the scars, as seen in this case (see the figures). Also, skin thickening may be seen in the lower breast, at the scar, and the ducts might appear to terminate lower than the nipple because the nipple has been moved up.
Technologists should always chronicle any breast surgery on the patient’s history form before performing the mammogram.
Some patients present with a palpable lump after reduction mammoplasty, often along the scar tissue. Mammographic views of the palpable finding should be performed, and if fat necrosis is seen, no further work-up is necessary. The patient might want to be seen by her plastic surgeon for reassurance. If the mammogram shows no signs of fat necrosis, ultrasound of the palpable finding should be performed. Fat necrosis on ultrasound may have the appearance of a hypoechoic round mass with a thick echogenic border, but ultrasound appearance can be variable. Fat lucency is required on the mammogram to dismiss the palpable finding as fat necrosis. If there is uncertainty about the etiology of the palpable mass, biopsy should be considered.
CASE 97
History: A 64-year-old asymptomatic woman underwent a routine screening mammogram. She has a family history of breast cancer in her daughter, diagnosed at age 37, and in her grandmother, age unknown. She is recalled for additional views.
1. What should be included in the differential diagnosis for the images shown? (Choose all that apply.)
B. Invasive ductal carcinoma (IDC)
C. Invasive lobular carcinoma (ILC)
D. Ductal carcinoma in situ (DCIS)
2. Why is the distortion better seen on one view?
A. The craniocaudal (CC) view has more compression.
C. The mediolateral oblique (MLO) view does not include the far medial and lateral breast.
D. The MLO view is technically more difficult to perform and is more limited.
3. Which of the following is not the reason that ILC is detected at a larger size than IDC?
A. ILC does not form a mass that is well seen on two views.
B. ILC is often seen as an asymmetric density, on only one view.
C. ILC is not typically palpable.
D. ILC does not typically cause a desmoplastic reaction in the surrounding tissues.
4. Which of the following statements is true about ILC?
A. ILC has a higher risk of bilaterality compared with IDC.
B. ILC has a lower risk of bilaterality compared with IDC.
C. The prognosis for ILC is worse than for IDC.
D. Treatment of ILC is more likely to require chemotherapy.
ANSWERS
CASE 97
Invasive Lobular Carcinoma
1. A, B, and C
2. B
3. D
4. A
References
Brem RF, Ioffe M, Rapelyea JA, et al. Invasive lobular carcinoma: detection with mammography, sonography, MRI, and breast-specific gamma imaging. AJR Am J Roentgenol. 2009;192(2):379–383.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:101.
Comment
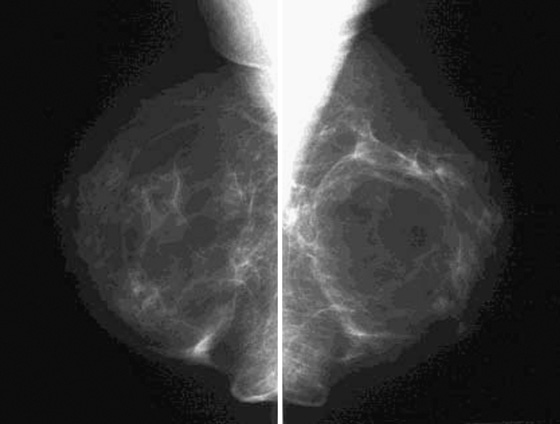
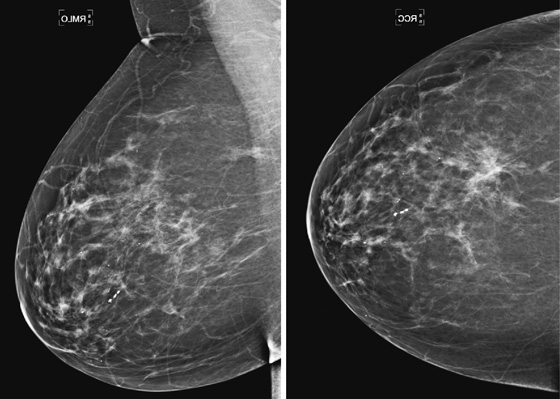
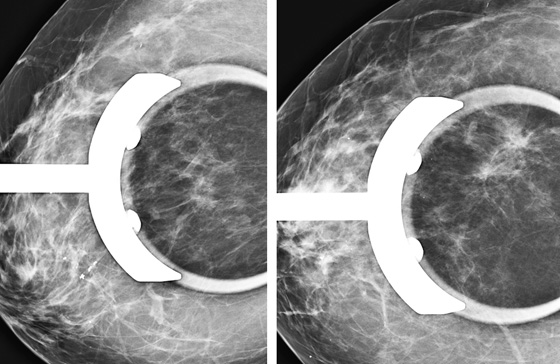
The mammogram shown demonstrates how ILC can be difficult to detect because it may be seen on only one view. When seen on one view, it is more commonly seen on the CC view, as in this patient (see the figures). The features most commonly seen on mammography are an equal-density developing asymmetry with distortion, or a spiculated mass. Calcifications are rare (seen in 1% to 11% of cases). Growth of the tumor is insidious, spreading in a single file of cells and not inciting a desmoplastic reaction. These features explain why the tumor is so difficult to detect on mammography. In one study, ILC was missed on the mammogram in 21% of patients. It is detected at a later stage owing to its difficulty in detection. In one study, 14% were larger than 5 cm at detection compared with 9% of IDC being larger than 5 cm at detection.
ILC represents approximately 10% of all breast cancers and is much less common than IDC. It is more commonly multifocal, multicentric, and bilateral. Bilateral rate is approximately 10% to 15%. For this reason, the ipsilateral and contralateral breasts must be evaluated for additional sites of disease; this evaluation is commonly done with MRI.
When ILC is suspected on mammography, spot compression views should be obtained (see the figures). On ultrasound, there is typically a hypoechoic shadowing mass that is similar to IDC. On MRI, ILC usually enhances, but it may not enhance more than the surrounding glandular tissue, leading to a false-negative study.
CASE 98
History: Two different patients present for routine screening.
1. What is the differential diagnosis for both patients? (Choose all that apply.)
A. Both have saline implant leak.
B. Both have silicone implant leak.
C. Both patients have a rupture of the left implant.
D. Patient A has an extracapsular rupture; patient B has a complete collapse of her implant.
2. Are the implants in these two patients subpectoral or subglandular?
A. Both patients have subpectoral implants.
B. Patient A has subglandular; patient B has subpectoral.
C. Both patients have subglandular implants.
D. Patient A has subpectoral implants; patient B has subglandular.
3. How does a silicone leak manifest itself?
B. The implant shell does not collapse but rather holds the silicone gel in place.
C. The implant shell collapses, and the silicone always leaks into the breast tissue.
D. Silicone leaks are always seen on the mammogram.
4. How does a saline implant leak manifest itself?
A. The saline stays in place, inside the fibrous capsule.
B. The shell collapses, and the saline is quickly reabsorbed.
C. The saline very slowly leaks from the implant over a period of years.
D. The breast size remains the same after rupture.
ANSWERS
CASE 98
Implant Leak
1. C and D
2. C
3. A
4. B
References
Berg WA, Caskey CI, Hamper UM, et al. Diagnosing breast implant rupture with MR imaging, US, and mammography. Radiographics. 1993;13(6):1323–1336.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:341. 346
Comment
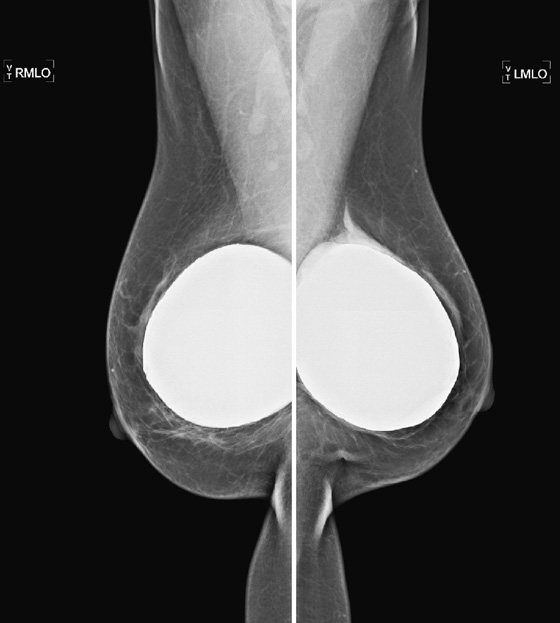
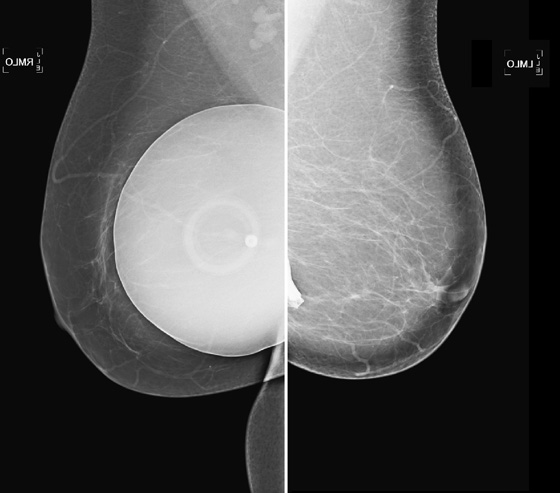
These two patients illustrate the appearance of implant rupture. Patient A has subglandular silicone implants (see the first figure). To judge the location of the implant, look for the implant’s position relative to the pectoral muscle. If it is anterior to the muscle, as in the first set of figures, it is in the subglandular or prepectoral position. In the mammogram of patient A, there is a wispy area of silicone-density material adjacent to the superior aspect of the left silicone implant. This material is seen external to the contour of the implant; it is silicone that has escaped the confines of the silicone implant shell, as well as the confines of the fibrous capsule.
In the mammogram of patient B (see the second figure) an intact saline implant is seen in prepectoral position in the right breast. On the left side, there is a triangular density near the chest wall. This represents the collapsed saline implant.
Implants are essentially bags, usually made of silicone, with silicone gel, saline, or other material (peanut oil was used for a brief period) inside the shell. The bag is placed inside the body, either in front of the pectoralis muscle (see the figures) or posterior to the muscle. The anterior position is easier to place, but it more commonly leads to implant contracture. The contracture is due to constriction of the fibrous capsule, which is made by the host, as a foreign body reaction to the implanted silicone bag. This capsule is essentially fibrous tissue and has a tendency to constrict.
When discussing silicone implant rupture, it is important to classify the rupture as intracapsular or extracapsular. In the first instance, the bag ruptures, and the silicone gel is held in place by the host’s fibrous capsule. This might not be recognized mammographically because the shape may be the same. It can be seen on MRI and can also be recognized on ultrasound. In the case of extracapsular rupture of a silicone implant, the silicone gel escapes from the fibrous capsule and extrudes into the breast tissue. It can then be picked up by the lymphatics and deposited into the lymph nodes (not seen in this patient). This can be recognized on mammography as in this case, on ultrasound (“snowstorm” appearance in the breast tissue), and on MRI.
In saline implant rupture, such distinction is not necessary. If the bag ruptures, the saline is reabsorbed by the body. The saline is not retained inside the capsule. The collapsed bag is seen. Patients who have saline implants do not need ultrasound or MRI for evaluation of the implant. The mammogram is diagnostic. Usually, the clinical exam is diagnostic, because the breast size decreases dramatically.
CASE 99
History: Two patients have mammograms. The first patient is 45 years old and asymptomatic; she had a screening mammogram and is recalled for additional views (see the first figure). The second patient is 60 years old and has not had a mammogram for 3 years; she feels a lump in her left breast and comes in for evaluation (see the second figure).
1. What should be included in the differential diagnosis for these two patients? (Choose all that apply.)
A. Fibrocystic change with sclerosing adenosis
B. Ductal carcinoma in situ (DCIS)
D. Atypical ductal hyperplasia
2. What terminology would you use to describe the form and distribution of calcification in the first patient?
B. Coarse and fine heterogeneous, in segmental distribution
C. Fine linear branching, segmental
D. Clusters of pleomorphic calcifications in a regional distribution
3. What terminology would you use to describe the form and distribution of calcification in the second patient?
A. Clustered, round, and amorphous
B. Linear calcifications in a regional distribution
C. Fine linear branching calcifications in a focal cluster
D. Amorphous calcifications in a cluster
4. Which of the following is not a reason to use the lexicon for describing calcifications?
A. The form of the calcifications suggests the grade of DCIS.
B. The lexicon describes the level of concern for the mammographic finding.
C. The terminology describes the extent of the abnormality for the surgeon.
D. It is a good academic exercise.
ANSWERS
CASE 99
Ductal Carcinoma In Situ in Two Patients
1. B and C
2. B
3. C
4. D
References
Allred DC. Ductal carcinoma in situ: terminology, classification, and natural history. J Natl Cancer Inst Monogr. 2010;2010(41):134–138.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:63. 74
Comment
The term DCIS encompasses a heterogeneous group of breast malignancies characterized by the clonal proliferation of epithelial cells originating in the terminal ductal lobular unit. It is a nonobligate precursor to invasive ductal carcinoma, which is lethal. The disease varies by the nuclear grade of the malignant epithelial cells and by the presence or absence of necrosis. When necrosis is present (also called comedonecrosis), the disease is more aggressive; the higher the nuclear grade (Van Nuys classification I, II, and III), the more aggressive. The local recurrence and chance of progression to invasive disease are related to the nuclear grade. Other histologic classifications used include cribriform, solid, papillary, and micropapillary forms. These types are typically less aggressive than the comedo type and usually well to moderately differentiated (grades I and II).
The distinctions are important in the management of the disease. There is a risk of local recurrence if the disease is incompletely resected at breast conservation therapy. The risk is increased in patients who have a high nuclear grade and comedonecrosis, patients who present with a lump or bloody nipple discharge, and young patients. The radiologist must recognize the characteristics of suspicious calcifications and the disease extent and obtain adequate biopsy samples of all of the lesions seen.
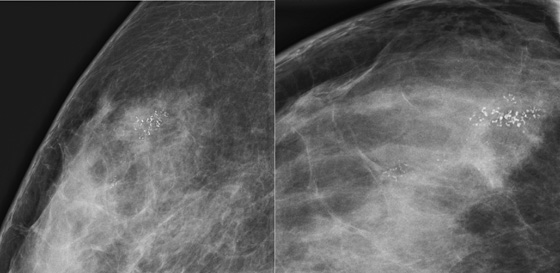
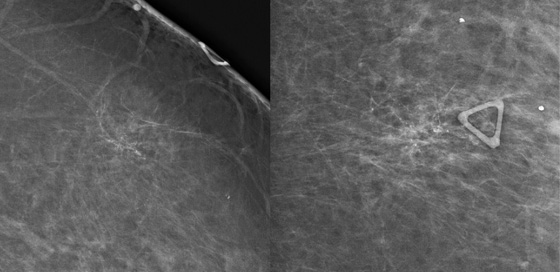
The first patient had coarse and fine heterogeneous calcifications in multiple groupings in the right upper outer breast (see the figures). These appeared within a segmental distribution. The histologic classification of coarse and fine heterogeneous calcifications is variable but tends to be low and intermediate nuclear grade. The patient may be able to be treated with breast conservation therapy, with relatively low risk of recurrence.
The first patient underwent ultrasound of the right breast, which showed several hypoechoic areas associated with calcifications, consistent with DCIS. Ultrasound can be used to evaluate for the presence of an associated mass and to target biopsy. In this patient, biopsy was performed with ultrasound guidance, and a diagnosis of DCIS, solid and cribriform types, nuclear grade II, was made, concordant with the imaging findings.
The second patient presented with a palpable mass. DCIS can be palpable if associated with stromal fibrosis around the intraductal malignancy, or if DCIS is present in an existing mass, such as fibroadenoma. The mammogram showed fine linear branching calcifications in a focal area and no mass (see the figures). This type of calcification has been shown to be associated with high–nuclear grade DCIS and necrosis, which has a higher incidence of local recurrence. Stereotactic biopsy results were concordant with the appearance of the calcifications: grade III DCIS with comedonecrosis.
CASE 100
History: A 56-year-old woman presents with pain in both breasts. Silicone implants were placed 10 years ago.
1. What is the differential diagnosis based on the MR images?
A. Bilateral intracapsular silicone implant rupture
B. Bilateral extracapsular silicone implant rupture
C. Bilateral intracapsular saline implant rupture
D. Bilateral intact silicone implants
2. What imaging modality is the most sensitive for detecting intracapsular rupture?
C. Breast-specific gamma imaging (BSGI)
3. What term is not used to describe intracapsular rupture on MRI?
4. What is the most important feature of intracapsular implant rupture diagnosis on MRI?
A. Silicone on both sides of the implant capsule
B. Folding of the implant capsule
C. The position of the implant in front of the pectoralis muscle
D. T2-bright fluid around the implant capsule
ANSWERS
CASE 100
MRI of Intracapsular Rupture of Silicone Implant
1. A
2. D
3. C
4. A
References
Collis N, Litherland J, Enion D, Sharpe DT. Magnetic resonance imaging and explantation investigation of long-term silicone gel implant integrity. Plast Reconstr Surg. 2007;120(5):1401–1406.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:351–354.
Comment
Implants are common. One estimate is that 2.5 million women in the United States have implants. Implants are placed for cosmetic augmentation (approximately 80%) and for reconstruction after mastectomy (approximately 20%). Implants consist of an envelope made of silicone elastomer membrane or shell, filled with saline or silicone gel. The patient’s host response to the implant is to form a fibrous capsule around it.
Complications of silicone implants are common. Women can present with pain, capsular contracture, inflammation, and infection. Rupture of the implant is not unusual, and it can occur within the host’s fibrous capsule (intracapsular), or it can break through the fibrous capsule (extracapsular). In intracapsular rupture, only the implant shell is ruptured. In extracapsular rupture, both the implant shell and the fibrous capsule rupture. In one retrospective study, 24% of women with implants (both silicone and saline) had a complication needing a surgical procedure during the first 5 years after receiving the implants. Women having implants for reconstruction were more likely to need reoperation, compared with women having cosmetic augmentation.
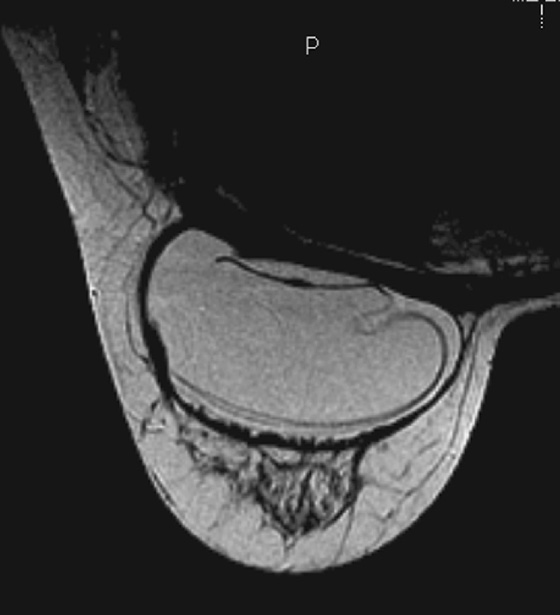
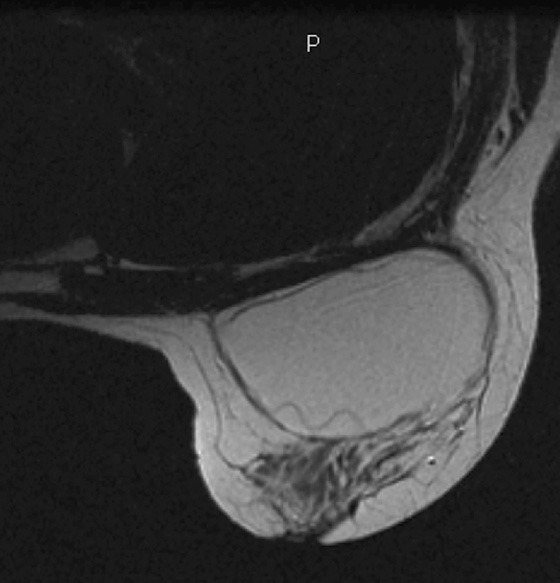
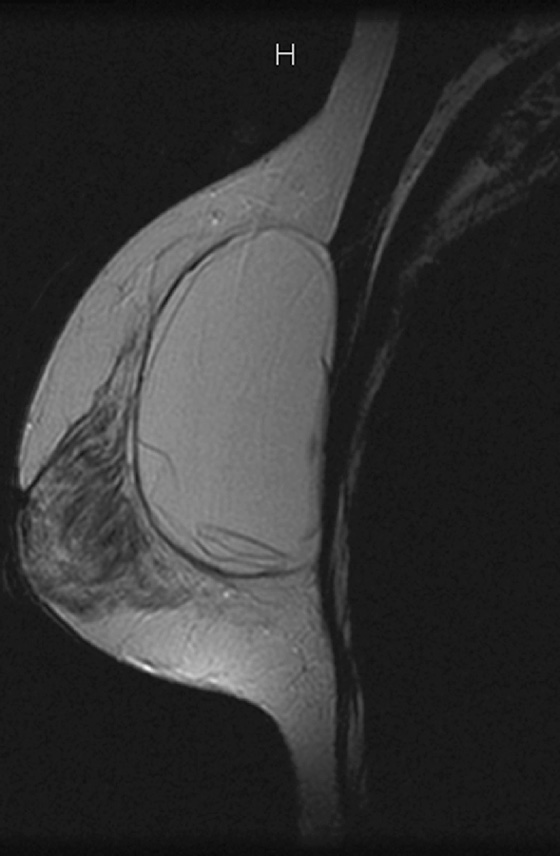
MRI is the most sensitive imaging study for evaluating implant rupture. Extracapsular rupture may be seen on the mammogram as dense material outside the confines of the implant capsule, and it can be recognized on ultrasound as the “snowstorm” appearance. However, intracapsular rupture is not well seen on the mammogram, and it can be difficult to recognize on ultrasound (the stepladder sign is not always obvious). When intracapsular rupture is pronounced, the silicone shell is collapsed, and it is seen floating free in the silicone gel. This is the stepladder sign in ultrasound and the linguine sign in MRI (see the figures). When the rupture is of a lesser degree, the implant shell is seen pulled slightly away from the fibrous capsule in loops or nooses, and silicone is seen on both sides of the silicone shell (see the figures).
Folds in the implant shell are common and must be differentiated from rupture of the shell. Look for the presence of silicone on both sides of the shell; this will not be present with folds. Also common is water-density fluid surrounding the capsule in intact implants. This will not be seen on a water-saturated, fat-saturated sequence, where only silicone is bright. It is not related to rupture.
In this patient, there is bilateral intracapsular silicone implant rupture (see the figures).
CASE 101
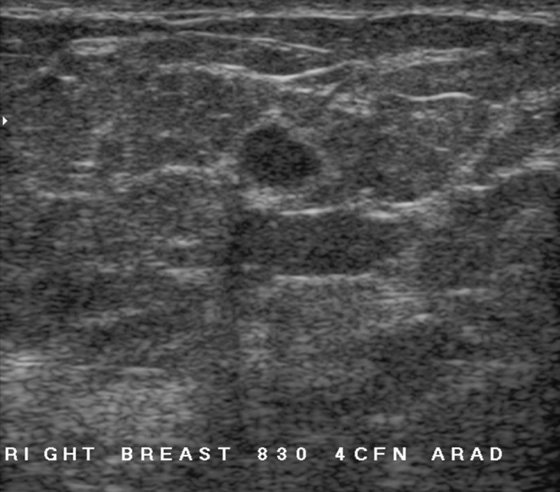
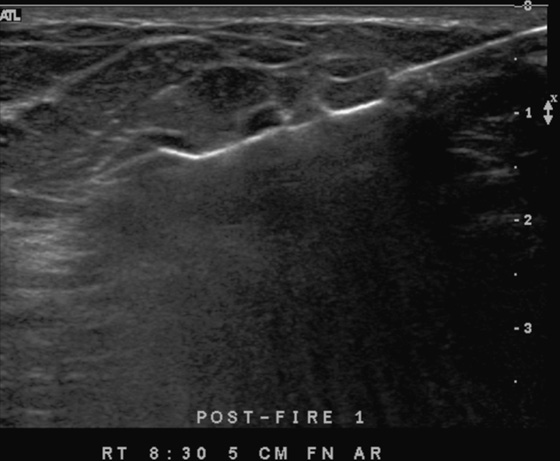
History: A 62-year-old woman presents for routine screening mammogram. The right breast only is shown; the finding is new since her last mammogram. She has a family history of breast cancer in her mother at age 62.
1. What should be included in the differential diagnosis of the right mammogram? (Choose all that apply.)
2. What differentiates central and peripheral papillomas?
A. They are the same lesion but in different locations.
B. Peripheral papillomas are more likely to manifest with bloody nipple discharge.
D. Both types are equally likely to be multiple.
3. What is unusual about the location of the mass on the mammogram and the location noted on ultrasound?
B. The mass is lateral on the mammogram, and the 8:30 position is in the medial breast.
C. Nothing—the locations of the mass on the mammogram and ultrasound are concordant.
4. What is the next step in management of a peripheral papilloma on core biopsy?
ANSWERS
CASE 101
Peripheral Papilloma with Ductal Carcinoma In Situ
1. A, B, and C
2. C
3. C
4. A
References
Al Sarakbi W, Worku D, Escobar PF, et al. Breast papillomas: current management with a focus on a new diagnostic and therapeutic modality. Int Semin Surg Oncol. 2006;3:1.
Ibarra JA. Papillary lesions of the breast. Breast J. 2006;12(3):237–251.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:120. 383
Comment
Papillary masses have various manifestations in the breast. The most commonly encountered is large duct papilloma, which is in the central, subareolar breast, is rarely palpable, and often manifests with nipple discharge. These are typically benign, and their surgical management is controversial.
Peripheral papilloma arises from the terminal ductal lobular unit, which is the location that gives rise to epithelial proliferation, including atypical ductal hyperplasia, ductal carcinoma in situ, and invasive ductal carcinoma. There is a higher association with malignancy in peripheral papilloma compared with large duct papilloma. In one large study, more than 37% of peripheral papillomas were associated with malignancy. They manifest less often with nipple discharge (about 20% of the time) and are more frequently palpable.
When a new mass is seen on routine mammography (see the figures), additional work-up is needed. The patient is recalled for additional spot compression views. Ultrasound is then performed to identify if there is a cystic or solid mass to correspond to the mammographic finding (see the figures). If the mass is cystic but has a thick wall, papillary projections, or thick internal septations, malignancy is suspected, and a biopsy is performed. If the mass is solid, biopsy is performed (see the figures). Biopsy can be averted only if the mass is typical of a simple cyst. Color flow Doppler should be used to ensure that the mass is not an anechoic solid tumor. Cyst aspiration can be performed if there is any doubt. The mass in the patient in the present case was a papilloma with associated ductal carcinoma in situ on 12-gauge vacuum core biopsy.
Papillomas on mammography manifest as round masses, with or without calcifications, as in the patient in this case, but also can manifest as foci of microcalcifications, clusters of nodules, and asymmetric density. Although additional abnormalities are not seen on this patient’s mammogram, bilateral MRI may be performed to assess for additional disease. On MRI, papillomas are typically small round masses with brisk early enhancement and washout on kinetic curve.
CASE 102
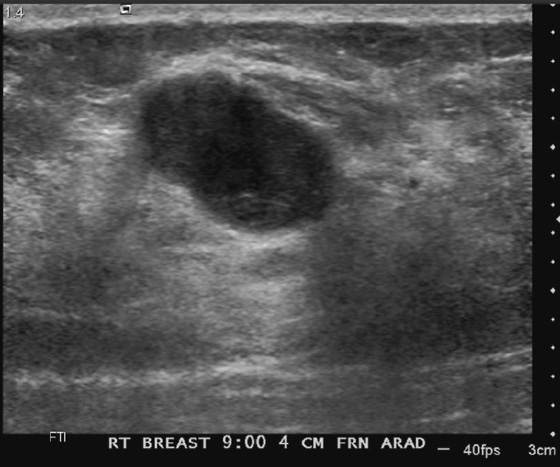
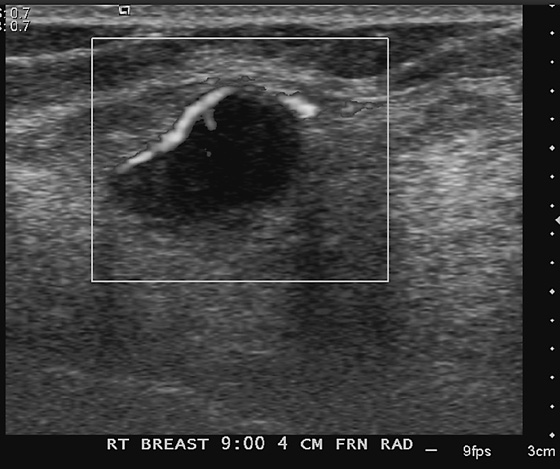
History: A 46-year-old woman has a family history of breast cancer in her sister at age 40. She has had multiple benign breast biopsies. She underwent breast MRI for high-risk screening, and multiple masses were seen, most consistent with fibroadenomas. One of the masses seen on MRI had brisk early enhancement and was considered indeterminate. She then had a targeted ultrasound with vacuum-assisted ultrasound-guided biopsy.
1. What should be included in the differential diagnosis for the mass in the right breast? (Choose all that apply.)
A. Infiltrating ductal carcinoma
2. What is the next step in management?
A. Additional imaging with breast-specific gamma imaging
C. Follow-up ultrasound examination in 6 months
D. Needle biopsy using ultrasound guidance
3. Why is Doppler assessment useful before core biopsy?
A. It adds no useful information if biopsy is to be performed anyway.
B. If a mass is avascular on Doppler, no biopsy is indicated.
C. It can help map the location of arteries and veins within the mass.
D. A very vascular mass should not be biopsied with percutaneous technique.
4. Which of the following is not an advantage of vacuum-assisted, ultrasound-guided needle biopsy?
A. The cores in a vacuum-assisted biopsy are larger.
B. Ultrasound guidance is easier for the patient than stereotactic biopsy.
C. There is no radiation exposure during an ultrasound-guided procedure.
D. The ultrasound-guided procedure cannot cause a pneumothorax.
ANSWERS
CASE 102
Vacuum-Assisted Biopsy
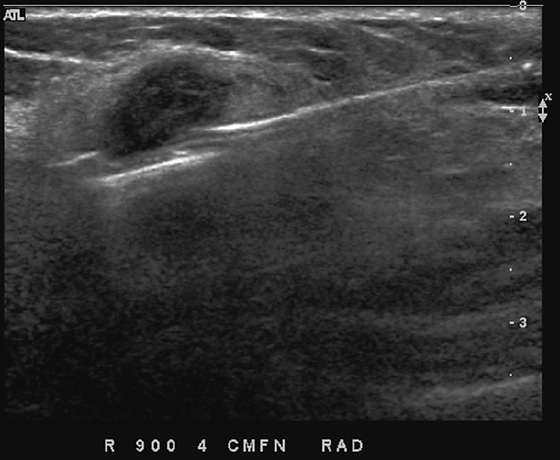
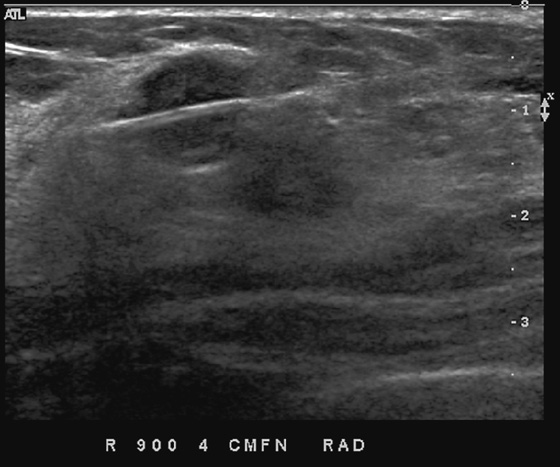
1. A and B
2. D
3. C
4. D
References
Chon N, Moon WK, Cha JH, et al. Sonographically guided core biopsy of the breast: comparison of 14-gauge automated gun and 11-gauge directional vacuum-assisted biopsy methods. Korean J Radiol. 2005;6(2):102–109.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:215.
Comment
Ultrasound-guided core biopsy is a cost-effective, efficient method for determining the histology of an indeterminate lesion seen on ultrasound (see the figures). Compared with stereotactic guidance, it is more comfortable for the patient, uses no specialized equipment such as the stereotactic table, has no ionizing radiation, and is generally faster. The needle is seen in “real time” during the procedure. It is possible to evaluate for blood vessels using color Doppler (see the figures).
Some specific aspects of the ultrasound-guided procedure must be taken into consideration, making this procedure more difficult to learn than stereotactic biopsy. The location of the needle insertion site, relative to the mass, is important (see the figures). The needle must be seen at all times when in the breast and must be kept away from the chest wall to prevent pneumothorax. Ideally, the needle is inserted in such a way that it can be kept roughly parallel to the skin (see the figures).
The automated core biopsy device uses a spring to advance the needle into the lesion. The needle typically projects out 2.5 cm when the spring is activated. Multiple insertions are used to obtain the tissue needed compared with the single insertion of a vacuum device. The vacuum-assisted device is generally placed into or below the lesion (see the figures), and cores of tissue are taken after the vacuum device has “pulled” the tissue into the cutting slot of the needle (see the figures). Because there is no “throw” of the needle, there is a lower chance of the needle inadvertently penetrating the chest wall or lung. The vacuum device samples are larger for the same gauge needle compared with the automated device cores.
With image-guided biopsy, there is a possibility of false-negative diagnosis. The false-negative rate of ultrasound-guided biopsy is very low, 0.5% to 1%, whether using a 14-gauge automated core device or larger vacuum-assisted device. There is also the possibility of underestimation of disease, whether obtaining a core diagnosis of atypical ductal hyperplasia when ductal carcinoma in situ exists or obtaining a core diagnosis of ductal carcinoma in situ when invasive disease is present. The underestimation rate is lower (36% vs. 55% in one large series) for the vacuum-assisted device compared with the automated spring-activated device for calcified lesions. For this reason, the vacuum-assisted device is preferred when performing biopsy for calcifications, using either stereotactic or ultrasound guidance.
This patient’s mass was a fibroadenoma.
CASE 103
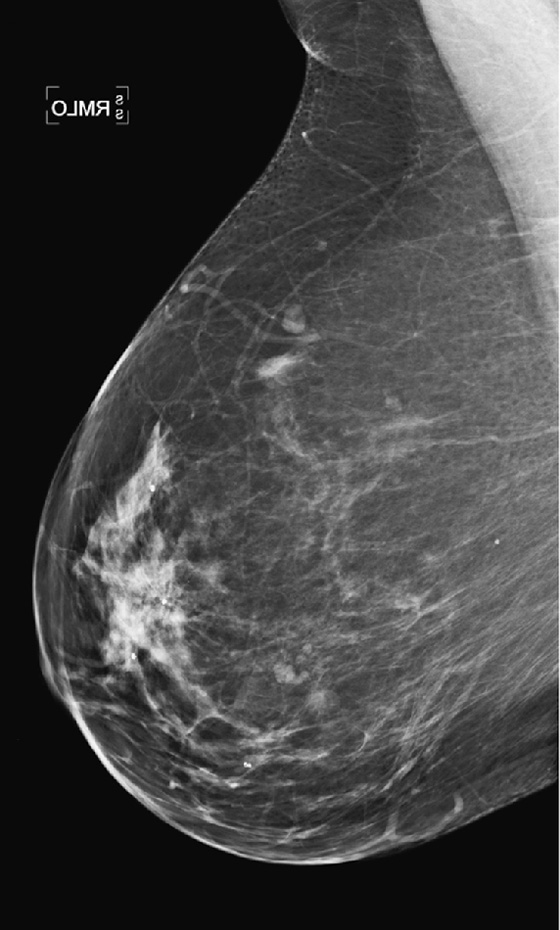
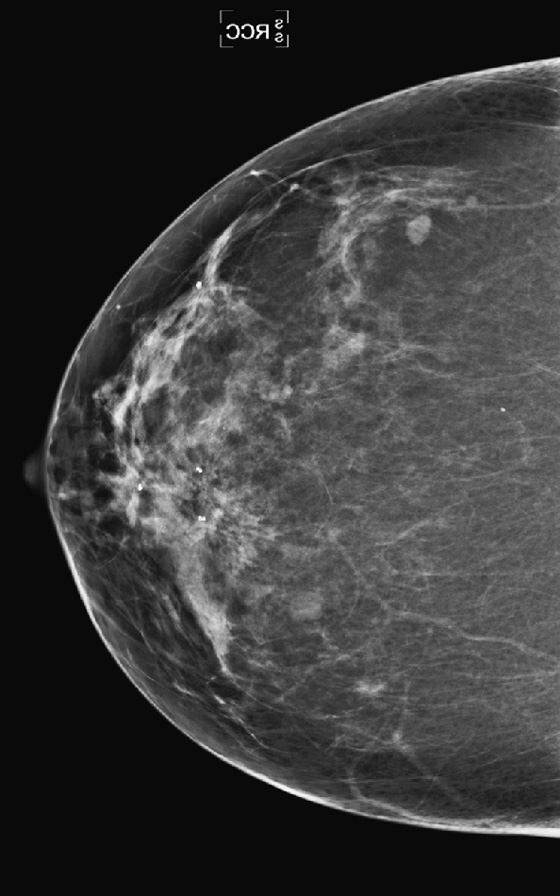
History: A 53-year-old woman with no history of prior breast surgery undergoes routine screening mammogram.
1. What should be included in the differential diagnosis for the distortion in the right upper breast? (Choose all that apply.)
A. Infiltrating ductal carcinoma
C. Infiltrating lobular carcinoma
2. If this finding were stable over many years and at the site where a benign biopsy had been performed, what would be your BI-RADS (Breast Imaging Reporting and Data System) score of this mammogram?
D. BI-RADS 5—highly suspicious
3. What is the next step in management of this finding on screening mammogram?
A. MRI to check for abnormal enhancement
B. Spot compression views or spot magnification views
C. Follow-up mammogram at 6 months
D. Referral to a surgeon for excision
4. After additional views, the distortion is more obvious, and distortion is also seen on ultrasound. What is the next step?
A. Referral to a surgeon for excision
B. Needle core biopsy with vacuum-assisted technique
C. Fine-needle aspiration biopsy using ultrasound
D. Needle core biopsy using the smallest gauge needle available, owing to risk of bleeding
ANSWERS
CASE 103
Radial Scar
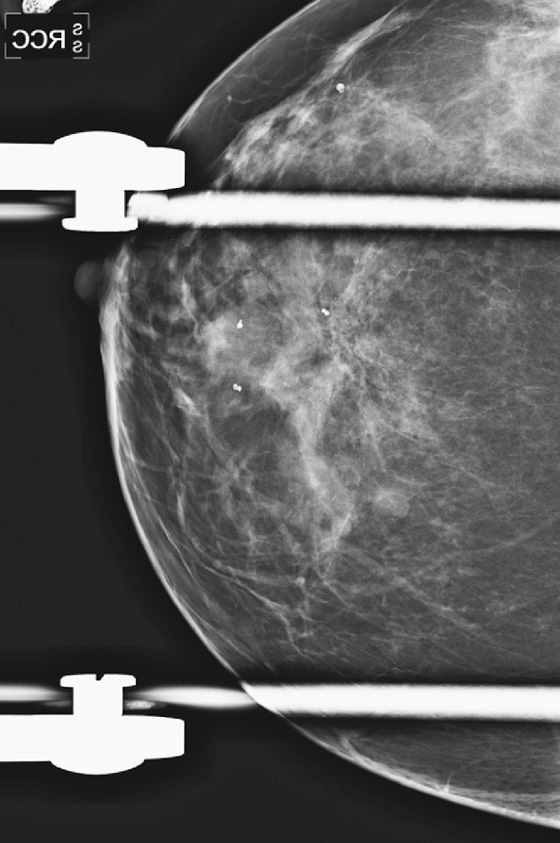
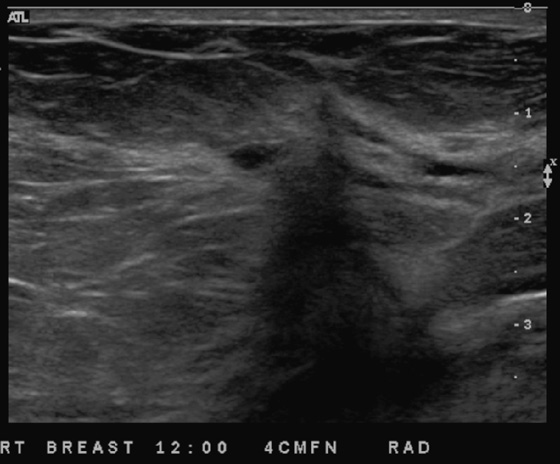
1. A, B, and C
2. B
3. B
4. B
References
Brenner RJ, Jackman RJ, Parker SH, et al. Percutaneous core needle biopsy of radial scars of the breast: when is excision necessary?. AJR Am J Roentgenol. 2002;179(5):1179–1184.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:111.
Comment
Radial scar is a pathologic abnormality consisting of a central sclerotic core, with radiating bands of proliferating ducts and lobules, which may entrap surrounding fat. A radial scar is also called a complex sclerosing lesion. Atypical cells may be within the benign stromal cells. The radiating bands of stromal cells cause the spiculated appearance on mammography, an appearance that overlaps with invasive carcinoma (see the figures).
When a spiculated mass is seen and there is no history of benign surgery at the site, work-up should proceed with additional spot compression or magnification views (see the figures), and histology must be obtained. A radial scar is a benign lesion but is associated with atypia and malignancy: atypical ductal hyperplasia, ductal carcinoma in situ, atypical lobular hyperplasia, and lobular carcinoma in situ. In the past, surgical excision was recommended instead of needle core biopsy because of fear of sampling error, missing the associated atypia, or malignancy. In addition, the lesion can be misinterpreted as a tubular carcinoma by the pathologist because of similar features. It is also thought that the lesion may be a precursor to tubular carcinoma.
Because invasive carcinoma is a possibility with this appearance, performing needle biopsy before surgery is beneficial. Knowledge of the pathology before definitive surgery allows the surgeon to plan to perform the necessary excision and possible lymph node surgery in one step, rather than needing multiple surgical procedures. Core biopsy should be performed with vacuum assistance (9-gauge, 10-gauge, or 11-gauge needle) with multiple cores taken to increase the diagnostic yield of the biopsy. Ultrasound can be used to guide the biopsy if the lesion is seen. These lesions often appear subtle on mammography and may be better seen on ultrasound.
This patient underwent needle core biopsy with vacuum assistance, and histology showed a complex sclerosing lesion. The lesion was excised after a needle localization procedure. Final histology of the excised tissue included markedly atypical lobular hyperplasia, bordering lobular carcinoma in situ, and a complex sclerosing lesion (radial scar).
CASE 104
History: A 46-year-old woman underwent breast MRI for screening because of a strong family history of early-onset breast cancer.
1. What should be included in the differential diagnosis for the MRI views and time-intensity curve shown? (Choose all that apply.)
2. What do the colors on a computer-assisted detection (CAD) image of the breast denote?
D. Suspicious shapes of masses
3. What is the effect of CAD on breast MRI interpretation?
A. Increased sensitivity for suspicious lesions
B. Increased time spent in reviewing hundreds of images for each MRI examination of the breast
C. Decreased biopsy rate of benign breast lesions
D. Increased specificity for breast cancer detection
4. What is the role of breast MRI in screening?
A. All women should be screened with MRI because of low sensitivity of mammography.
C. Screening with MRI is reserved only for women who have had breast cancer previously.
D. Screening MRI is best used in women with fatty breasts on mammography.
ANSWERS
CASE 104
MRI of Breast Carcinoma
1. A, B, and C
2. C
3. A
4. B
References
Bassett LW, Dhaliwal SG, Eradat J, et al. National trends and practices in breast MRI. AJR Am J Roentgenol. 2008;191(2):332–339.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:239.
Comment
Breast MRI has been shown to detect malignancies not detected on standard imaging with mammography and ultrasound. Because of its high cost, need for intravenous contrast agent and the inherent risk of the contrast agent, and other medical reasons for not tolerating MRI, use of MRI is best confined to patients at increased risk. The American Cancer Society has published guidelines for the groups of women who should be recommended for screening MRI. These include women with BRCA1 or BRCA2 mutation or first-degree relative with this mutation; 20% to 25% or greater lifetime risk for breast cancer; radiation to the chest between ages 10 and 30; history of Li-Fraumeni syndrome, Cowden disease, or Bannayan-Zonana syndrome; or first-degree relative with the aforementioned syndromes.
In this patient, a round mass with irregular margins was detected in the left breast on screening MRI (see the figures). The left breast 12 o’clock region was subsequently evaluated with ultrasound, and a suspicious lesion was found. Biopsy with needle core technique was performed. Histology showed well-differentiated invasive ductal carcinoma with tubular features. Mammography was negative, even after the location of the tumor was known.
MRI has been shown to increase the detection of breast cancer compared with mammography and ultrasound. Sensitivity of MRI is reported to be 94% to 100%, sensitivity of mammography alone is 25% to 59%, and sensitivity of mammography plus ultrasound is approximately 49% to 67%. The specificity of MRI is lower and may necessitate additional studies and biopsies. The use of CAD can increase the efficiency and sensitivity of the MRI reading because color coding is applied to breast lesions, allowing rapid assessment of the kinetics of the lesion. In the CAD system shown here, red color is applied to lesions with a rapid wash-in, wash-out curve; green color is assigned to lesions that have a plateau; and blue color is assigned to lesions that have persistent enhancement over time. This patient has a malignant mass with red on color assignment, indicating rapid wash-out.