CASE 8 STEMI Intervention and Stent Thrombosis
Cardiac catheterization
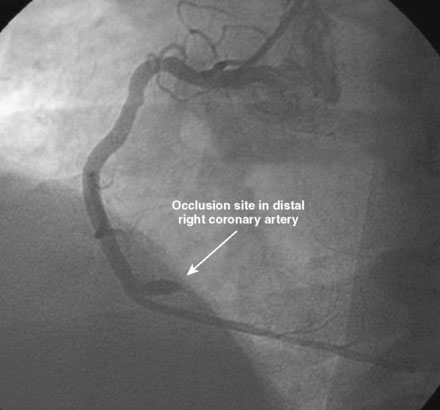
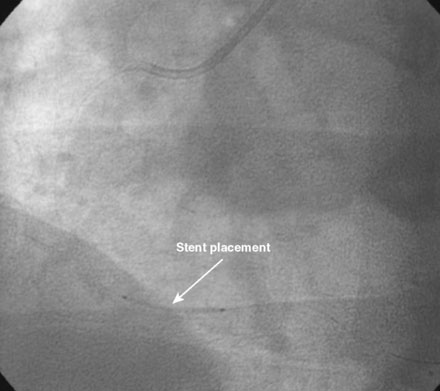
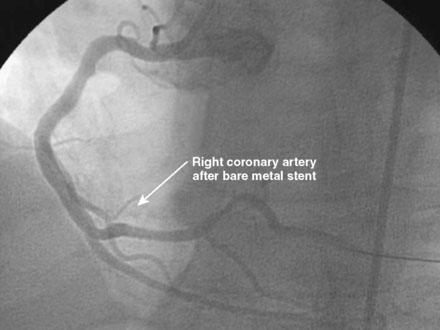
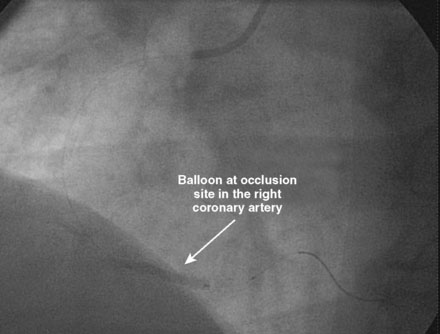
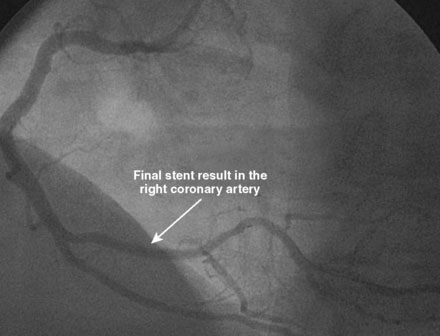
An ACT was measured and additional heparin administered to achieve an ACT between 250 and 300 seconds. Based on the ECG, the right coronary artery was the suspected infarct artery and was engaged first using a 6 French JR4 guiding catheter. Right coronary angiography revealed occlusion of the distal vessel, with retained contrast at the occlusion site (Figure 8-1 and Video 8-1). Eptifibatide was administered (two boluses, each of 180 mcg per kg, and an infusion of 2.0 mcg/kg per minute for 14 hours). The operator crossed the occlusion with a 0.014 inch floppy-tipped guidewire and TIMI-3 flow was restored after balloon dilatation with a 2.5 mm diameter by 15 mm long, compliant balloon. Following this, a 3.0 mm diameter by 23 mm long bare-metal stent was selected based on visual determination of the proximal and distal reference segments (Figure 8-2 and Video 8-2). A bare-metal stent was selected due to uncertainty about future medication compliance. The stent was positioned across the narrowed arterial segment and deployed at 14 atmospheres of pressure. The operator achieved a satisfactory angiographic result (Figure 8-3 and Video 8-3); intravascular ultrasound was not used.
Postprocedural course
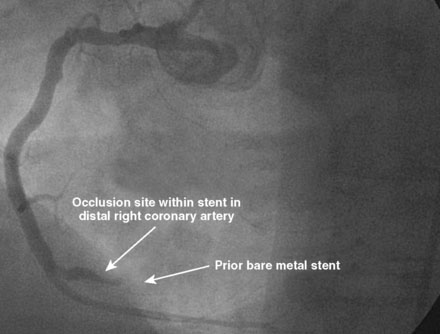
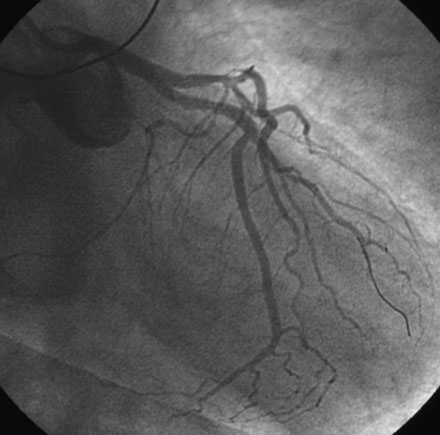
Three months later, the patient again presented to the emergency department after suffering chest pains for 5 hours. The initial ECG demonstrated 3 to 4 mm ST elevations in leads 2, 3, and aVF with Q waves in those leads. The patient had not kept his previous medical follow-up appointments and admitted to stopping all of his medications after running out of prescriptions 3 weeks prior to the current presentation. Again, he was promptly treated in the emergency department with aspirin (325 mg), clopidogrel (600 mg), and unfractionated heparin (60 U/kg) and brought emergently to the cardiac catheterization laboratory. Right coronary angiography confirmed the suspected occlusion at the site of the previously-placed bare-metal stent (Figure 8-4 and Video 8-4). Adjunctive pharmacology consisted of therapeutic levels of unfractionated heparin along with eptifibatide double bolus and infusion. The operator crossed the occluded stent with a conventional, 0.014 inch floppy-tipped guidewire, taking great care to avoid passing the wire behind stent struts. Similar to the first event, TIMI-3 flow was promptly restored after balloon dilatation with a 2.5 diameter by 15 mm long, compliant balloon (Figure 8-5 and Video 8-5). The operator chose to further expand the stent with a 3.0 mm diameter by 20 mm long, noncompliant balloon inflated to 18 atmospheres of pressure. Angiography confirmed the lack of any distal embolization and an acceptable angiographic result (Figure 8-6). Hemostasis was achieved with a closure device and the patient was transferred to the coronary care unit. Again, the patient was treated during “off hours” with a door-to-balloon time of 74 minutes.
Discussion
The approach to PCI reperfusion has been a rapidly evolving field. There is increasingly compelling data to suggest that thrombus extraction with a simple aspiration catheter improves clinical outcomes prior to stenting for acute ST-elevation MI.1 This was not done in our laboratory at the time this case occurred but has currently become the preferred approach, when technically feasible. Similarly the optimal adjunctive pharmacologic therapy is in a state of evolution. In this case, unfractionated heparin and eptifibatide were used, but other heparins and GP IIb/IIIa inhibitors are also appropriate. The HORIZON trial has established the role of bivalirudin without routine GP IIb/IIIa inhibitors in such cases.2 Although early stent thrombosis was increased with bivalirudin, this was not significant at later time points and the reduction in bleeding with bivalirudin was associated with decreased mortality at 1 year in patients with STEMI. In a separate publication, the HORIZON’s investigators also established the role of drug-eluting stents in ST-elevation MI and showed significantly reduced angiographic evidence of restenosis and recurrent ischemia necessitating repeat revascularization procedures with no safety concerns apparent at 1 year with drug-eluting stents.3 The challenge of such cases is to establish the bleeding risk and patient compliance issues during the “fast-forward” environment of an acute PCI. In this case, there were concerns raised regarding compliance with medications and a bare-metal stent was selected.
This patient did well for 2 months, but then became noncompliant with his follow-up and stopped taking all his medications, including aspirin and clopidogrel. Predictably, he developed late stent thrombosis within 3 weeks. The role of aspirin and clopidogrel (dual antiplatelet therapy) in the prevention of late stent thrombosis is well established. Clopidogrel has replaced ticlopidine as the ADP-receptor blocker of choice due to its improved safety profile. However, there is a defined incidence of clopidogrel resistance which results from multiple factors including its complex pharmacology and need for conversion from a pro-drug to its active form. Stent thrombosis has been associated with clopidogrel resistance as measured by platelet aggregation studies and with bedside platelet monitoring. Newer agents such as prasugrel (Triton TIMI 38) and ticagrelor (PLATO) may have improved efficacy against stent thrombosis.4
Stent thrombosis complicates approximately 2% of coronary interventional procedures and is associated with significant morbidity and mortality and a high risk of myocardial infarction. Certain situations are known to have increased risk of stent thrombosis including unstable angina, diabetes mellitus, low ejection fraction, renal failure, small vessel diameter, long lesions (with multiple stents), bifurcation lesions, downstream poorly-functioning myocardium, residual uncovered dissection, poor distal runoff, and suboptimal final postprocedure lumen. Proper stent deployment with optimal sizing and avoidance of malapposition are important technical factors in avoiding stent thrombosis. Intravascular ultrasound may be helpful in defining risk factors for stent thrombosis, including improper sizing, stent malapposition, and edge dissections. Whether the “on label” use of drug-eluting stents treated with dual antiplatelet therapy results in increased late and very late thrombosis compared with bare-metal stents remains uncertain.5
1 Svilaas T., Vlaar P.J., van der Horst I.C., Diercks G.F.H., de Smet B.J.G.L., van den Heuvel A.F.M., Anthonio R.L., Jessurun G.A., Tan E.S., Suurmeijer A.J.H., Zijlstra F. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358:557-567.
2 Stone G.W., Witzenbichler B., Guagliumi G., Peruga J.Z., Brodie B.R., Dudek D., Kornowski R., Hartmann F., Gersh B.J., Pocock S.J., Dangas G., Wong S.C., Kirtane A.J., Parise H., Mehran R., the HORIZONS-AMI Trial Investigators. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358:2218-2230.
3 Stone G.W., Lansky A.J., Pocock S.J., Gersh B.J., Dangas G., Wong S.C., Witzenbichler B., Guagliumi G., Peruga J.Z., Brodie B.R., Dudek D., Mockel M.O., Andrzej K., Alison P.H., Mehran R., the HORIZONS-AMI Trial Investigators. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med. 2009;360:1946-1959.
4 Wallentin L., Becker R.C., Budaj A., Cannon C.P., Emanuelsson H., Held C., Horrow J., Husted S., James S., Katus H., Mahaffey K.W., Scirica B.M., Skene A., Steg P.G., Storey R.F., Harrington R.A., the PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-1057.
5 Marroquin O.C., Selzer F., Mulukutla S.R., Williams D.O., Vlachos H.A., Wilensky R.L., Tanguay J.F., Holper E.M., Abbott J.D., Lee J.S., Smith C., Anderson W.D., Kelsey S.F., Kip K.E. A comparison of bare-metal and drug-eluting stents for off-label indications. N Engl J Med. 2008;358:342-352.