CHAPTER 8. SLEEP
Catherine Vena
Sleep is an essential human need and an important component of human homeostasis. Despite an explosion of scientific discovery about sleep in the past 50 years, there is much to be discovered about the role of sleep in health and illness. However, research has shown that the lack of adequate sleep results in poor physical and mental function (Pilcher & Huffcutt, 1996). Because sleep has also been found to be important for immune, endocrine, and metabolic functions, insufficient or poorly timed sleep affects health and well-being (Akerstedt & Nilsson, 2003). Yet sleep problems are common in chronic illnesses such as cancer (Clark, Cunningham, McMillan, et al., 2004; Vena, Parker, Cunningham et al., 2004), cardiopulmonary disease (Gay, 2004; Parker & Dunbar, 2002), and neurological disorders such as Parkinson’s disease (Rye, 2004) and Alzheimer’s disease (Ancoli-Israel, Gehrman, Martin et al., 2003). Furthermore, sleep is frequently disturbed in populations characterized by chronic pain (Landis, Frey, Lentz et al., 2003) and is often associated with psychosocial distress such as anxiety, depression, or worry (Bixler, Vgontzas, Lin et al., 2002; Hall, Buysse, Nowell et al., 2000; Morin, Rodrigue, & Ivers, 2003). For the person experiencing a chronic or life-threatening illness, disturbed sleep has important implications for clinical outcomes. The sleep of family members, especially those who are caregivers for the chronically and terminally ill, is also at risk for being disrupted or inadequate due to either the patient’s sleep problems or the burdens of caregiving, which places the family unit at risk for adverse outcomes (Carter, 2002, 2003). Therefore, it is important that advanced practice nurses (APNs) be proactive in assessing and promoting sleep in their palliative care practice. This chapter reviews normal sleep and sleep regulation, discusses factors that lead to disturbed sleep, and presents evidence-based interventions to promote sleep.
OVERVIEW OF NORMAL SLEEP
Despite the fact that a third of human existence is spent in the sleep state, there is little understanding of why sleep is needed or what mechanisms underlie its capacities for physical and mental restoration. As late as the early twentieth century, sleep was believed to be a passive, quiescent state intermediate between wakefulness and death. However, the discovery of rapid eye movement (REM) and the identification of the dramatic effects of sleep deprivation in the mid-twentieth century prompted an escalation in research concerning the physiology, neurobiology, and biobehavioral aspects of sleep (Akerstedt & Nilsson, 2003). It is now known that sleep is an active process characterized by a distinct architecture and rhythmicity. There are actually three discrete, functional states of the brain: waking, non-REM (NREM) sleep, and REM sleep. The cycling of these states is at two levels: the basic wake/sleep cycle and the within-sleep cycle of REM and NREM sleep. A complex array of behavioral, neuroendocrine, and central nervous system factors regulates these cycles. In optimal conditions, waking is consolidated during the day and sleeping is consolidated at night. In the presence of pathology or physical illness, it is possible to see intrusion of wakefulness into the sleeping period and, conversely, intrusion of sleep into the wake period.
Normal sleep consists of a sequence of various stages distinguished by electroencephalographic (EEG) activity, muscle tone, and eye movements (Silber, Krahn, & Morgenthaler, 2004). Sleep is initiated after an initial stage of drowsiness. The first stage, NREM sleep, is divided into stages 1 through 4, roughly corresponding to sleep depth. Each stage has its own “signature” of EEG activity. Stage 1 is characterized by mixed-frequency low-amplitude waves; stage 2 is characterized by K complexes and spindles; and stages 3 and 4 are characterized by progressively greater numbers of slow, high-amplitude waves. Stages 3 and 4 are also collectively termed slow-wave sleep (SWS) and are associated with the highest arousal threshold. REM sleep is characterized by EEG activity somewhat similar to stage 1 or wakefulness and by episodic bursts of eye movements and a loss of muscle tone. While often associated with dreaming, it is now known that dreaming can also occur during NREM sleep. Normal sleep architecture across the night consists of NREM alternating with REM sleep in approximately 90- to 120-minute cycles. A young adult will typically have four to six of these cycles as illustrated in Figure 8-1. Most SWS generally occurs during the first half of the night, while more REM sleep occurs during the second half. Stage 1 sleep represents 2% to 5% of total sleep; stage 2, 45% to 55%; and stages 3 and 4, about 13% to 23%. Typically, REM sleep accounts for 20% to 25% of total sleep (Carskadon & Dement, 2005). Brief arousals punctuate normal sleep, although only the longest of these are remembered. Arousals tend to increase in frequency as sleep quality deteriorates. Common terms used in conjunction with sleep are listed in Box 8-1.
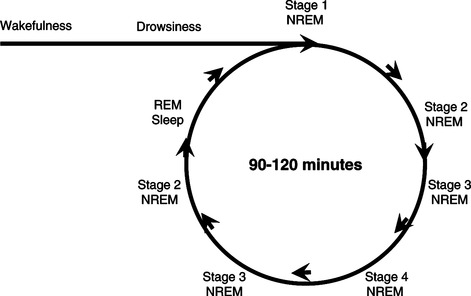 |
| Figure 8-1 |
Box 8-1
Apnea Cessation of airflow at the nostrils and mouth lasting at least 10 seconds.
Arousal Abrupt change from sleep to wakefulness, from a “deeper” stage of non-REM sleep to a “lighter” stage.
Circadian rhythm Innate, daily fluctuation of behavioral and physiological functions, including sleeping and waking, generally tied to the 24-hour day-night cycle but sometimes to a different (e.g., 23- or 25-hour) periodicity when light/dark and other time cues are removed.
Excessive daytime sleepiness Difficulty in maintaining the alert, awake state.
Insomnia Sleep problems characterized by difficulty falling asleep, frequent awakenings during the night, or waking up earlier than desired that can result in getting up in the morning feeling nonrested and experiencing drowsiness during the day. Insomnia may be primary or secondary to other conditions.
K complex Sharp, negative, high-voltage EEG wave, followed by a slower, positive component. K complexes occur spontaneously during NREM sleep and define stage 2.
Light sleep Term used to describe non-REM sleep stage 1 and sometimes stage 2.
NREM sleep Non–rapid eye movement sleep; divided into four stages, 1 through 4. A stage of sleep in which brain activity and bodily functions slow down. NREM sleep accounts for the largest portion of the sleep cycle.
Periodic limb movement disorder Disorder characterized by periodic episodes of repetitive and highly stereotyped limb movements that occur during sleep.
Polysomnogram (PSG) Gold standard for objective measurement of sleep. PSG involves the continuous and simultaneous recording of physiological variables during sleep, i.e., EEG, EOG, EMG (the three basic stage scoring parameters), ECG, respiratory air flow, respiratory excursion, lower limb movement, and other electrophysiological variables.
REM density Function that expresses the frequency of eye movements per unit of time during REM sleep.
REM sleep Rapid eye movement sleep. REM sleep occurs in brief spurts of increased activity in the brain and body. REM is considered the dreaming stage of sleep. It is characterized by the darting of the eyes under the eyelids.
Restless legs syndrome (RLS) Disorder characterized by sensations in the legs that tend to occur when an individual is not moving and are usually worse in the evening. There is an almost irresistible urge to move the legs; the sensations are relieved by movement.
Sleep apnea syndrome Disorder characterized by repetitive episodes of reduced or absent respiratory airflow that occur during sleep and that are usually associated with a reduction in blood oxygen level. Symptoms include loud snoring and a gasping or snorting sound when the sleeping individual starts to breathe again. Although the individual may not be aware of having sleep apnea, the condition can disrupt the quality of sleep and result in daytime fatigue. The most common type, obstructive sleep apnea, occurs when the tongue or other soft tissue blocks the airway.
Sleep architecture NREM-REM sleep-stage and cycles.
Sleep debt Result of recurrent sleep deprivation that occurs over time when an individual does not experience a sufficient amount of the restorative daily sleep that is required to maintain a sense of feeling rested and refreshed.
Sleep deprivation Acute or chronic lack of sufficient sleep.
Sleep diary (log) Daily, written record of an individual’s sleep-wake pattern containing such information as time of retiring and arising, time in bed, estimated total sleep period, number and duration of sleep interruptions, quality of sleep, daytime naps, use of medications or caffeine beverages, nature of waking activities, and other data.
Sleep disorders Broad range of illnesses arising from many causes, including dysfunctional sleep mechanisms, abnormalities in physiological functions during sleep, abnormalities of the biological clock, and sleep disturbances that are induced by factors extrinsic to the sleep process.
Sleep efficiency (SE) Proportion of sleep in the episode filled by sleep; the ratio of TST to time in bed.
Sleep fragmentation Brief arousals occurring throughout the night, reducing the total amount of time spent in the deeper levels of sleep.
Sleep latency (SL) Time period measured from “lights out,” or bedtime, to the beginning of sleep.
Sleep onset Transition from wake to sleep, normally into NREM stage 1.
Sleep pattern (24-hour sleep-wake pattern) Individual’s clock hour schedule of bedtimes and rise times as well as nap behavior: may also include time and duration of sleep interruptions.
Slow-wave sleep (SWS) Common term for NREM stages 3 and 4 (also called delta or deep sleep).
Total sleep time (TST) Amount of actual sleep time in a sleep episode; the time is equal to the total sleep episode less the awake time.
Zeitgeber Environmental time cue that entrains biological rhythms to a specific periodicity. Known Zeitgebers are light, melatonin, and physical activity.
Data from Kryger, M.H., Roth, T., & Dement, W.C. (Eds.). (2005) Principles and practice of sleep medicine (4th ed.). Philadelphia: Elsevier Saunders; American Academy of Sleep Medicine (2005). The international classification of sleep disorders: Diagnostic and coding manual, ICSD-2 (2nd ed.). Westchester, Ill: Author; and Silber, M.H., Krahn, L.E., & Morgenthaler, T.I. (2004). Sleep medicine in clinical practice, London: Taylor & Francis.
As humans age, the variabilities in sleep architecture and sleep-wake patterns increase. In general, relative to younger people, older people frequently have difficulty maintaining sleep and experience early morning awakenings, resulting in more time spent in bed relative to the amount of sleep. They also have more stage 1 sleep and less stage 2, 3, and 4 sleep. While total REM time decreases by a small percentage, the proportion of time spent in REM sleep to total sleep remains relatively unchanged into healthy old age (Bliwise, 2005). Objective changes in sleep architecture occur earlier and are more pronounced in men; however, women are more likely to voice subjective sleep complaints (Blazer, Hays, & Foley, 1995; Middelkoop, Smilde-van den Doel, Neven et al., 1996).
Normal aging plays a role in changes in sleep, but there is also evidence that psychosocial factors and medical illness may be significant contributors to disturbed sleep in the elderly. In a large survey, elders’ sleep complaints were associated with respiratory symptoms, physical disability, depressive symptoms, and poor perceived health (Foley, Monjan, Brown et al., 1995). Other studies have demonstrated that in the absence of psychological, medical, and social factors that affect sleep, sleep problems in elders were much less frequent or even nonexistent (Bliwise, King, Harris et al., 1992; Ford & Kamerow, 1989; Gislason & Almqvist, 1987; Vitiello, Moe, & Prinz, 2002).
The Function of Sleep
A period of prolonged sleep loss is most likely to precipitate overwhelming sleepiness, which suggests that sleep is appetitive and fulfills essential needs. Despite the rapid increase in understanding of sleep physiology and pathology, the exact function of sleep remains unknown. Research has shown that REM and NREM sleep may serve specific biological functions. When individuals are restricted from entering REM sleep, they tend to spend longer periods in REM sleep during their next sleeping period. Furthermore, these REM periods are more intense and have more eye movements per minute than normal REM sleep. Similarly, individuals deprived of NREM sleep spend more time in NREM sleep during a recovery sleep period. EEGs measuring brain activity show that this “rebound” NREM sleep also differs from normal NREM sleep. This research suggests that the body needs adequate levels of both REM and NREM sleep and that the two kinds of sleep serve different biological purposes (Finelli, 2005).
While the exact function of sleep remains unclear, there are a number of theories. Some have suggested that sleep, especially NREM sleep, is necessary to reverse and/or restore biochemical and/or physiological processes through protein synthesis, cell division, and growth. Others have postulated that sleep serves to conserve energy through reduced metabolic rate and body temperature (Silber et al., 2004). There have also been numerous speculations on the functions of REM sleep, including brain restoration, consolidation of memory, and erasure of inappropriate memories (Siegel, 2001). Sleep also appears to be important for immune and endocrine function, metabolism, and thermoregulation (Krueger, Majde, & Obal, 2003; McGinty, Alam, Szymusiak et al., 2001; Steiger, 2003; Van Someren, 2000). None of these theories is supported unequivocally by current evidence. Most likely, sleep serves many functions.
Sleep Regulation
A basic understanding of the mechanisms that regulate sleep is necessary to appreciate how the sleep-wake cycle may be disrupted in palliative care patients. Two basic processes govern physiological sleepiness and wakefulness: process S and process C (Borbely, 1982). Process S is the homeostatic pressure to sleep that increases with the length of time an individual is awake and is eliminated by sleep. Process C, the circadian rhythm, is regulated by a clocklike mechanism in the brain (located in the suprachiasmatic nucleus). Process C represents the drive for wakefulness that is lowest in the early morning hours and highest at mid-day. The natural sleep-wake rhythm cycle is about 25 hours. However, cues from the environment (zeitgebers) entrain or “set” sleep’s rhythm to a 24-hour schedule. As a result, humans depend on external cues such as exposure to light, regular meals, and social interactions to keep their diurnal cycle “on time.”
Sleep propensity, sleep structure, and waking are regulated by a subtle and complex interaction of the two processes. Process C serves to maintain sleep as process S declines during the night and to maintain alertness as process S increases during the day (Borbely & Achermann, 2005). NREM sleep intensity is determined primarily by the homeostatic process (process S), and REM sleep by the circadian process (process C), while the ratio of REM to NREM sleep depends on both homeostatic and circadian factors. Factors that either interfere with or support these processes have the potential to significantly affect the timing, duration, and structure of sleep as well as daytime functioning.
EFFECTS OF IMPAIRED SLEEP
Impaired sleep may be the result of an inadequate amount of sleep or frequent disruption or fragmentation of sleep. The amount of sleep each person needs depends on many factors, including age. For most adults, 7 to 8 hours a night appears to be the best amount of sleep, although some people may need as few as 5 hours or as many as 10 hours of sleep each day (National Institute of Neurological Disorders and Stroke, 2005). The most common causes for inadequate amounts of sleep are self-imposed sleep restriction or lifestyle or work demands that require staying awake at night (Lee, Landis, Chasens et al., 2004). Caregivers are especially at risk for this type of sleep loss (Carter, 2002, 2003). Getting too little sleep creates a “sleep debt,” which eventually demands repayment. Contrary to popular belief, humans do not adapt to getting less sleep than needed. Although individuals may become accustomed to a sleep-depriving schedule, adverse effects remain apparent. Sleep fragmentation can occur because of environmental conditions, but it is frequently the consequence of acute or chronic illness or primary sleep disorders. Both inadequate amounts of sleep and frequently interrupted sleep result in sleep loss, a condition with known adverse consequences. In addition to daytime sleepiness, sleep loss has been shown to produce impairment in cognitive function, including reduced attention, short-term memory, and problem-solving ability (Bonnet, 2005). Sleep fragmentation can also result in elevation of blood pressure, increases in urinary and serum catecholamines (an indicator of acute stress), arrhythmias, and progression of heart failure (Leung & Bradley, 2001; Sin, Logan, Fitzgerald et al., 2000). In addition, sleep loss has been shown to produce a catabolic state leading to negative nitrogen balance, alter immune function, increase oxygen consumption and carbon dioxide production, and disrupt thermoregulation (Bonnet, 2005). Thus, the lack of consolidated, restorative sleep places the palliative care patient and their caregivers at risk for adverse outcomes.
Factors Contributing to Disturbed Sleep in the Palliative Care Setting
Disturbed sleeping and waking are usually multifactoral. Some factors such as a history of primary sleep disorder and demographic or lifestyle factors predispose the patient to further problems with sleep when confronted with a chronic or life-threatening illness. Other factors, such as psychosocial, disease-related, and treatment-related factors, precipitate sleep-wake disturbance through further interference with sleep regulatory processes (process S and process C).
PRIMARY SLEEP DISORDERS
From population-based surveys, the National Sleep Foundation (NSF) estimates that at least 34% of Americans are at risk for primary sleep disorders such as insomnia, sleep apnea, and restless legs syndrome (NSF, 2005a). This means that tens of millions of Americans have undiagnosed and untreated sleep disorders. In addition, the average amount of sleep obtained by adults in this country is 6.9 hours per weeknight. Seventy-one percent of adults say they sleep less than 8 hours per night; 40% report sleeping less than 7 hours (NSF, 2005a). Thus, a significant number of patients seen in a palliative care practice may have a preexisting sleep disorder or chronic sleep deprivation, conditions that could worsen the effect of numerous precipitating factors associated with their disease process or treatments.
Demographic Factors
Researchers have identified increased reports of sleep problems in people who are older, female, single, and of lower socioeconomic status (Bliwise, 2005; Breslau, Roth, Rosenthal et al., 1997; Moore, Adler, Williams et al., 2002; Steptoe & Marmot, 2003). Of these factors, age appears to have the most significant impact on sleep. Elders have increased fragmentation of sleep, decreased amounts of deep sleep, and increased daytime napping that are linked to age-related changes that alter both homeostatic (process S) and circadian (process C) cycles, including (1) nocturia, (2) elevated autonomic activity that results in a greater susceptibility to arousal, and (3) decreased strength of circadian rhythms (Bliwise, 2005). Furthermore, primary sleep disorders such as sleep apnea (Young, Skatrud, & Peppard, 2004), periodic limb movements during sleep (Montplaisir, Allen, Walters et al., 2005), and insomnia are more prevalent in the elderly (Benca, Ancoli-Israel, & Moldofsky, 2004). While older men have more objective changes in sleep architecture, older women are more likely to complain of sleep difficulties (Rediehs, Reis, & Creason, 1990). Thus, elders in the palliative care setting may be at greater risk for disturbed sleep.
Lifestyle Factors
Lifestyle behaviors can influence quantity and quality of sleep (Lee-Chiong, 2002). Two factors that can interfere with both process S and process C are (1) the timing and duration of sleep periods and (2) conditions or agents that cause arousal in the sleep setting. Because the homeostatic drive for sleep is influenced by the amount of prior sleeping or waking, long naps taken during the daytime hours, especially late in the day, decrease the drive to sleep during the nocturnal sleep period (Monk, Buysse, Carrier et al., 2001). In addition, irregular bedtimes, staying longer in bed, and decreased daytime activity interfere with circadian activity-rest patterns (Lee-Chiong, 2002). Maintaining regular bed and rise times, as well as regular daily activity, enhances nocturnal sleep. Environmental factors and ingestion of common substances can interfere with process S by eliciting arousal. Room temperature, noise, and light level, as well as use of stimulants (such as caffeine in coffee and soft drinks and nicotine in tobacco products), can interfere with sleep onset or sleep continuity. Although alcohol ingestion is generally associated with sedation, falling blood alcohol levels produce sympathetic arousal so that sleep continuity after ingestion becomes a problem (Gillin, Drummond, Clark et al., 2005). These behaviors or conditions in combination can produce a profound effect on sleep.
Psychosocial Factors
Anxiety and depression are commonly encountered in palliative care practices. Researchers have found that anxiety related to a general stress burden is associated with numerous changes in sleep architecture, including a reduced amount of total sleep time, difficulty getting to sleep, reduced SWS, increased microarousals and stage 1 sleep, and reduced REM density (Hall et al., 2000; Kecklund & Akerstedt, 2004). These findings suggest that anxiety can interfere with process S by causing increased physiological arousal. Disturbed sleep is also a key feature of depression. Sleep in persons diagnosed with major depression and dysthymia is characterized by changes in sleep architecture including decreased SWS, reduced REM latency, prolonged first REM period, and higher REM density (total eye movements/total REM time), as well as by sleep continuity disturbances and excessive daytime sleepiness (Benca, 2005). It has been hypothesized that these changes in sleep architecture may be due to deficiencies in process S (Borbely & Wirz-Justice, 1983) and changes in the circadian rhythm of body temperature (Avery, Wildschiodtz, Smallwood et al., 1986; Schultz & Lund, 1983). However, the role of depression and anxiety in sleep disturbance is not always clear. Increasing data indicate that sleep problems often precede clinical depression and anxiety (Breslau, Roth, Rosenthal et al., 1996; Ohayon & Roth, 2003).
Disease-Related Factors Chronic Illness
Many persons with chronic illnesses, including cardiac diseases, lung diseases, renal failure, and cancer, report sleep problems (Parker, Bliwise, & Rye, 2000; Parker & Dunbar, 2002; Serber, Sears, Sotile et al., 2003; Silber et al., 2004; Vena et al., 2004). Common complaints among patients with these conditions include difficulty getting to sleep, frequent interruptions in sleep, daytime sleepiness, and fatigue. These complaints are likely the result of the disease process or treatments that have the potential to disturb sleep regulatory processes.
Pain
Disturbed sleep is a key complaint of patients experiencing both acute and chronic pain (Roehrs & Roth, 2005). Researchers have discovered that areas in the brain that process pain signals also regulate NREM sleep. This phenomenon may at least partially explain the interaction between pain and poor sleep (Basbaum & Jessell, 2000). Persons with pain have reduced amounts of SWS and often experience fragmented sleep related to the arousing property of pain (Lavigne, Zucconi, Castronovo et al., 2000). These findings suggest alterations in process S.
Sleep loss can also affect pain perception. Persons deprived of SWS or undergoing a cumulative sleep deficit (33% reduction in habitual amount) demonstrate a lowered pain threshold and increased somatic complaints (headache, sore joints) (Dinges, Pack, Williams et al., 1997; Lentz, Landis, Rothermel et al., 1999). Conversely, a good night of sleep may enhance mood and coping with pain. One group of investigators found that sleep quality was more likely to predict the following day’s mood and physical symptoms than were mood and symptoms able to predict the following night’s sleep quality (Totterdell, Reynolds, Parkinson et al., 1994).
Fatigue
Fatigue is a prevalent and disruptive symptom of many chronic illnesses, including heart failure, chronic lung disease, and cancer (Ancoli-Israel et al., 2003; Nordgren & Sorensen, 2003; Reishtein, 2005). There are many potential causes of fatigue in chronic illness, including preexisting physical conditioning, physical and psychological symptoms, and the consequences of treatments (Sharpe & Wilks, 2002). Several studies have demonstrated a link between fatigue and nocturnal sleep disturbance (Ancoli-Israel et al., 2003; Reishtein, 2005). Fatigue can lead to inactivity and daytime napping which interferes with process S. In turn, inactivity can also lead to a disruption in circadian activity/rest cycles (process C). Daytime sleepiness and “feeling drowsy” have also been identified as factors in the perceived level of fatigue (Ahsberg & Furst, 2001; Hwang, Chang, Rue et al., 2003). One study found that patients use the terms “lack of energy,” “tiredness,” and “fatigue” rather that “sleepiness” to describe their problem (Chervin, 2000). It may be that both patients and clinicians operationalize daytime sleepiness and fatigue in ways that contribute to confusing rather than distinguishing between the conditions (Pigeon, Sateia, & Ferguson, 2003).
Other Symptoms
Palliative care patients are likely to present with a number of other symptoms that interfere with sleep, including dyspnea, cough, nausea, gastrointestinal disturbances, and/or hot flashes (Albert, Davis, & Young, 2002; Blackler, Mooney, & Jones, 2004; Sachs, Shega, & Cox-Hayley, 2004; Walsh, Donnelly, & Rybicki, 2000). Although there is little research into the interaction of common nonpain symptoms and sleep, physical discomfort can delay onset of sleep or interrupt sleep (process S). The APN needs to carefully assess for the presence of these symptoms and their impact on sleep.
Treatment-Related Factors
It is becoming increasingly apparent that medical treatments have the potential to interfere with sleep regulatory processes and thus precipitate sleep loss. Sleep disturbances have been associated with hospitalization (Carvalhaes-Neto, Ramos, Suchecki et al., 2003; Redeker, 2000), surgery and anesthesia (Cronin, Kiefer, Davies et al., 2001; Redeker, Ruggiero, & Hedges, 2004; Rosenberg-Adamsen, Skarbye, Wildschiodtz et al., 1996), cancer therapies (Berger & Higginbotham, 2000; Capuron, Ravaud, & Dantzer, 2000; Miaskowski & Lee, 1999; Stein, Jacobsen, Hann et al., 2000), and hemodialysis (Parker et al., 2000). In addition, most palliative care patients received a number of medications to control the disease process or alleviate associated symptoms. A number of these may interfere with sleep or wakefulness. Impaired renal, hepatic, or neurological function in the chronically ill can further sensitize patients to adverse effects from their medications. It is beyond the scope of this chapter to provide a comprehensive review of the effect of medications on the sleep-wake cycle. However, the major types of drugs known to effect sleep and wakefulness are summarized in Table 8-1. It is common to think of the impact of analgesics, sedatives, and hypnotics on sleep and wakefulness. However, numerous other agents can have adverse effects on sleep regulation, particularly those with central nervous system effects (Schweitzer, 2005).
| REM, Rapid eye movement sleep; SWS, slow-wave sleep (stage 3-4 non-REM sleep); TS, total sleep time. | ||
| Category | Drug | Effect |
|---|---|---|
| Analgesics | Opioids | ↓REM sleep |
| ↓ Stage II | ||
| ↓ Arousal | ||
| Nonsteroidal antiinflammatory drugs (aspirin, ibuprofen, naproxen) | ↑ Stage II | |
| ↓ SWS | ||
| Altered thermoregulation | ||
| Antidepressants | Tricyclics (amitriptyline, doxepin, imipramine, trimipramine, desipramine, nortriptyline) | ↓ REM, ↑ TST |
| Selective serotonin reuptake inhibitors (fluoxetine, paroxetine, fluvoxamine) | ↓ REM, ↓ TST | |
| Antiemetics | Dopamine antagonists (phenothiazines, metoclopramide) | Drowsiness, sedation |
| ↓ REM | ||
| Anticholinergic agents (scopolamine) | Delayed REM onset | |
| ↓ REM sleep | ||
| ↑ Stage II | ||
| ↑ Body movement | ||
| 5-Hydroxytryptamine 3 (5-HT 3) antagonists (ondansetron, granisetron) | Drowsiness | |
| Antihypertensives | β-Adrenergic receptor antagonists (propranolol, nadolol) | Insomnia, daytime sleepiness, nightmares, and vivid dreams |
| α-Adrenergic agonists (clonidine, methyldopa) | Daytime sleepiness, insomnia, ↓ TST, ↓ SWS, REM changes | |
| Anxiolytics | Benzodiazepines (alprazolam, diazepam, lorazepam) | ↓ SWS/REM |
| ↑ Stage II | ||
| Shortened REM latency | ||
| Bronchodilators | Theophylline | ↑ Arousals, ↓ TST |
| Corticosteroids | Prednisone, dexamethasone | Insomnia |
| Bad dreams | ||
| Hypnotics | Benzodiazepines (flurazepam, triazolam, temazepam) | ↓ SWS/REM (mild) |
| Nonbenzodiazepines (zaleplon, zolpidem, zopiclone) | Minimal to no effects on SWS and REM | |
| ↓ Sleep latency | ||
ASSESSMENT OF SLEEP
In light of the growing awareness of the potential for sleep loss and its adverse effects on the palliative care patient, it is important that the APN regularly assess for disturbed nocturnal sleep and daytime sleepiness. Although objective measures of sleep are available, an initial assessment relies on subjective information from the patient. Several questionnaires have been designed to assess sleep quantity and quality, including the Pittsburgh Sleep Quality Index (PSQI) (Buysse, Reynolds, Monk et al., 1989), the St. Mary’s Hospital Sleep Questionnaire (Ellis, John, Lancaster et al., 1981), the General Sleep Disturbance Scale (Lee, 1992), and the Sleep Questionnaire and Assessment of Wakefulness (SQAW) (Miles, 1982). The use of these questionnaires in primary care is difficult due to various factors, including length, complexity in scoring, and lack of defined cut-points in scores. Primary information is best obtained from a careful sleep-wake history. Collateral history from the patient’s bed partner or caregiver can also provide vital information of which the patient may be unaware.
A sleep history should contain a subjective and behavioral account of a typical 24-hour day. Good sleep is characterized by the ability to fall asleep without difficulty (5 to 10 minutes), remain asleep throughout the night, awaken refreshed without the aid of an alarm clock, and remain alert and awake throughout the day. The BEARS system is a useful tool to organize taking a sleep history (see Table 8-2) (Owens, 2005). Another useful tool for assessing sleep patterns across time is the sleep diary or sleep log. Sleep diaries allow the APN to see night-to-night sleep patterns, sleep quality, and daily practices that affect sleep such as activity, naps, medications, and caffeine. Several sleep diaries are available online at the following Web sites.
| Parameter | Objective | Initial Question | Follow-up Question |
|---|---|---|---|
| Bedtime | Find out what happens at sleep onset | Do you have difficulty falling asleep? | How long does it take you to fall asleep? |
| Review: intake of alcohol, nicotine, chocolate, caffeine, medications | What prevents you from falling asleep? | ||
| How long have you had this problem? | |||
| Excessive daytime sleepiness | Determine the extent of daytime sleepiness | Do you find yourself falling asleep during the day when you don’t want to? | How likely are you to fall asleep while reading, watching TV, during a conversation, while driving? |
| Give Epworth Sleepiness Scale | |||
| Awakenings | Nightime: characterize the extent of awakenings | Are you having difficulty sleeping through the night? | What awakens you (pain, shortness of breath, etc)? |
| How often and for how long are you awake? | |||
| What keeps you from falling back asleep? | |||
| Early morning: screen for mood disorder | Are you having any difficulty sleeping until the morning? | At what time do you usually awaken? | |
| What is your mood like in the morning? | |||
| Regularity and duration of sleep | Delineate sleep habits | What time do you usually go to bed and get up in the morning? | Do you feel that you usually get enough sleep? |
| Sleep disorders | Screen for common sleep disorders | Have you or anyone else noticed that you snore loudly? | Have you or anyone else noticed that you stop breathing in your sleep? |
| Have you or anyone else noticed that your legs kick or twitch at night? | Does your bed partner go to another room of the house to sleep? | ||
| Do you have an irresistible urge to move or other types of sensations in your arms or legs? | Does this urge to move or sensation in your arms or legs become worse in the evening? | ||
| Are these sensations relieved by moving or walking? |
▪ National Heart, Lung, and Blood Institute (NIH): www.nhlbi.nih.gov/health/public/sleep/starslp/teachers/sleep_diary.htm
▪Shuteye.com (Sanofi Aventis): www.shuteye.com/solutions_patterns_diary.asp
Daytime sleepiness is also an important aspect of any sleep history. Often patients will not be cognizant of their level of sleepiness. As mentioned before, patients often characterize sleepiness as feeling “tired” or “fatigued.” These complaints warrant a thorough investigation of nocturnal sleep and sleepiness. The Epworth Sleepiness Scale (ESS) (Johns, 1991) is widely used in practice to evaluate the level of daytime sleepiness in patients. The questionnaire contains eight items and measures the likelihood of falling asleep in hypothetical situations. Patients who score greater than 10 have excessive daytime sleepiness. The National Sleep Foundation (NSF) has also developed a Sleepiness Diary (NSF, 2005b). Like the sleep diary, this tool allows the APN to follow patterns of sleepiness across time.
Most sleep disorders are treatable. Many sleep problems can be solved with behavioral or brief pharmacological interventions. However, there are instances when a referral to a sleep specialist is in order for further evaluation. The decision to refer should be based on patient-family goals and the patient’s place in the disease trajectory, which include:
▪ Sleep loss (insufficient sleep/fragmented sleep) not responsive to therapy and accompanied by daytime sleepiness
▪ Unexplained daytime sleepiness: ESS >10, falling asleep during the day without deliberate effort
▪ Symptoms of restless legs syndrome
▪ Symptoms of sleep apnea: loud snoring, awakening with gasping for breath, witnessed apneas during sleep
▪ Symptoms of periodic limb movement disorder: excessive limb movements or jerks during sleep (usually partner report)
INTERVENTIONS TO IMPROVE SLEEP IN PALLIATIVE CARE
Promoting Healthy Sleep Practices
Because disturbed sleep is due to multiple interacting factors, no single intervention is likely to produce desired outcomes. Given a basic understanding of the factors in palliative care patients that potentially alter sleep regulatory processes, the APN can implement patient-specific strategies to minimize disruption of sleep-wake cycles. It has been shown that poor sleep practices can complicate any disease or treatment factors that precipitate sleep-wake disturbances (Zarcone, 2000). Poor sleep habits tolerated in a healthy state may be less tolerable in the disease state. Therefore, APNs must consider evaluation of patient sleep habits and promotion of behaviors that enhance both process S and process C as first steps in any treatment plan. Sleep-promoting behaviors are listed in Table 8-3.
| Promotes | ||
|---|---|---|
| Process S | Process C | |
| Establish Routine Activity-Rest Patterns | ||
| Get up and go to bed the same time every day. | X | |
| Reduce time in bed to the average number of hours slept in the previous week. | X | X |
| Limit daytime sleep to brief naps (less than 1 hour) no later than 8 hours after waking. | X | |
| Engage in light exercise or increase activity in the morning or afternoon. Limit exercise 4 hours before bedtime. | X | X |
| Get at least 30 minutes exposure to sunlight or bright light within a half hour of arising. | X | X |
| Establish Dietary Habits that Promote Sleep | ||
| Do not eat heavily for 3 hours before bedtime. | X | |
| A light bedtime snack is appropriate. Tryptophan content in dairy products and turkey may act as a natural sleep inducer. | X | |
| A hot drink may help to relax and warm you. | X | X |
| Make Sure Your Bed and Bedroom Are Pleasant and Relaxing | ||
| Keep room dark. Avoid exposure to bright light if you have to get up at night. | X | X |
| If environmental noise is a problem, use earplugs or a “white noise” machine. | X | |
| Keep room well ventilated and temperature at a comfortable level. | X | |
| Make sure your mattress is comfortable and the pillow the correct height. | X | |
| Associate your bed with sleep. Try not to use the bed to watch television, listen to the radio, or read. | X | |
| Establish a Relaxing Bedtime Routine | ||
| Avoid emotionally upsetting conversations or activities before bedtime. | X | |
| Make a list of pressing problems and simple solutions to be implemented the next day, then set your problems aside. | X | |
| Listen to relaxing music. | X | |
| Do relaxation exercises or meditate. | X | |
| Read something soothing until you are sleepy. | X | |
| Take a bath hot enough to raise your body temperature about 90 minutes before bedtime. Declining body temperature promotes sleepiness. | X | X |
| Avoid Stimulants at least 4 to 6 Hours before Bed | ||
| Avoid caffeine: coffee, tea, cola, cocoa, chocolate. | X | |
| Check over-the-counter medications for caffeine content. | X | |
| Do not smoke after 7:00 p.m. or avoid smoking altogether. | X | |
| Use alcohol sparingly. Alcohol makes you sleepy, but it interrupts sleep in the second half of the sleep period. | X | |
Treatment of Disturbing Symptoms
Controlling symptoms is a goal of palliative care and a means to improve quality of life. As discussed previously, a number of symptoms have been associated with sleep problems, including pain, dyspnea/cough, gastrointestinal disturbances (reflux, diarrhea, nausea/vomiting), and psychological manifestations (depression/anxiety). Research suggests that symptoms rarely occur in isolation but instead occur in groups or clusters (Dodd, Miaskowski, & Lee, 2004). Furthermore, it appears that the association between symptoms may be interactive (Parker, Kimble, Dunbar et al. 2005). In approaching symptoms that cluster together (e.g., sleep/pain/depression or sleep/dyspnea/anxiety), the APN should plan for behavioral and pharmacological interventions that address multiple symptoms.
Medications
When behavioral interventions alone do not resolve sleep-wake problems, the addition of medications may be warranted. The use of hypnotics in palliative care populations does not have evidence from rigorous randomized controlled trials (Hirst & Sloan, 2005); therefore, the choice of medications depends on the pharmacokinetics of the agent, goals of treatment, symptom presentation, and patient response. Commonly prescribed medications are listed in Table 8-4.
| Medication | Dose | Half-life | Comments |
|---|---|---|---|
| Nonbenzodiazepine Hypnotics | |||
| Zolpidem (Ambien) | 5-10 mg | 1.5-2.4 hours | Use caution in presence of liver failure and in the elderly. May cause rebound insomnia if withdrawn abruptly. May cause rebound morning anxiety. Eszopiclone has been shown effective and safe for long-term use. |
| Zaleplon (Sonata) | 5-10 mg | 1 hour | |
| Eszopiclone (Lunesta) | 1-3 mg | 6 hours | |
| Benzodiazepine Hypnotics | |||
| Tamezepam (Restoril) | 15-30 mg | 8-20 hours | May develop dependency. Caution in patients with liver failure. Longer-acting medications associated with daytime sleepiness and impaired performance. Avoid rapid withdrawal. Use with caution in the elderly. |
| Estazolam (ProSom) | 1-2 mg | 8-24 hours | |
| Triazolam (Halcion) | 0.125-0.25 mg | 2-6 hours | |
| Melatonin Receptor Agonist | |||
| Remelteon (Rozerem) | 8 mg | 0.5-1.5 hours | Contraindicated in severe liver failure. Do not take after a high-fat meal. For use with sleep-onset insomnia. |
| Antidepressants | |||
| Tricyclic | |||
| Amitriptyline (Elavil) | 25-50 mg (Maximum 150) | 10-50 hours | Cautious use with elders due to anticholinergic effects. May be used as adjunct for neuropathic pain. Very sedating. Not FDA approved for primary insomnia. |
| Selective Serotonin Reuptake Inhibitors | |||
| Trazodone (Desyrel) | 25-100 mg | 5-9 hours | Not FDA approved for insomnia. Doses usually used for insomnia are below recommended doses for depression. Not considered best antidepressant due to excessive daytime sedation at therapeutic doses. |
| Other | |||
| Mirtazapine (Remeron) | 15-45 mg | 20-40 hours | Not FDA approved for insomnia. May be useful for sleep with coexisting mood disorder due to sedating properties. Increases appetite. Weight gain common. |
| Antihistamines | |||
| Diphenhydramine (Benadryl) | 25-50 mg | 3-12 hours | Not much data to support use for sleep. May cause morning sedation. Should only be used short term. Use with great caution in elders. Has been shown to cause cognitive impairment and delirium. |
| Doxylamine (Unisom) | 12.5-25 mg | 9 hours | Potent antihistamine with strong anticholinergic effects. Use with caution in elders. |
CONCLUSION
Palliative care patients are susceptible to severe disturbances in sleep that have the potential to impair tissue repair, immune function, endocrine function, and metabolism—conditions that affect overall morbidity and mortality. Some of the factors that underlie sleep disturbances include predisposing factors, such as a history of a primary sleep disorder, demographic factors, and lifestyle factors, and precipitating factors, such as psychosocial, disease-related, treatment-related, and environmental conditions. These factors have the potential to disrupt sleep regulatory mechanisms. APNs can promote sleep by recognizing which factors may be operable in individual patients and implementing strategies to minimize disruption in sleep and promote daytime alertness.
American Academy of Sleep Medicine
• Education site: www.sleepeducation.com
• Education for health professionals: www.aasmnet.org/MedSleep_Resources.aspx (look under specific authors)
National Sleep Foundation
Ahsberg, E.; Furst, C.J., Dimensions of fatigue during radiotherapy, Acta Oncol 40 (2001) 37–43.
Akerstedt, T.; Nilsson, P.M., Sleep as restitution: An introduction, J Intern Med 254 (2003) 6–12.
Albert, N.M.; Davis, M.; Young, J., Improving the care of patients dying of heart failure, Cleve Clin J Med 69 (2002) 321–328.
Ancoli-Israel, S.; Gehrman, P.; Martin, J.L.; et al., Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients, Behav Sleep Med 1 (2003) 22–36.
Avery, D.H.; Wildschiodtz, G.; Smallwood, R.G.; et al., REM latency and core temperature relationships in primary depression, Acta Psychiatr Scand 74 (1986) 269–280.
Basbaum, A.I.; Jessell, T.M., The perception of pain, In: (Editors: Kandel, E.R.; Schwartz, J.H.; Jessell, T.M.) Principles of neuroscience4th ed. ( 2000)McGraw-Hill, New York, pp. 472–491.
Benca, R.M., Mood disorders, In: (Editors: Kryger, M.H.; Roth, T.; Dement, W.C.) Principles and practice of sleep medicine3rd ed. ( 2005)Elsevier Saunders, Philadelphia, pp. 1311–1326.
Benca, R.M.; Ancoli-Israel, S.; Moldofsky, H., Special considerations in insomnia diagnosis and management: Depressed, elderly, and chronic pain populations, J Clin Psychiatry 65 (Suppl 8) ( 2004) 26–35.
Berger, A.M.; Higginbotham, P., Correlates of fatigue during and following adjuvant breast cancer chemotherapy: A pilot study, Oncol Nurs For 27 (2000) 1443–1448.
Bixler, E.O.; Vgontzas, A.N.; Lin, H.M.; et al., Insomnia in central Pennsylvania, J Psychosom Res 53 (2002) 589–592.
Blackler, L.; Mooney, C.; Jones, C., Palliative care in the management of chronic obstructive pulmonary disease, Br J Nurs 13 (2004) 518–521.
Blazer, D.G.; Hays, J.C.; Foley, D.J., Sleep complaints in older adults: A racial comparison, The Journals of Gerontology. Series A, Biol Sci Med Sci 50 (1995) M280–M284.
Bliwise, D.L., Normal aging, In: (Editors: Kryger, M.H.; Roth, T.; Dement, W.C.) Principles and practice of sleep medicine4th ed. ( 2005)Elsevier Saunders, Philadelphia, pp. 24–38.
Bliwise, D.L.; King, A.C.; Harris, R.B.; et al., Prevalence of self-reported sleep in a healthy population aged 50-65, Soc Sci Med 34 (1992) 49–55.
Bonnet, M.H., Acute sleep deprivation, In: (Editors: Kryger, M.H.; Roth, T.; Dement, W.C.) Principles and practice of sleep medicine4th ed. ( 2005)Elsevier Saunders, Philadelphia, pp. 51–66.
Borbely, A.A., A two-process model of sleep regulation, Hum Neurobiol 1 (1982) 195–204.
Borbely, A.A.; Achermann, P., Sleep homeostasis and models of sleep regulation, In: (Editors: Kryger, M.H.; Roth, T.; Dement, W.C.) Principles and practice of sleep medicine4th ed. ( 2005)Elsevier Saunders, Philadelphia, pp. 405–417.
Borbely, A.A.; Wirz-Justice, A., Sleep, sleep deprivation and depression: A hypothesis derived from a model of sleep regulation, Hum Neurobiol 1 (1983) 205–210.
Breslau, N.; Roth, T.; Rosenthal, L.; et al., Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young adults, Biol Psychiatry 39 (1996) 411–418.
Breslau, N.; Roth, T.; Rosenthal, L.; et al., Daytime sleepiness: An epidemiological study of young adults, Am J Pub Health 87 (1997) 1649–1653.
Capuron, L.; Ravaud, A.; Dantzer, R., Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy, J Clin Oncol 18 (2000) 2143–2151.
Carskadon, M.A.; Dement, W.C., Normal human sleep: An overview, In: (Editors: Kryger, M.H.; Roth, T.; Dement, W.C.) Principles and practice of sleep medicine4th ed. ( 2005)Elsevier Saunders, Philadelphia, pp. 13–23.
Carter, P.A., Caregivers’ descriptions of sleep changes and depressive symptoms, Oncol Nurs For Online 29 (2002) 1277–1283.
Carter, P.A., Family caregivers’ sleep loss and depression over time, Cancer Nurs 26 (2003) 253–259.
Carvalhaes-Neto, N.; Ramos, L.R.; Suchecki, D.; et al., The effect of hospitalization on the sleep pattern and on cortisol secretion of healthy elderly, Exp Aging Res 29 (2003) 425–436.
Chervin, R.D., Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea, Chest 118 (2000) 372–379.
Clark, J.; Cunningham, M.; McMillan, S.C.; et al., Sleep-wake disturbances in people with cancer. Part II: Evaluating the evidence for clinical decision making, Oncol Nurs For 31 (2004) 747–771.
Cronin, A.J.; Keifer, J.C.; Davies, M.F.; et al., Postoperative sleep disturbance: Influences of opioids and pain in humans, Sleep 24 (2001) 39–44.
Dinges, D.F.; Pack, F.; Williams, K.; et al., Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night, Sleep 20 (1997) 267; 267.
Dodd, M.J.; Miaskowski, C.; Lee, K.A., Occurrence of symptom clusters, J Nat Cancer Inst Monogr 32 (2004) 76–78.
Ellis, B.W.; Johns, M.W.; Lancaster, R.; et al., The St. Mary’s Hospital Sleep Questionnaire: A study of reliability, Sleep 4 (1981) 93–97.
Finelli, L.A., Sleep deprivation: Cortical and EEG changes, In: (Editor: Kushida, C.A.) Sleep deprivation: Basic science, physiology, and behavior ( 2005)Marcel Dekker, New York, pp. 223–264.
Foley, D.J.; Monjan, A.A.; Brown, S.L.; et al., Sleep complaints among elderly persons: An epidemiologic study of three communities, Sleep 18 (1995) 425–432.
Ford, D.E.; Kamerow, D.B., Epidemiologic study of sleep disturbance and psychiatric disorders: An opportunity for prevention?JAMA 262 (1989) 1479–1484.
Gay, P.C., Chronic obstructive pulmonary disease and sleep, Respira Care 49 (2004) 39–51.
Gillin, J.C.; Drummond, S.P.A.; Clark, C.P.; et al., Medication and substance abuse, In: (Editors: Kryger, M.H.; Roth, T.; Dement, W.C.) Principles and practice of sleep medicine4th ed. ( 2005)Elsevier Saunders, Philadelphia, pp. 1345–1358.
Gislason, T.; Almqvist, M., Somatic diseases and sleep complaints, Acta Med Scand 221 (1987) 475–481.
Hall, M.; Buysse, D.J.; Nowell, P.D.; et al., Symptoms of stress and depression as correlates of sleep in primary insomnia, Psychosom Med 62 (2000) 227–230.
Hirst, A.; Sloan, R., Benzodiazepines and related drugs for insomnia in palliative care, Cochrane Database System Review 3 (2005).
Hwang, S.S.; Chang, V.T.; Rue, M.; et al., Multidimensional independent predictors of cancer-related fatigue, J Pain Symptom Manage 26 (2003) 604–614.
Johns, M.W., A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale, Sleep 14 (1991) 540–545.
Kecklund, G.; Akerstedt, T., Apprehension of the subsequent working day is associated with a low amount of slow wave sleep, Biol Psychiatry 66 (2004) 169–176.
Krueger, J.M.; Majde, J.A.; Obal, F., Sleep in host defense, Brain Behav Immun 71 (Suppl 1) ( 2003) S41–S47.
In: (Editors: Kryger, M.H.; Roth, T.; Dement, W.C.) Principles and practice of sleep medicine4th ed. ( 2005)Elsevier Saunders, Philadelphia.
Landis, C.A.; Frey, C.A.; Lentz, M.J.; et al., Self-reported sleep quality and fatigue correlates with actigraphy in midlife women with fibromyalgia, Nurs Res 52 (2003) 140–147.
Lavigne, G.; Zucconi, M.; Castronovo, C.; et al., Sleep arousal response to experimental thermal stimulation during sleep in human subjects free of pain and sleep problems, Pain 84 (2000) 283–290.
Lee, K.A., Self-reported sleep disturbances in employed women, Sleep 15 (1992) 493–498.
Lee, K.A.; Landis, C.; Chasens, E.R.; et al., Sleep and chronobiology: Recommendations for nursing education, Nurs Outlook 52 (2004) 126–133.
Lee-Chiong, T.L., Manifestation and classification of sleep disorders, In: (Editors: Lee-Chiong, T.L.; Sateia, M.J.; Carskadon, M.A.) Sleep medicine ( 2002)Hanley & Belfus, Philadelphia, pp. 125–142.
Lentz, M.J.; Landis, C.A.; Rothermel, J.; et al., Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women, J Rheumatol 26 (1999) 1586–1592.
Leung, R.S.; Bradley, T.D., Sleep apnea and cardiovascular disease, Am J Respir Crit Care Med 164 (2001) 2147–2165.
McGinty, D.; Alam, M.N.; Szymusiak, R.; et al., Hypothalamic sleep-promoting mechanisms: Coupling to thermoregulation, Arch Ital Biol 139 (2001) 63–75.
Miaskowski, C.; Lee, K.A., Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: A pilot study, J Pain Symptom Manage 17 (1999) 320–332.
Middelkoop, H.A.; Smilde-van den Doel, D.A.; Neven, A.K.; et al., Subjective sleep characteristics of 1,485 males and females aged 50-93: Effects of sex and age, and factors related to self-evaluated quality of sleep, J Gerontol 51 (1996) M108–M115.
Miles, L., Sleep Questionnaire and Assessment of Wakefulness (SQAW), In: (Editor: Guilleminault, C.) Sleeping and waking disorders: Indications and techniques ( 1982)Addison-Wesley, Menlo Park, Calif., pp. 384–413.
Monk, T.H.; Buysse, D.J.; Carrier, J.; et al., Effects of afternoon “siesta” naps on sleep, alertness, performance, and circadian rhythms in the elderly, Sleep 24 (2001) 680–687.
Montplaisir, J.; Allen, R.P.; Walters, A.S.; et al., Restless legs syndrome and periodic limb movement disorder, In: (Editors: Kryger, M.H.; Roth, T.; Dement, W.C.) Principles and practice of sleep medicine4th ed. ( 2005)Elsevier Saunders, Philadelphia, pp. 839–852.
Moore, P.J.; Adler, N.E.; Williams, D.R.; et al., Socioeconomic status and health: The role of sleep, Psychosom Med 64 (2002) 337–344.
Morin, C.M.; Rodrigue, S.; Ivers, H., Role of stress, arousal, and coping skills in primary insomnia, Psychosom Med 65 (2003) 259–267.
National Institute of Neurological Disorders and Stroke, Brain basics: Understanding sleep, Retrieved May 20, 2006, from www.ninds.nih.gov/disorders/brain_basics/understanding_sleep.htm ( 2005).
National Sleep Foundation, Sleep in America, Retrieved May 20, 2006, from www.sleepfoundation.org/hottopics/index.php?secid=16&id=245 ( 2005).
National Sleep Foundation, Sleepiness diary, Retrieved May 20, 2006, from www.sleepfoundation.org/quiz/index.php?secid=&id=110 ( 2005).
Nordgren, L.; Sorensen, S., Symptoms experienced in the last six months of life in patients with end-stage heart failure, Eur J Cardiovasc Nurs 2 (2003) 213–217.
Ohayon, M.M.; Roth, T., Place of chronic insomnia in the course of depressive and anxiety disorders, J Psychiatr Res 37 (2003) 9–15.
Owens, J., Taking a sleep history, Retrieved May 20, 2006, from www.aasmnet.org/Resources.aspx?Resource_ID=1008 ( 2005).
Parker, K.P.; Bliwise, D.L.; Rye, D.B., Hemodialysis disrupts basic sleep regulatory mechanisms: Building hypotheses, Nurs Res 49 (2000) 327–332.
Parker, K.P.; Dunbar, S.B., Sleep and heart failure, J Cardiovasc Nurs 17 (2002) 30–41.
Parker, K.P.; Kimble, L.P.; Dunbar, S.B.; et al., Symptom interactions as mechanisms underlying symptom pairs and clusters, J Nurs Schol 37 (2005) 209–215.
Pigeon, W.R.; Sateia, M.J.; Ferguson, R.J., Distinguishing between excessive daytime sleepiness and fatigue: Toward improved detection and treatment, J Psychosom Res 54 (2003) 61–69.
Pilcher, J.J.; Huffcutt, A.I., Effects of sleep deprivation on performance: A meta-analysis, Sleep 19 (1996) 318–326.
Redeker, N.S., Sleep in acute care settings: An integrative review, J Nurs Schol 32 (2000) 31–38.
Redeker, N.S.; Ruggiero, J.; Hedges, C., Patterns and predictors of sleep pattern disturbance after cardiac surgery, Res Nurs Health 27 (2004) 217–224.
Rediehs, M.H.; Reis, J.S.; Creason, N.S., Sleep in old age: Focus on gender differences, Sleep 13 (1990) 10–24.
Reishtein, J.L., Relationship between symptoms and functional performance in COPD, Res Nurs Health 28 (2005) 39–47.
Roehrs, T.; Roth, T., Sleep and pain: Interaction of two vital functions, Semin Neurol 25 (2005) 106–116.
Rosenberg-Adamsen, S.; Skarbye, M.; Wildschiodtz, G.; et al., Sleep after laparoscopic cholecystectomy, Br J Anaesth 77 (1996) 572–575.
Rye, D.B., The Two Faces of Eve: Dopamine’s modulation of wakefulness and sleep, Neurology 6 (8 Suppl 3) ( 2004) S2–S7.
Sachs, G.A.; Shega, J.W.; Cox-Hayley, D., Barriers to excellent end-of-life care for patients with dementia, J Gen Internal Med 19 (2004) 1057–1063.
Schultz, H.; Lund, R., Sleep onset REM episodes are associated with circadian parameters of body temperature. A study in depressed patients and normal controls, Biol Psychiatry 18 (1983) 1411–1426.
Schweitzer, P.K., Drugs that disturb sleep and wakefulness, In: (Editors: Kryger, M.H.; Roth, T.; Dement, W.C.) Principles and practice of sleep medicine4th ed. ( 2005)Elsevier Saunders, Philadelphia, pp. 499–518.
Serber, E.R.; Sears, S.F.; Sotile, R.O.; et al., Sleep quality among patients treated with implantable atrial defibrillation therapy: Effect of nocturnal shock delivery and psychological distress, J Cardiovasc Electrophysiol 14 (2003) 960–964.
Sharpe, M.; Wilks, D., Fatigue, Br Med J 325 (2002) 480–483.
Siegel, J.M., The REM sleep-memory consolidation hypothesis, Science 294 (2001) 1058–1063.
Silber, M.H.; Krahn, L.E.; Morgenthaler, T.I., Sleep medicine in clin practice. ( 2004)Taylor & Francis, London.
Sin, D.D.; Logan, A.G.; Fitzgerald, F.S.; et al., Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne-Stokes respiration, Circulation 102 (2000) 61–66.
Steiger, A., Sleep and endocrinology, J Intern Med 254 (2003) 13–22.
Stein, K.D.; Jacobsen, P.B.; Hann, D.M.; et al., Impact of hot flashes on quality of life among postmenopausal women being treated for breast cancer, J Pain Symptom Manage 19 (2000) 436–445.
Steptoe, A.; Marmot, M., Burden of psychosocial adversity and vulnerability in middle age: Associations with biobehavioral risk factors and quality of life, Psychosom Med 65 (2003) 1029–1037.
Totterdell, P.; Reynolds, S.; Parkinson, B.; et al., Associations of sleep with everyday mood, minor symptoms and social interaction experience, Sleep 17 (1994) 466–475.
Van Someren, E.J., More than a marker: Interaction between the circadian regulation of temperature and sleep, age-related changes, and treatment possibilities, Chronobiol Internat 17 (2000) 313–354.
Vena, C.; Parker, K.P.; Cunningham, M.; et al., Sleep-wake disturbances in people with cancer. Part I: An overview of sleep, sleep regulation, and effects of disease and treatment, Oncol Nurs For 31 (2004) 735–746.
Vitiello, M.V.; Moe, K.E.; Prinz, P.N., Sleep complaints cosegregate with illness in older adults: Clinical research informed by and informing epidemiological studies of sleep, J Psychosom Res 53 (2002) 555–559.
Walsh, D.; Donnelly, S.; Rybicki, L., The symptoms of advanced cancer: Relationship to age, gender, and performance status in 1,000 patients, Support Care Cancer 8 (2000) 175–179.
Young, T.; Skatrud, J.; Peppard, P.E., Risk factors for obstructive sleep apnea in adults, JAMA 291 (2004) 2013–2016.
Zarcone, V.P., Sleep hygiene, In: (Editors: Kryger, M.H.; Roth, T.; Dement, W.C.) Principles and practice of sleep medicine3rd ed. ( 2000)Saunders, Philadelphia, pp. 657–661.