CHAPTER 79
Stress Fractures of the Lower Limb
Sheila Dugan, MD; Sol M. Abreu Sosa, MD, FAAPMR
Definition
Stress fractures are complete or partial bone fractures caused by the accumulation of microtrauma [1]. Normal bone accommodates to stress through ongoing remodeling. If this remodeling system does not keep pace with the force applied, stress reaction (microfractures) and, finally, stress fracture can result. Stress fracture is the end result of a continuum of biologic responses to stress placed on bone. Adolescent, young adult, and premenopausal women athletes have a higher incidence of stress injuries to bone than men do [2,3]. Stress fractures in juveniles are rare [4]. Both extrinsic and intrinsic factors have been implicated in this imbalance between bone resorption and bone deposition [5]. Malalignment and poor flexibility of the lower extremities (intrinsic factors) and inadequate footwear, changes in training surface, and increases in training intensity and duration without an adequate ramp-up period (extrinsic factors) can lead to stress fractures [6].
Stress fractures in athletes vary by sports and are most common in the lower extremities [2,7]. The most common sites are the tibia, metatarsals, and fibula, and they affect most commonly runners and dancers. The fracture site is the area of greatest stress, such as the origin of lower leg muscles along the medial tibia [8]. A narrower mediolateral tibial width was a risk factor for femoral, tibial, and foot stress fractures in a study of military recruits [9]. Studies of female runners demonstrated greater loading rates in those with history of tibial stress fractures compared with those without injury [10,11]. In contrast, in comparison of runners with and without history of tibial stress fracture, no difference in ground reaction forces, bone density, or tibial bone geometric parameters was found between groups [12].
Military recruits have been extensively studied in regard to lower extremity stress fractures. In a study of 179 Finnish military recruits aged 18 to 20 years, tall height, poor physical conditioning, low hip bone mineral content and density, and high serum parathyroid hormone level were risk factors for stress fractures [13]. The authors postulated that given the poor vitamin D status, intervention studies of vitamin D supplementation to lower serum parathyroid hormone levels and possibly to reduce the incidence of stress fractures are warranted. Prospective studies of vitamin D and calcium in stress fracture prevention, one in female young athletes and the second in female military recruits, were the focus of a recent review paper. A longitudinal study of female athletes aged 18 to 26 years showed that greater baseline intakes of dietary calcium, dairy products, and milk were linked to significant reductions in fracture incidence; fracture risk decreased by 62% per additional cup of skim milk consumed per day, and women who consumed less than 800 mg of calcium per day had nearly six times the stress fracture rate of women who consumed more than 1500 mg of calcium and more than double the rate for women who consumed between 800 and 1500 mg [14]. The second study of female military recruits who were prescribed an 8-week trial of supplementation with 2000 mg of calcium and 800 IU of vitamin D demonstrated a statistically significant 20% reduction in fracture injuries compared with women given a placebo [14]. The study concluded that evaluation of age-appropriate dietary guidelines for calcium and vitamin D levels is needed to promote bone health, to reduce the risk of stress fracture injury in the young athlete, and to achieve peak bone density that will promote lifelong bone health [14].
A database of systematic reviews, including 13 randomized prevention trials, concluded that shock-absorbing insert use in footwear probably reduces the incidence of stress fractures in military personnel [15]. There was insufficient evidence to determine the best design of such inserts.
Stress fractures may be related to abnormalities of the bone, such as in female athletes with low bone density due to exercise-induced menstrual abnormalities [16–19]. Premature osteoporosis leads to an increased risk for stress fractures. One study looked at premenopausal women runners and collegiate athletes and concluded that those with absent or irregular menses were at increased risk for musculoskeletal injuries while engaged in active training [17]. Muscle deficits in the gastrocnemius-soleus complex in jumping athletes have also been implicated in causing tibial stress fractures. Bone injury may be a secondary event after a primary failure of muscle function [20].
Recent literature review of the influence of sports participation on bone health in the young athlete concluded that high-impact and weight-bearing activities enhance bone density, particularly in anatomic locations directly loaded by those sports [21]. It also showed that participation in sports during the age range in which growth and skeletal maturity occur may result in a higher peak bone density [21]; in particular, athletes aged 10 to 30 years who participate in impact sports (particularly high-impact or odd-impact sports) may enjoy enhanced bone density and improvements in bone geometry. Nonimpact sports such as cycling and swimming are not associated with improvements in bone health, and prolonged participation in endurance sports, including long-distance running and cycling, may be associated with decreased peak bone density [21].
During the last few years, researchers have shown a link between stress fractures and long-term bisphosphonate use. Bisphosphonate medications are indicated for patients with postmenopausal osteoporosis, and it has been shown that bisphosphonate use improves bone mineral density, prevents bone loss, and reduces the number of fractures [22,23]. Bisphosphonates inhibit osteoclastic bone resorption, and therefore bone turnover, by inducing osteoclast apoptosis. The combined and coordinated action of resorbing damaged bone and laying down new bone is fundamental to the process of bone remodeling [23]. If this coupling is impaired, the microdamage that occurs under physiologic conditions that normally is repaired may accumulate, resulting in a major reduction in the energy required to cause fracture [23]. These fractures are low-energy injuries and have characteristic findings observed on femoral radiographs: a transverse fracture line originating from the lateral tension side of the cortex and lateral cortical thickening adjacent to the fracture [23,24]. In addition, prodromal thigh pain from the insufficiency changes may be present. The subsequent minimal trauma that often is required to complete the fracture is characteristic, with patients often sustaining a spontaneous nontraumatic fracture during activities of daily living [23].
Individuals who are nonambulatory or have limited ambulation due to disability represent another population with abnormal bone and premature osteoporosis. In stroke patients, there is significant bone loss on the paretic side, which is greatest in those patients with the most severe functional deficits [25]. Spinal cord injury may not only cause bone loss but also alter bone structure and microstructure [26]. Practitioners caring for individuals with limited mobility should consider stress fracture in the differential diagnosis of overuse injuries.
Symptoms
Patients may report an increase in training or activity level or a change in training conditions preceding the onset of symptoms. Because of pain in the affected region of the bone, patients may seek medical attention during the microfracture or stress reaction phase of injury. Should they forego relative rest (avoidance of the pain-provoking activity), they can progress to stress fracture or even complete fracture. The pain will gradually increase with activity and may occur with less intense exercise, such as walking, or even at rest. In general, however, the pain will improve with rest. The pain can lead to a decline in performance. The individual may also note swelling in the affected region of the bone. Symptoms of paresthesias and numbness should alert the clinician to consider an alternative diagnosis.
Physical Examination
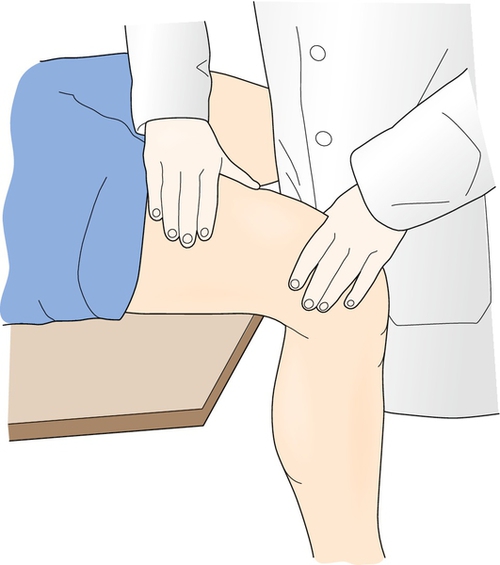
On physical examination, the clinician will find an area of exquisite, well-localized tenderness, warmth, and edema over the affected region of the bone. Ecchymosis along the plantar aspect may be present with foot involvement. Percussion of the nearby region can cause pain. Placement of a vibrating tuning fork over the fracture site intensifies the pain [27]. In the tibia, stress fractures primarily occur along the medial border; the frequency, in order, is upper, lower, and mid shaft. In the fibula, they usually occur one handbreadth proximal to the lateral malleolus [5]. Tarsal or metatarsal stress fractures are manifested with localized foot tenderness. Weight-bearing activity, such as a one-legged hop test, can provoke the pain by increasing the ground reaction forces. For a presumed femoral stress fracture, the clinician can provoke pain by applying a downward force on the distal femur while the affected individual is seated with the distal femur extending beyond the edge of the seat (Fig. 79.1).
The physical examination must include an examination of the lumbar spine and lower limbs to evaluate for any anatomic malalignment or biomechanical abnormalities. For instance, an individual with rigid supinated feet or weak foot intrinsic muscles may transmit more ground reaction forces to the tibia. On physical examination, one can identify problems that must be addressed in treatment planning.
Strength should be normal but occasionally limited by pain. Sensation and muscle stretch reflexes should also be normal.
Functional Limitations
Recreational and athletic activities requiring weight bearing through the affected lower limb may be limited by pain. For instance, running results in the transmission of increased ground reaction forces through the leg. These forces can increase if one runs on a concrete surface versus an all-weather track. In acute cases, ambulation can be painful.
Diagnostic Studies
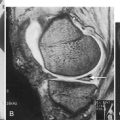
Plain films may take as long as 6 weeks to demonstrate fracture, and films are initially normal in up to two thirds of patients [28]. Technetium Tc 99 m diphosphonate bone scanning will yield the earliest confirmatory data for stress fractures, demonstrating a “hot spot” 1 to 4 days after fracture [18]. The fracture site may not return to normal on a bone scan for 5 months or longer, so it is not clinically useful to assess recovery. Computed tomography is necessary to differentiate stress fractures of the sacrum and the tarsal navicular bone [29]. Magnetic resonance imaging (MRI) delineates the fracture location and status of healing (Fig. 79.2). MRI provides soft tissue definition, which can be helpful in the setting of stress reaction or tendinitis. Although it can confirm the diagnosis in the acute phase, it is generally not indicated initially. MRI findings can help with clinical decision-making about return to activity or play. A retrospective review of military recruits presenting with anterior lower leg pain showed that only 56% had positive findings on MRI testing, implicating tissues other than bone as the pain generator [30]. In a study, 12% of asymptomatic feet in male college basketball players demonstrated bone marrow edema on MRI; the authors noted that this early detection might lead to preventive strategies to avoid injury [31].
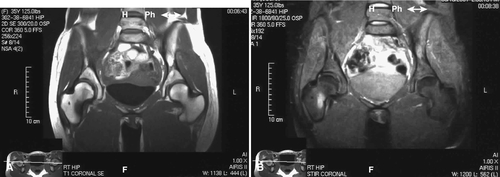
MRI grading systems have been developed with short T1 inversion recovery (STIR) sequences. Four grades of abnormality have been described from grade 1 (demonstration of periosteal edema on STIR or T2-weighted images) through grade 4 (visible injury line on T1- or T2-weighted images) [32,33]. Radiographic grading scales of stress injuries to bone can be useful in clinical trials to more specifically delineate response to management. One should consider bone density testing in a female patient with a history of amenorrhea [3].
Treatment
Initial
Pain and edema should be managed initially with PRICE (protection, rest, ice, compression, and elevation). Use of nonsteroidal anti-inflammatory drugs (NSAIDs) is discouraged by several authors because of the negative impact of NSAIDs on tissue healing; in addition, masking of pain can compromise healing by reducing symptomatic feedback [34–37]. Acetaminophen may be helpful in patients who are resting but still have pain. Activities that provoke pain are eliminated. If ambulation is painful, athletes are placed on non–weight-bearing status or full crutch walking to eliminate painful weight bearing [38]. In non–weight-bearing subjects, a trial of walking is performed every 2 days, and once walking is pain free, full ambulation with crutches is begun. Fractures with the propensity to progress to nonunion, such as midshaft tibial stress fractures and tarsal navicular fractures, may require immediate immobilization with a bivalved orthotic boot. Femoral neck fractures on the tension (superior) aspect can become displaced and require strict non–weight-bearing status with axillary crutches initially [39]. Metatarsal stress fractures can be treated with a stiff shoe and a straight cane or a rigid orthosis. Navicular fractures may require immobilization in a short leg cast [34].
In the setting of female athletes with exercise-induced amenorrhea, nutritional counseling and correction of any energy debt must be included in the stress fracture treatment program. If the menstrual cycle does not return with these interventions, there is controversy about the use of an oral contraceptive pill to restore menses. Fewer athletes with fractures were using oral contraceptive pills than were athletes without fractures in one study [40]. In addition, women without stress fractures had a higher intake of calcium than did those with stress fractures. Nine elite runners with stress fractures were compared with matched control subjects without stress fractures, and significant differences in the number of menses per year (less in the fracture group) and the age at onset of menses (delayed in the fracture group) were identified [41].
In the setting of bisphosphonate-related stress fracture in the femoral subtrochanteric or high diaphyseal region, there is still debate as to whether it should be treated nonoperatively or operatively. A study suggested prophylactic fixation because eventually these fractures may become complete and displaced, requiring an eventual fixation [22].
Rehabilitation
Physical therapy modalities such as heat and interferential electrical stimulation are used to increase local blood flow and to promote healing; however, there is a lack of controlled studies to prove their efficacy. Deep soft tissue massage, including transverse friction massage, may be indicated and complement stretching for the muscles that originate along the medial tibia. Ongoing cardiovascular and strengthening activities should continue if they produce no pain; aqua jogging, stationary bicycling, or use of the elliptical machine can be substituted for running. Athletes can return to running once they are pain free with ambulation and cross-training activities; however, training schedules should be modified, and pain should be used as the guide to progression of the program [42]. In the setting of low-risk stress fractures, athletes may continue to participate if activity can be modified to minimize stress at the fracture site [43]. Sports-specific training must be addressed before return to play.
Careful attention to the training surface and equipment is mandatory. In the setting of significant forefoot or rearfoot biomechanical abnormalities, custom foot orthotics may be indicated. Taping may be used temporarily to provide stability of the ankle and foot. Local lower extremity strengthening is progressed from static exercises to concentric to eccentric training on the basis of symptoms. Plyometric (weight-bearing eccentric) training should precede return to play. Shock-absorbing insoles and running on shock- absorbing surfaces are recommended and thought to decrease the ground reaction forces transmitted to the bones of the lower extremity [15,44]. In a prospective study of athletes without control subjects, immobilization with a pneumatic leg brace was used to allow participation in a modified training schedule earlier. The authors concluded that the brace promoted healing and limited the forces across the fracture site [45].
Rocker-bottom shoes and steel shanks can be used to prevent and to treat lower extremity stress fractures in susceptible disabled individuals.
Procedures
There is no specific nonsurgical procedure for this injury.
Surgery
Conservative management successfully treats lower extremity stress fractures with a few exceptions. Femoral neck stress fractures on the tensile (superior) aspect may require pinning if they do not heal after a course of non–weight bearing. Midshaft tibial fractures are at risk for nonunion and must be immobilized and observed closely; an open bone grafting procedure may be indicated in the setting of nonunion. Tarsal navicular stress fractures that do not respond to conservative treatment and demonstrate displacement, comminution, or nonunion may require open reduction with internal fixation [46]. In 26 subjects with 32 fractures treated for 2 years or more, surgical fixation of navicular stress fractures appears to be as effective as conservative management in the longer term [47].
Potential Disease Complications
If biomechanical and training principles are not addressed during treatment, stress fractures can recur. In female athletes with menstrual abnormalities and premature osteoporosis, failure to treat these conditions might also lead to recurrent stress fractures.
Potential Treatment Complications
Immobilization can lead to loss of joint range of motion and reduced muscle strength. Treatment risks with NSAIDs include gastrointestinal, hepatic, and renal side effects; in addition, detrimental effects on bone healing must be considered. A large retrospective study found that use of NSAIDs is associated with a 1.47 relative risk of fractures (nonvertebral) compared with control subjects who did not receive NSAIDs [48]. Treatment of amenorrhea with oral contraceptive pills involves increased risk for blood clots and their sequelae. Treatment of premature osteoporosis with bisphosphonates includes risk for esophageal erosion or ulceration. Complications of surgery include nonunion and other typical infrequent complications (e.g., infection, bleeding). Subjects who underwent surgical treatment for tarsal navicular stress fracture were more likely to continue to be tender over the navicular than were nonoperative subjects [47].