CASE 7 Saphenous Vein Graft Disease
Case presentation
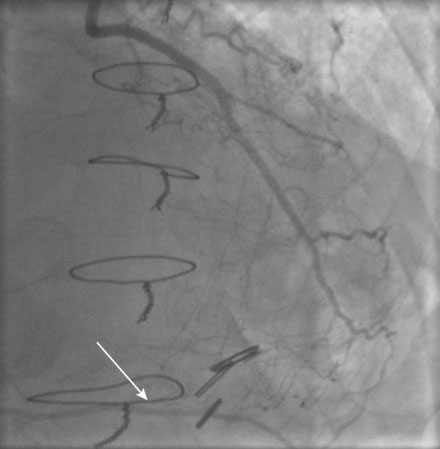
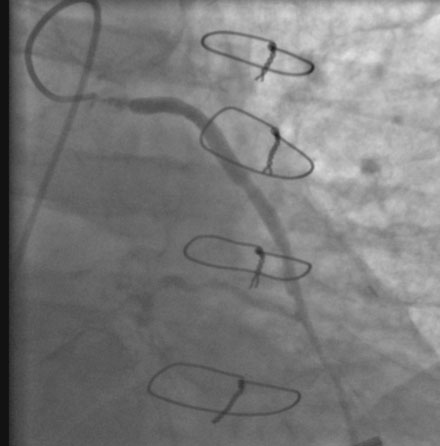
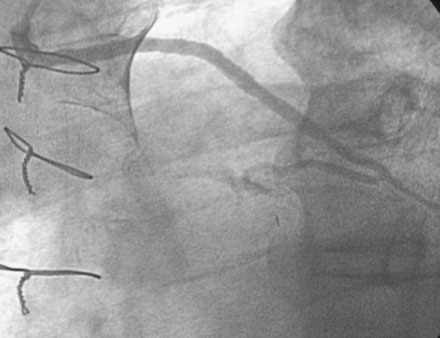
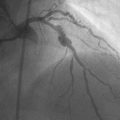
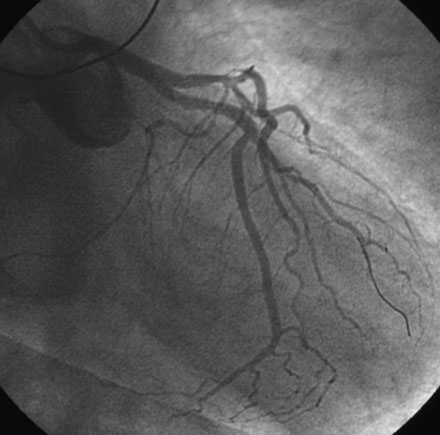
He had known left ventricular dysfunction with an ejection fraction of 40%, but had remained asymptomatic since his bypass operation until 2 to 3 weeks before presentation, when he noted progressive shortness of breath with any exertion associated with chest tightness. He developed rest dyspnea and then became unable to lie flat without developing a sense of suffocating. He presented to the emergency room and was promptly admitted with a diagnosis of congestive heart failure and unstable angina. His electrocardiogram showed nonspecific abnormalities that were unchanged from prior ECGs, and serial troponin assays remained in the normal range. However, an echocardiogram showed deterioration in his left ventricular function with an ejection fraction of 15% to 20%. He subsequently underwent cardiac catheterization, which found a chronically-occluded right coronary artery and a patent left internal mammary to the LAD with large collateral vessels to the right coronary (Figure 7-1 and Video 7-1). The native proximal LAD and circumflex arteries were completely occluded. The saphenous vein graft to the ramus had a very severe stenosis located in the proximal segment near the aortic anastomosis (Figures 7-2, 7-3 and Videos 7-2, 7-3). He was referred for percutaneous coronary intervention of the saphenous vein graft.
Cardiac catheterization
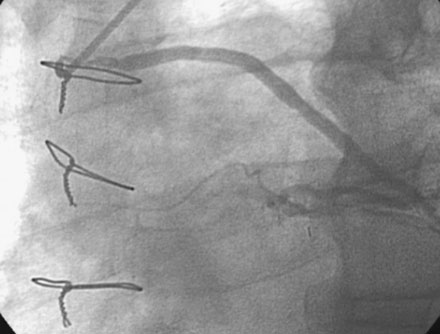
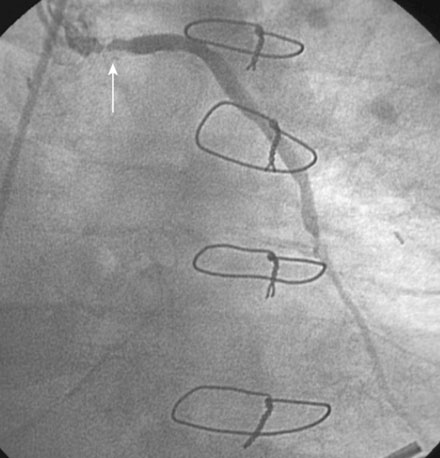
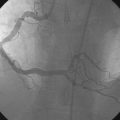
The night prior to the procedure, the patient received a loading dose of 600 mg clopidogrel and, after obtaining arterial access, the operator administered bivalirudin as the procedural anticoagulant. A 6 French Judkins right 4.0 guide catheter was engaged in the saphenous vein graft. To achieve distal embolic protection, the operator advanced a filter wire past the stenosis and positioned the filter in the distal portion of the vein graft (Figure 7-4). The lesion in the proximal vein graft was first treated with a 3.0 mm diameter by 20 mm long compliant balloon and then with a 4.0 mm diameter by 23 mm long bare-metal stent. The stent was postdilated with a 4.5 mm diameter noncompliant balloon to high atmospheres. The filter wire was retrieved and angiography showed normal flow in the ramus with no evidence of distal embolization and an excellent luminal result. The final angiographic results are shown in Figure 7-5 and Video 7-4.
Postprocedural course
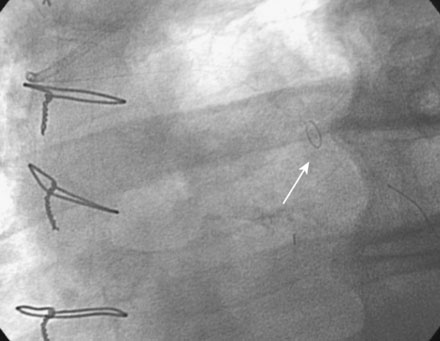
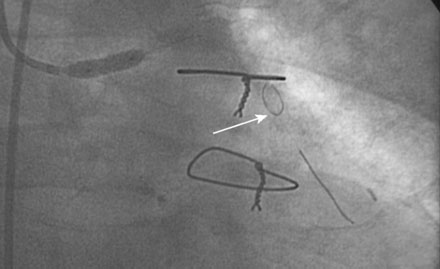
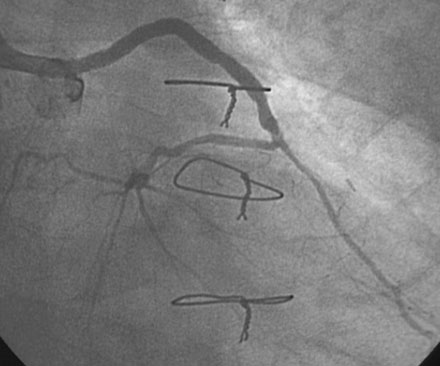
He had no postprocedure complications and was discharged the next morning on lisinopril, atenolol, furosemide, rosuvastatin, aspirin, clopidogrel, and insulin. At follow-up visits 6 weeks and 3 months later, he remained free of chest pain, orthopnea, or significant dyspnea on exertion. Repeat echocardiography found no change in his severe left ventricular dysfunction, and his physician planned to refer him for an implantable defibrillator. However, 4 months after the intervention, he experienced recurrent chest tightness and presented to the emergency room after a prolonged episode. He became pain-free after nitroglycerin administration, and his troponin peaked at 4.0 ng/mL. Repeat catheterization showed no change in the left internal mammary to the LAD, but severe focal in-stent restenosis was seen in the saphenous vein graft to the ramus (Figure 7-6 and Video 7-5). Again, a distal embolic protection device was placed and intervention performed first with a 3.5 mm balloon (Figure 7-7). A sirolimus-eluting stent (3.5 mm diameter by 18 mm long, postdilated with a 4.0 mm noncompliant balloon) was deployed within the stent (Figure 7-8 and Video 7-6). There were no complications, and, after an overnight observation, he was discharged the next morning. He remained symptom-free at follow-up visits 3 and 9 months after this second intervention.
Discussion
Saphenous vein grafts are subject to accelerated atherosclerosis. This phenomenon limits the long-term efficacy of coronary bypass surgery, as more than half of saphenous vein grafts are either occluded or have severe disease 10 years after surgery.1 Graft failure occurring very early after bypass surgery (i.e., within a month) is due to thrombotic occlusion of the graft and is often due to technical issues related to graft harvest, graft quality, or the nature of the distal vessel. Beyond the first month, lesions developing within the first year of coronary bypass surgery are typically caused by intimal proliferation, and are usually located at the aorto-ostium or distal anastomosis. After 1 year, lesions are due to more typical atherosclerosis; however, these lesions behave differently than those that affect native coronary arteries. Once atherosclerosis begins, saphenous vein graft lesions tend to rapidly progress. More importantly, unlike lesions in native coronary arteries, lesions in saphenous vein grafts are friable and prone to distal embolization when treated percutaneously. This later feature is the primary reason contributing to the high risk of saphenous vein graft interventions.
Various methods to improve the safety and efficacy of saphenous vein graft interventions have been tried. Atherectomy devices and covered stents are associated with an increased risk of distal embolization and have essentially been abandoned for this indication. In contrast to other high-risk lesions, the glycoprotein IIb/IIIa receptor antagonists have not shown any benefit in this subgroup.2 The only approach that has shown significant benefit in reducing the risk of distal embolization and periprocedural myocardial infarction in saphenous vein graft interventions is the use of distal embolic protection devices.3–5 The first generation device consisted of distal balloon occlusion during intervention, followed by aspiration of the embolic material.3 This technique has mostly been replaced by the use of distal filters, such as that used in the present case. Proximal protection is also possible, in the event that disease involves the distal segment of the graft and a filter cannot be placed beyond the lesion.5 All these devices have reduced the risk of periprocedural MI and the incidence of no-reflow and should be routinely used when performing an intervention on an atherosclerotic lesion within a vein graft. As noted above, lesions occurring at the anastomosis within the first year after bypass surgery are usually due to intimal proliferation and do not have the tendency to embolize. Similarly, although a distal protection device was used during treatment of the restenotic lesion present in this case, restenosis lesions have a very low likelihood of distal embolization and a distal protection device may not be necessary.6
For the initial intervention, a bare-metal stent was used. Unfortunately, despite the fact that the stent was postdilated to 4.5 mm diameter, the patient developed in-stent restenosis and required an additional revascularization procedure. The routine use of drug-eluting stents to treat saphenous vein graft lesions and reduce rates of restenosis is controversial. One randomized, controlled trial found a lower rate of target lesion revascularization at 6 months in patients treated with sirolimus-eluting stents compared to patients treated with bare-metal stents (5.3% vs. 27%).6 However, this effect disappeared during long-term follow-up, and, in fact, there was an unexpected higher mortality seen in the drug-eluting stent group.7 Additional studies did not show an increase in mortality among patients treated with drug-eluting stents but did not find any benefit over bare-metal stents.8,9 The most recent randomized trial comparing paclitaxel-eluting stents to bare metal stents found a similar mortality but lower rates of angiographic restenosis and target vessel failure in patients treated with drug-eluting stents.10 Thus, it is unclear whether drug-eluting stents should be routinely used in this population. As done in this case, many operators planning to use large-diameter stents employ bare-metal stents initially and reserve the use of drug-eluting stents for restenotic lesions.
1 Motwani J.G., Topol E.J. Aortocoronary saphenous vein graft disease: Pathogenesis, predisposition, and prevention. Circulation. 1998;97:916-931.
2 Roffi M., Mukherjee D., Chew D.P., Bhatt D.L., Cho L., Robbins M.A., Ziada K.M., Brennan D.M., Ellis S.G., Topol E.J. Lack of benefit from intravenous platelet glycoprotein IIb/IIIa receptor inhibition as adjunctive treatment for percutaneous interventions of aortocoronary bypass grafts. A pooled analysis of five randomized clinical trials. Circulation. 2002;106:3063-3067.
3 Baim D.S., Wahr D., George B., et alon behalf of the Saphenous vein graft Angioplasty Free of Emboli Randomized (SAFER) Trial Investigators. Randomized trial of a distal embolic protection device during percutaneous intervention of saphenous vein aorto-coronary bypass grafts. Circulation. 2002;105:1285-1290.
4 Stone G.W., Rogers C., Hermiller J., et alfor the FilterWire EX Randomized Evaluation (FIRE) Investigators. Randomized comparison of distal protection with a filter-based catheter and a balloon occlusion and aspiration system during percutaneous intervention of diseased saphenous vein aorto-coronary bypass grafts. Circulation. 2003;108:548-553.
5 Mauri L., Cox D., Hermiller J., Massaro J., Wahr J., Tay S.E., Jonas M., Popma J.J., Pavliska J., Wahr D., Rogers C. The PROXIMAL Trial: Proximal protection during saphenous vein graft intervention using the proxis embolic protection system. A randomized, prospective, multicenter clinical trial. J Am Coll Cardiol. 2007;50:1442-1449.
6 Vermeersch P., Agostoni P., Verheye S., Van den Heuvel P., Convens C., Bruining N., Van den Branden F., van Langenhove G. Randomized double-blind comparison of sirolimus-eluting stent versus bare-metal stent implantation in diseased saphenous vein grafts. Six-month angiographic, intravascular ultrasound, and clinical follow-up of the RRISC Trial. J Am Coll Cardiol. 2006;48:2423-2431.
7 Vermeersch P., Agostoni P., Verheye S., Van den Heuvel P., Convens C., Van den Branden F., van Langenhove G., for the DELAYED RRISC (Death and Events at Long-term follow-up AnalYsis. Extended Duration of the Reduction of Restenosis in Saphenous vein grafts with Cypher stent) Investigators: Increased late mortality after sirolimus-eluting stents versus bare-metal stents in diseased saphenous vein grafts. Results from the randomized DELAYED RRISC Trial. J Am Coll Cardiol. 2007;50:261-267.
8 Okabe T., Lindsay J., Buch A.N., Steinberg D.H., Roy P., Slottow T.L.S., Smith K., Torguson R., Xue Z., Satler L.F., Kent K.M., Pichard A.D., Weissman N.J., Waksman R. Drug-eluting stents verus bare-metal stents for narrowing in saphenous vein grafts. Am J Cardiol. 2008;102:530-534.
9 Vignali L., Saia F., Manari A., Santarelli A., Rubboli A., Varani E., Piovaccari G., Menozzi A., Percoco G., Benassi A., Rusticali G., Marzaroli P., Guastaroba P., Grilli R., Maresta A., Marzocchi A. Long-term outcomes with drug-eluting stents verus bare metal stents in the treatment of saphenous vein graft disease (Results from the REgistro Regionale AngiopLastiche Emilia-Romagna Registry). Am J Cardiol. 2008;101:947-952.
10 Brilakis E.S., Lichenwalter C., de Lemos J.A., et al. A randomized controlled trial of a paclitaxel-eluting stent versus a similar bare-metal stent in saphenous vein graft lesions. The SOS (Stenting of Saphenous Vein Grafts) Trial. J Am Coll Cardiol. 2009;53:919-928.