Topic 7 Pericardial structure and function
The visceral serous pericardium covers the left and right ventricles, and right atrium. It extends over the first 1–2 cm of the great vessels entering and leaving the heart, before reflecting back as the parietal serous pericardium. Posterior to the left atrium, the reflection occurs at the oblique sinus, leaving the posterior aspect of the left atrium as extrapericardial (see Figure 7.1).
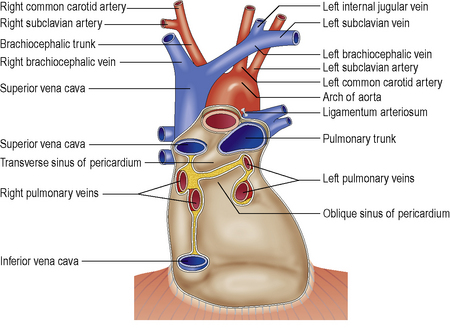
Figure 7.1 Schematic of the pericardial anatomy and reflections on the posterior aspect of the normal heart.
The E/E′ ratio is increased in CP, and is inversely proportional to PCWP (annulus paradoxus).