Introduction 151
History of blood glucose monitoring 152
Monitoring in type 2 diabetes 153
Monitoring options 160
Testing for ketones 167
Other factors that can influence monitoring of diabetes 168
Conclusion 169
References 169
Research has clearly demonstrated the link between elevated blood glucose levels and diabetic microvascular complications in people with type 1 diabetes (The Diabetes Control and Complications Trial (DCCT) 1993) and type 2 diabetes (UK Prospective Diabetes Study (UKPDS) 1998). There is evidence that elevated blood glucose levels are also linked to macrovascular complications (UKPDS 1998) (see Chapter 8). Self-monitoring, by measuring glucose levels in either blood or urine, can help people with diabetes make sense of and contribute to the decisions about self-management. Self-monitoring improves the individual’s understanding of his or her diabetes and assists the person to maintain day-to-day control of his or her blood glucose. This can enable the individual to explore the impact of exercise, diet and treatment on blood glucose levels. The value of self-monitoring of blood glucose in people with type 1 diabetes is well established, however, self-monitoring – especially blood glucose monitoring by people with type 2 diabetes – remains fiercely debated (Gallichan 1997, Reynolds & Strachan 2004, Reynolds & Webb 2006). One of the challenges for both the person with diabetes and the healthcare professional is to make this serious and complicated disease become ‘real’ so that it can be controlled successfully enough to avoid diabetic complications. It would seem reasonable, therefore, that self-monitoring, which allows the person with diabetes to recognise and correct abnormal results, could be a cornerstone to self-management of diabetes.
The two methods for self-monitoring of glucose in diabetes are through blood glucose monitoring or urine testing for glucose. An alternative to self-monitoring would be to provide a blood test at the surgery or hospital for measurement of glycated haemoglobin (HbA1c) (Owens et al 2005).
This chapter addresses monitoring in type 1 diabetes and discusses some of the controversies of monitoring in type 2 diabetes.
Self-monitoring of blood glucose (SMBG) became available to people with type 1 diabetes only in the late 1970s, initially with the availability of reagent strips that required a lengthy complex procedure including visual reading of colour change for an accurate result. In the UK, provision of strips came from the hospital pharmacy budget and there was no provision for them in primary care.
In recent years, blood glucose meters have been developed and refined and are available at relatively low cost to individuals or obtainable at no cost from many hospital clinics and GP surgeries. Strips and lancets to prick a finger to obtain a blood sample require to be prescribed by the GP. Alternatively, they can be bought at significant cost from the pharmacist (£15–£25 per box of 50 strips). Results are precise providing technique is accurate although are less exact at whole blood glucose levels less than 3.5 mmols/L. Blood glucose meters have improved significantly over recent years with more sophisticated technology and are easier and quicker to use. The procedure involves obtaining a fingertip drop of blood placing it onto a strip that has been sited or about to be sited, in a meter. The result is available anywhere within 5–30 seconds. More recently, some pharmaceutical companies have modified their meters to accept blood from alternative, less painful sites to the fingertips. The amount of blood required for an accurate result has reduced significantly and is as little as 0.3 microlitres.
URINE TESTING
Before blood glucose monitoring (BGM) becoming available, people with diabetes were asked to perform tests to measure glucose in the urine. As with measuring blood glucose, urine testing has also become a less complex procedure, which involves dipping a reagent strip into urine for 2 seconds then waiting up to 30 seconds for the strip to change colour if glucose is present. No glycosuria indicates that the blood glucose level several hours before was probably under 10 mol/L and likely to be in the acceptable range for preventing long-term complications. People with diabetes do not like urine testing, saying that repeated ‘negative’ results can lead them to believe that diabetes has gone away (Lawton et al 2004). Urine testing does not give any indication of hypoglycaemia, which can be an immediate complication of too much insulin or oral hypoglycaemic therapy. Caution should be exercised in interpreting urine testing results in the older population. The renal threshold is higher in this group and hence an individual might have a high blood glucose but no glycosuria. In this situation, regular blood glucose monitoring would detect any progressing hyperglycaemia.
HBA1C MEASUREMENT
Alternatives to self-monitoring are to do no monitoring at all and instead have a test performed by the healthcare professional known as a ‘long-term’ test: glycated haemoglobin or HbA1c. Red blood cells containing haemoglobin are made in the bone marrow, circulate for 120 days and are then removed by the spleen and liver. These millions of red cells have a mean life of around 49 days when prevailing blood glucose binds irreversibly with haemoglobin to form glycated haemoglobin or HbA1c. Measuring glycated haemoglobin is done in most laboratories and the result offers a very good test of how average the blood glucose level has been in the previous seven weeks. The DCCT (1993) demonstrated that to minimise complications in type 1 diabetes, the HbA1c should be below 7.5%. The UKPDS (1998) demonstrated that to minimise complications in type 2 diabetes the HbA1c should be below 7.1%. Nearly all research looking at glycaemic control outcomes uses HbA1c as the outcome measure. The disadvantage of the HbA1c is that it does not indicate swings in blood glucose or hypoglycaemia, nor give immediate feedback to enable the individual to make decisions regarding self-treatment or lifestyle adjustments. It remains debatable as to whether swings in blood glucose levels are damaging (Buse 2003, Davidson 2003).
The Clinical Standards Board for Scotland (2001) recommends an absolute minimum of an annual HbA1c measurement and more often as indicated. The exception to this is in pregnant women with diabetes, in whom it is important to know the trend in glycaemic control on a month-to-month basis. For someone with changing diabetes management the HbA1c would be checked not more than 2-monthly and 3- to 6-monthly in others.
FRUCTOSAMINE
This is another form of assay used to measure glucose binding to proteins in the blood. It reflects the average blood glucose level over the preceding 2–3 weeks. It is really only of value in people who suffer from a haemoglobinopathy in whom an HbA1c measurement is not accurate (Owens et al 2005).
MONITORING IN TYPE 2 DIABETES
In the early years, BGM was taught to people with type 1 diabetes to enable the regulation of glycaemic control through adjustment of insulin by the person with diabetes. In recent years, as the seriousness of type 2 diabetes has become increasingly recognised, so has the move to encourage people with type 2 diabetes to self-monitor (European NIDDM Policy Group 1994).
A major factor contributing to the increase in BGM in type 2 diabetes has been the recognition that type 2 diabetes is a progressive disease that, over time, requires increasing and changing treatment. Although someone with type 2 diabetes might start treatment with a modified diet, the UKPDS (1998) demonstrated progression to one or more types of oral hypoglycaemic agent and then to insulin therapy.
Pharmacists are keen to support people with diabetes and this includes educating individuals on how to use a meter.
The American Diabetes Association declared that SMBG has revolutionised management of diabetes (ADA 2003):
Using SMBG, patients with diabetes can work to achieve and maintain specific glycemic goal targets.
Although the above quote is questionable in terms of the evidence available, the ADA also states that SMBG can monitor the efficacy of diet, medications and exercise. The Association recommends that all people on insulin or oral hypoglycaemic agents should perform SMBG on a daily basis. The number of tests a day should be agreed between the healthcare professional and the individual. The ADA affirms that the role of SMBG in people who control their diabetes with diet alone is not known.
The European consensus guidelines states that urine testing can be useful for people who cannot manage blood testing where this is indicated in people whose glucose control is deteriorating or who take insulin (European Diabetes Policy Group 1999). This suggests that BGM is the first choice of monitoring but it also suggests that self-monitoring in people whose diabetes is stable might be unnecessary.
Within the UK there has been the continued development of a consensus statement around the debate of SMBG and recommendations are presented in Table 7.1 (Owens et al 2005). These recommendations have been developed around current research and expert opinion (Reynolds & Webb 2006).
| Diabetes type | Treatment group | Monitoring regimen |
|---|---|---|
| Type 1 diabetes | All people with type 1 diabetes |
SMBG should be regarded as an integral part of treating all people with type 1 diabetes
People with type 1 diabetes should be educated to SMBG and adjust treatment appropriately
The majority of patients with type 1 diabetes should consider SMBG four or more times per day to prevent hypoglycaemia and control hyperglycaemia Avoiding metabolic emergencies such as diabetic ketoacidosis might require frequent SMBG
|
| Diabetic pregnancy | Diabetic pregnancy |
Pregnant women with type 1 diabetes, plus those with type 2 diabetes requiring insulin and patients with gestational diabetes requiring insulin should SMBG at least four times per day to include both fasting and post-meal blood glucose measurements
In diet-treated patients it may be necessary to SMBG with the same frequency as insulin-treated patients to ensure strict glycaemic control
In insulin-treated patients increased frequency of testing may be necessary in the first trimester when the risk of hypoglycaemia is greatest
|
| Type 2 diabetes | Intensive insulin therapy |
People who adopt intensive insulin therapies require regular feedback regarding SMBG levels
People with type 2 diabetes who use a multiple daily insulin regimen should SMBG in the same way as those with type 1 diabetes
Fasting blood glucose should be tested daily during basal insulin dose titration
|
| Type 2 diabetes | Conventional insulin therapy | People with type 2 diabetes who are using a conventional insulin regimen and who have stable control should SMBG two or three times a week People with type 2 diabetes who are using a conventional insulin regimen and who have less stable control should SMBG at least once daily, varying the time of testing between fasting, pre-meal and post-meal Fasting blood glucose should be tested daily during basal insulin dose titration |
| Type 2 diabetes | Combined insulin and oral antidiabetic therapy |
Fasting blood glucose should be tested daily during basal insulin dose titration
People with type 2 diabetes who use insulin or oral hypoglycaemic agents should SMBG at least once daily, varying the time of testing between fasting, pre-meal and post-meal
|
| Type 2 diabetes | Diet and exercise |
People with type 2 diabetes who have good control on diet and exercise, metformin or glitazone treatment do not need SMBG monitoring, unless they are destabilized by other factors
Glycaemic control managed through diet and exercise in people with type 2 diabetes is best monitored through HbA1c testing
Patients with type 2 diabetes managed only by diet and exercise do not normally require routine SMBG
Informed patients might choose SMBG as a means of monitoring lifestyle changes
|
| Type 2 diabetes | Metformin (±glitazone) | As for diet and exercise |
| Type 2 diabetes | Glitazone (±metformin) | As for diet and exercise |
| Type 2 diabetes | Sulphonylurea alone (or in combination with oral antidiabetic agents) | Hypoglycaemia can be more common than assumed in people with type 2 diabetes on sulphonylureas and SMBG will reveal this situation |
THE EVIDENCE
A health technology assessment (HTA) systematic review examining blood glucose monitoring in diabetes was published in 2000 (Coster et al 2000). Part of the review related to BGM in type 2 diabetes. Only eight randomised controlled trials (RCTs) in relation to BGM in type 2 diabetes were identified as being robust enough to be included in the review. The eight RCTs included comparisons of blood testing, urine testing and no testing. Interventions were not standardised and training of individuals and adherence were not addressed systematically. No trial required subjects to modify their drug therapy in accordance with self-monitoring results. Six studies were included in a meta-analysis. The review concluded:
…that the results do not provide evidence for clinical effectiveness of an item of care with appreciable costs. Further work is needed to evaluate self-monitoring so that resources for diabetes care can be used more efficiently. (Coster et al 2000)
A more recent meta-analysis of SMBG in people with type 2 diabetes not using insulin looked at six RCTs and concluded cautiously that SMBG has a small beneficial effect on glycaemic control but, again, stated that there were methodological issues with the studies (Welschen et al 2005). From these studies it would appear that some people benefit more from SMBG than others; however, the cost of SMBG is very likely to be offset against the longer-term costs of care for the same people (Bandolier 2005). Given that BGM in type 2 diabetes remains so controversial, it is prudent at this point to consider why clinicians appear to be ignoring the evidence, or, rather, ignoring the lack of evidence (Barraclough 2003). There are several ways of interpreting ‘lack of evidence’:
▪ The research has not measured what it should have measured.
▪ The intervention might have had positive effects for some, but not all subjects and an RCT would not necessarily identify these differences.
▪ The research might not have been done.
▪ There is no supporting evidence, even when trials with adequate power have been properly conducted.
▪ Some people believe that the only evidence worth considering is that obtained from RCTs.
▪ Some studies might not have been able to separate other factors that could influence glycaemic control; they might not have had control groups (Gallichan 1997).
Clues about ‘other factors’ are hinted at in the three qualitative studies described below.
A qualitative study of people with type 1 diabetes found that, although there is no doubt that blood glucose monitoring is acceptable and useful for some, it is clearly difficult and painful, both physically and psychologically, for others (Fox et al 1994). Some people are unable to make the connection between testing and using test results to influence glycaemic control (Fox et al 1994).
A more recent grounded theory study of 40 people with type 2 diabetes diagnosed within the previous 6 months and interviewed twice during a 9-month period found that those who had optimal glycaemic control viewed monitoring in a positive light. However, the reverse was true for those who observed results outside the recommended range (Peel et al 2004). Positive aspects of monitoring were stated as modifying lifestyle and adjusting regimens based on results. Negative aspects identified were: evidence of the existence of their diabetes; additional distress if results were inexplicable; they did not know what to do with the results; monitoring for the benefit of the doctor, which was especially annoying if health professionals did not enquire about results. Not enquiring about results might reflect the reliance of healthcare professionals on HbA1c.
The same research group also recently completed a grounded theory study on urine testing (Lawton et al 2004). Forty people with newly diagnosed type 2 diabetes were interviewed three times over 1 year. They expressed ‘profoundly negative views’ on urine testing. Urine testing was perceived as less accurate, less hygienic and less convenient than BGM. Negative urine results were seen as an indicator that they did not have diabetes. It is interesting to compare these results with those of a study where a more positive view of urine testing was taken (Miles et al 1997). It suggests differences in diabetes education programmes and might reflect the personal views of the diabetes educators.
The fact that SMBG, from the point of view of the person with diabetes, has many costs and benefits is reflected in a survey of people with type 1 and type 2 diabetes who take insulin in the Tayside Region of Scotland (Evans et al 1999). This study showed that uptake of BGM strips from pharmacists was far below that prescribed, suggesting that people are not testing as often as requested by health professionals.
Another study showed that for those with type 2 diabetes taking insulin, SMBG was a useful aid and improved glycaemic control when insulin adjustment had been taught (Franciosi et al 2001). However, the same study showed that, in those not treated with insulin, SMBG was associated with higher HbA1c levels and higher psychological burden. The authors stated that SMBG should be part of wider educational programme but at the same time recommended that SMBG should be limited to those on insulin therapy.
Conversely, a prospective, multicentre randomised controlled study showed that glycaemic control improved significantly in the intervention group (Schwedes et al 2002). The intervention group received a structured diabetes education programme that included feedback. Participants maintained a diary of eating habits and SMBG results. Self-monitoring resulted in marked improvement in general well-being with significant improvement in the subitems depression and lack of well-being (Schwedes et al 2002). The researchers concluded that meal-related SMBG within a structured counselling programme improved glycaemic control in the majority of the intervention group of this study. The weakness of the study was that the final outcome measures were assessed at 6 months after the completion of the intervention and it is well recognised that an intervention effect can wear off after 6 months. However, the point is that it is still possible for SMBG to be effective if it is presented in the correct context of an effective education programme. In a later study, the same group demonstrated that SMBG was beneficial in the context of a short, structured education programme that included considering options for action (self-reflection) and believing in self-efficacy (self-regulation) (Siebolds et al 2006).
FINANCIAL IMPLICATIONS TO THE HEALTH SERVICES
Blood glucose monitoring is a costly procedure. The publication of the National Institute for Health and Clinical Excellence (NICE) guideline on blood glucose control in type 2 diabetes (NICE 2002) resulted in restrictions by GPs on the prescribing of blood glucose strips. The NICE guideline stated that self-monitoring:
▪ should not be considered as a stand-alone intervention
▪ should be taught if the needs and purpose are clear and agreed with the individual
▪ can be used in conjunction with appropriate therapy as part of integrated care.
Some primary care organisations have interpreted these guidelines to mean that as SMBG in type 2 diabetes has no evidence base, substantial cost savings can be gained from restrictions on prescribing SMBG strips. This has been refuted by Diabetes UK (2004). It is acknowledged that there are resource implications in utilising SMBG. Such resources include the actual equipment for BGM as well as the support required from health professionals to teach the skill and assist individuals to place SMBG within their own lifestyle context.
The NHS National Prescribing Centre (2002) produced a bulletin summarising the evidence for SMBG in type 2 diabetes. The first point was that the NHS spends 40% more on BGM materials than it does on oral hypoglycaemic agents (£90 million versus £64 million in 2001). The bulletin goes on to repeat the recommendations of the NICE report as above but also points out that there is no evidence that BGM is more effective than urine testing. It concludes that measurement of HbA1c is likely to provide more helpful information than day-to-day monitoring. This ignores the fact that SMBG and HbA1c are different measures measuring different aspects of glycaemic control. It is possible for people with diabetes to have HbA1c levels at target but be experiencing both frequent hypoglycaemia and/or hyperglycaemia. The above statement also raises the issue of patient empowerment.
EMPOWERMENT
Empowerment of individuals has been acknowledged at a government level and constitutes a significant role in the Diabetes National Service Framework documents for each country in the United Kingdom (Department of Health (DH) 2003, Northern Ireland Task Force 2003, Scottish Executive Health Department (SEHD) 2002, Welsh Department of Health 2003). However, there is a sense in these documents that a person with diabetes is empowered only if he or she follows the rules of diabetes management. There is a simplicity in the assumption that diabetes education results in optimal glycaemic control. There are all sorts of reasons why people with diabetes do not adhere to the ‘rules’ of self-management, not least the fear of hypoglycaemia.
A recent study has highlighted that the incidence of severe hypoglycaemia (requiring assistance from another) in people with either type 1 or type 2 diabetes is much higher than previously reported (Leese et al 2003). Severe hypoglycaemia is an accepted reason for not striving for glycaemia in the ‘normal’ range. Hypoglycaemia has long been recognised as an inevitable side-effect of the sulphonylurea oral agents and insulin therapy. The DCCT (1993) demonstrated that the achievement of near-normal glycaemia was at the expense of a two- to threefold increase in the incidence of severe hypoglycaemia. SMBG has therefore become even more critical for the person aiming for tight glycaemic control, although this is not the target for everyone (see Chapters 4 and 5). SMBG is necessary for the fine-tuning of insulin doses (or sulphonylurea) and allows the person with diabetes to maintain near-normal blood glucose levels with as few episodes of hypoglycaemia as possible.
To ensure appropriate use of the findings, individuals should be reviewed by professionals to discuss the meaning of results and their management options. Without support, people can become distressed by inexplicable results (Peel et al 2004). Whichever form of monitoring a person chooses, he or she should be supported in the choice and encouraged to take an active role in self-management. Self-monitoring should be continually reinforced, and support given to enable the chosen lifestyle changes to occur.
Self-monitoring does not improve a person’s diabetes control by itself (NICE 2002). A recent survey undertaken by Diabetes UK (2004) showed that only 28% of the sample of 361 people received any written information about testing. It was also found that key barriers to testing were associated pain, forgetfulness, inconvenience and anger when healthcare professionals appeared to show little interest in their results. The targets agreed should be specific to the individual person and be realistically achievable taking into account a variety of factors, including the risk of hypoglycaemia and diabetes-related complications, as well as the ability to undertake the skill (Home et al 2002). Agreed targets should be documented and an interest shown by the healthcare team in the individual’s self-monitoring results (Diabetes UK 2004).
MONITORING OPTIONS
The decision on type of self-monitoring should take place following discussion with the individual. Although the healthcare professional might be constrained by guidelines, the ideal scenario would be that the method chosen is as a result of the individual’s understanding of the costs and benefits of each method, including how self-monitoring fits into his or her responsibilities in relation to self-management options. The person with diabetes is entitled to consider no self-monitoring as an option and might choose a periodic HbA1c at the surgery or diabetic clinic.
Whether the person is physically able to undertake the test should be considered. Physical constraints, for example visual deficits or a lack of manual dexterity, can prevent or inhibit testing. A survey undertaken by Diabetes UK demonstrated that a significant number of people had a disability that affected their use of blood glucose meters (Diabetes UK 2004). Equally, an inability to read either the time or results, or a lack of understanding of either spoken or written English, will make monitoring more difficult to teach and to comprehend.
If monitoring is envisaged as being of value only to the healthcare team (either by the healthcare team and/or the individual with diabetes) clinic visits might become more frequent and self-monitoring becomes the focus of the consultation rather than the person with diabetes engaging in problem solving and decision making. Self-monitoring is a first step in facilitating self-empowerment.
URINE TESTING
Urine testing has the advantages of being cheap, easy to use and painless to carry out. Some people with diabetes do, however, find the concept of urine testing ‘dirty’ or unpleasant. In comparison with blood glucose monitoring, people find urine testing to be less convenient, less hygienic and less accurate (Lawton et al 2004). In the UK, urine testing has been predominantly replaced by BGM (Owens et al 2005); however, it may be the only form of monitoring in some other countries and is therefore included here for clarification.
Testing for glycosuria reflects only what has been happening to the blood glucose since the bladder was last emptied, hence it gives a relatively crude assessment of glycaemic control. It gives no indication of the current blood glucose level, nor does it demonstrate fluctuations in levels. The amount of glycosuria is also influenced by the renal threshold for glucose, which is affected by many factors (box 7.1). The renal threshold can be visualised as a dam for glucose. It is only when the blood glucose level exceeds the level of the dam that there will be any glycosuria. The double-voided sample is considered more accurate than the first ‘stored’ sample, especially first thing in the morning. The bladder should be emptied and, after drinking a glass of water, the person encouraged to pass urine that should be the sample used for testing.
Box 7.1
Factors affecting the renal threshold
▪ Lower in children and increases with age
▪ Lower during pregnancy
▪ Increases with duration of diabetes
▪ Time of day: it varies throughout the normal day
▪ Population groups: it varies among different populations
▪ Medications: ascorbic acid and salicylates give a false-positive result
▪ Fluid intake and urine concentration
Any glycosuria present usually reflects an elevated blood glucose level in the hours before the bladder was last emptied. In an adult this frequently means that the blood glucose has been above 10 mmol/L. A negative result for glycosuria cannot be related to a particular level of blood glucose. A negative result implies only that the blood glucose has probably been below the renal threshold since the last time the patient passed urine; it gives no indication of hypoglycaemia. It is possible that individuals could have glycaemic control that is considered ‘too tight’, resulting in the increased risk of hypoglycaemia. By contrast, those people who like to have ‘a touch of sugar in the urine’ might in fact have blood glucose levels well in excess of 10 mmol/L.
Self-monitoring should be supported by a periodic HbA1c to fully inform major therapy changes such as oral hypoglycaemic agents to insulin therapy.
Before proceeding to teach urine testing to Richard (in Case study 7.1), an estimation of his renal threshold should be obtained. This can be done by testing a double-voided urine sample for glucose, and at approximately the same time obtaining a blood glucose result. If the two results are compatible with a renal threshold of 10 mmol/l then, should he so choose, he would be taught this method of monitoring.
Case study 7.1
Richard is a 56-year-old man who has just been diagnosed as having type 2 diabetes. He has a strong family history of type 2 diabetes and is therefore not totally surprised at the diagnosis. He is aware of the long-term implications of the disease. Richard’s initial management is by diet alone. In discussion, he remembers his mother testing her urine for glucose. His brother, who also has type 2 diabetes, has just started testing his own blood glucose levels because his diabetes is going out of control. Richard asks how he ought to be monitoring his diabetes.
Richard appears eager and willing to monitor his diabetes. Through personal experience he is aware of two forms of monitoring. The healthcare professional will discuss with him the advantages and disadvantages of both methods and will facilitate his choice. The challenge is to be sure he is fully informed and to teach him his chosen method, the meaning of his results, how the results relate to his lifestyle and what action to take in relation to his results.
The testing of urine with dipsticks is familiar to most healthcare professionals and is given in Box 7.2 and Box 7.3.
Box 7.2
Equipment for urine testing
▪ Disposable gloves for the healthcare professional
▪ A clean container for the sample (optional)
▪ Urine testing dipsticks
▪ A watch or clock with a second hand
▪ Diary to record results
Box 7.3
Procedure for urine testing
▪ The healthcare professional wears gloves
▪ The expiry date of the urine testing strips is checked
▪ The urine to be tested should be the second sample passed at the required time and collected in a clean container
▪ One urine testing strip should be removed from the container and the lid replaced securely
▪ The strip should be dipped briefly into the urine sample and the time noted. When removing the strip it should be wiped along the rim of the container to remove any excess urine. Alternatively, the strip can be passed through the stream of urine at time of voiding and the time noted
▪ After the appropriate time, the colour reaction is compared with the side of the container according to the manufacturer’s instructions. Individuals with colour blindness might not be able to assess colour change
▪ Results should be recorded and reviewed at every consultation
▪ Store the urine test strips container in a safe, dry place away from children
Frequency of urine testing and its meaning
There is no consensus on the optimal frequency of urine testing for Richard (case study 7.1); however, it is generally recommended that four tests are performed each week. A pre-breakfast test reflects glycaemic control in the early hours of the morning and is a reflection of adequacy of insulin secretion in the individual. A test 2 hours after the main meal of the day (which may be lunch or evening meal) reflects how well the pancreas copes with a carbohydrate load. Testing at these times twice a week gives an overall guide of glycaemic control. The person should be taught how to record these results and advised to bring this record book to each clinic attendance. The significance of the results should be discussed.
SELF-MONITORING OF BLOOD GLUCOSE
When presenting monitoring management options to Richard, it would be pointed out that recent recommendations state that SMBG is not necessary (see Table 7.1). Whereas SMBG has the advantage of being accurate when the procedure is done properly, it does reflect the blood glucose concentration at a precise time. SMBG would also allow Richard to evaluate the effects of diet, exercise and lifestyle interventions on his blood glucose levels. Recent improvements in technology have made the procedure less complex: smaller amounts of blood are required and finger-pricking is less painful. If Richard asked to undertake SMBG, he should be facilitated in this choice. The equipment and procedure for undertaking SMBG are detailed in Box 7.4 and Box 7.5.
Box 7.4
Equipment for SMBG
▪ Disposable gloves for the healthcare professional
▪ A finger-pricking device
▪ Cotton wool or paper tissues
▪ A watch or clock with a second hand (if not using a timed meter)
▪ Blood glucose testing strips
▪ Disposal container for lancet
▪ Meter
▪ Diary to record results
Box 7.5
Procedure for SMBG
▪ The healthcare professional wears gloves
▪ The expiry date of the blood testing strips must be checked
▪ The person with diabetes washes his or her hands in warm water and dried. This not only cleanses the skin but also promotes blood flow to the fingertips
▪ To reduce the pain, the finger can be pricked on the side, using an appropriate device. Testing can be rotated around all the fingers but avoiding thumb and forefinger
▪ The drop of blood is applied to the blood glucose testing strip according to the manufacturer’s instructions
▪ The process is timed accurately
▪ Results should be recorded and referred to at every consultation
▪ The testing sticks container should be stored in a safe, dry place away from children
Visual reading of strips is no longer an option in the UK; however, strips for visual reading only are still available in other countries. Principles for SMBG, whether by meter or visually read remain the same. The procedure has to be carried out precisely as outlined in box 7.5 to obtain an accurate result.
Testing can be rotated around all the fingers but the thumbs and forefingers can become tender as these are used most in day-to-day activities. It is recommended that people do not squeeze their fingers to acquire a drop of blood as this can produce a mixture of serous fluid and blood and this may alter the result (Balance 2000). The blood drop is applied to the blood glucose test strip according to the manufacturer’s instructions and this will stimulate the timing mechanism on the blood glucose meter. Too little blood can result in a falsely low result and if a meter is not being used, a watch with a seconds hand should be used to ensure accurate timings before reading results.
Learning new skills requires practice. To help eliminate error in this procedure, the person should be taught how to perform SMBG, observed doing so, encouraged to practice (initially using glucose solution, which is included with the meter) and demonstrate the skill to the healthcare professional. Thereafter, an annual reassessment can be carried out, which would include technique and also a quality check of the meter itself.
Bringing blood monitoring equipment to all consultations will allow facilitation of any problem solving that people might require in relation to SMBG. This could include checking SMBG technique and cleaning and checking of the meter. Many people find the recording of results in a diary tedious and yet checking back in the memory bank of the meter can be frustrating because it can be difficult to see patterns of blood glucose results. Many pharmaceutical companies provide software that can be used by the individual with diabetes or the diabetic clinic to download results from the memory of the meter.
Finger-pricking lancets should be disposed of safely inside a firm plastic container, which should be secured before being deposited in the dustbin. In some areas, sharps containers are issued and local policies have been established whereby people deposit used lancets and needles at the local health centre. Finger-pricking devices are usually included with the purchase of a meter or can be purchased separately from manufacturers. To prevent cross-infection, finger-pricking devices should be used only by its owner and in accordance with the instructions of the manufacturer.
Frequency of SBMBG and its meaning
A recent consensus guideline on SMBG for all people with diabetes has been published (Owens et al 2005; see Table 7.1). In deciding on the frequency and timing of tests for those with type 1 diabetes and those with type 2 diabetes the authors took into account the differences between patterns in glycaemia in both conditions.
Type 1 diabetes is characterised by blood glucose levels that can range from hypoglycaemia to hyperglycaemia, often with little pattern from day to day. This has particularly been observed by continuous glucose monitoring systems (Sachedina & Pickup 2003). This means that a single blood glucose measure cannot relate to HbA1c results and the more erratic the blood glucose profile then the more frequent SMBG needs to be performed. This is necessary to understand treatment requirements and also for the individual to avoid extremes of blood glucose that might impair performance during day-to-day tasks such as driving. It is recommended that people with type 1 diabetes should consider SMBG at least four times per day.
People with type 2 diabetes are recommended to self-monitor their blood glucose according to their treatment regimen (see Table 7.1). The table gives recommendations and obviously individuals have to choose whether to follow them. In people with type 2 diabetes who are treated only with diet, both fasting and postprandial blood glucose levels are elevated but are also predictable from day to day (Pickup 2003). A more recent study has found that the correlation depends on the level of glycaemic control. The higher the fasting blood glucose, the more likely it is to relate to HbA1c. Owens et al (2005) conclude that a fasting blood glucose is a better measure of overall glycaemic control where control is suboptimal and that a postprandial blood glucose measure is better in those who have optimal glycaemic control.
Duncan has found it hard to accept his diagnosis of type 2 diabetes. He questioned his GP about the possibility that his diagnosis was a mistake. He had no symptoms and indeed felt very well. His GP suggested that performing blood glucose monitoring might enable Duncan to gain a sense of what happened to his blood glucose under varying lifestyle conditions. Duncan agreed to give this a try and after being taught how to SMBG by the community nurse he returned for a review, full of enthusiasm about what he had discovered. He had observed that his blood glucose levels rose above the target range 2 hours after a meal. He had also observed that his blood glucose level dropped in the late afternoon following a brisk walk. He had noted that during a period of illness his blood glucose levels rose despite his appetite being diminished. He found these results motivating in terms of the choices he could make about lifestyle and also precipitated questions such as glycaemic control in relation to illness, etc.
Although urine testing is easier to perform than SMBG, SMBG gives a more accurate picture of blood glucose levels at any particular point in time. Duncan might have seen some of the above changes if he had performed urine testing but not in the precise more quantifiable way that allowed him to see the impact of all lifestyle behaviours. Additionally, SMBG helped Duncan to accept that his diabetes was a reality.
SMBG is recommended as an integral part of treatment for everyone with type 1 diabetes. For those who are reluctant to SMBG, it is further recommended that they undertake SMBG for 2 weeks before any clinic appointment to facilitate the decision making at that meeting (Owens et al 2005).
SMBG is also recommended for those people who are heading towards the maximum dose of oral agents and/or whose control is suboptimal. There might be times when people with type 2 diabetes seem to be failing on oral hypoglycemic agents but in fact might be able to improve things with lifestyle adjustments. SMBG can be motivating in maintaining behaviour change but can also help the individual to accept that having done his or her best with lifestyle changes then insulin therapy might be the inevitable therapeutic option.
SMBG should also be considered in the younger person with type 2 diabetes who, by virtue of the age of onset of the disease, are more likely to live with the disease for longer and require insulin therapy at some point.
In those for whom there would not appear to be any real benefits from close monitoring of diabetes control, for example, someone with a reluctance to self monitor, no risk of hypoglycaemia, stable glycaemic control with an HbA1c level that is agreed to be optimal, then HbA1c alone might be an acceptable option (Owens et al 2005).
INTERPRETING FASTING/RANDOM GLUCOSE RESULTS IN THE CLINIC
To acquire some objective assessment of glycaemic control in the clinic, a fasting blood glucose or a random blood glucose can be checked.
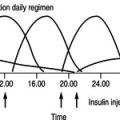
In type 1 diabetes, blood glucose profiles can show considerable variation throughout the day. This is because the primary causation of type 1 diabetes is lack of endogenous insulin and blood glucose is reliant on the balancing act between food, exercise, stress levels and insulin injections.
Most people with type 2 diabetes retain some secretion of insulin from their beta cells and are in fact insulin resistant as opposed to insulin deficient, hence a 24-hour glucose profile of the person with type 2 diabetes is very similar to the normal person’s profile, although the levels are significantly higher. A fasting blood glucose or random blood glucose will give a fairly accurate reflection of glucose levels throughout the day (Pickup 2003). As the fasting blood glucose is less likely to be influenced by what has recently been eaten, this result is probably the most important.
METERS FOR THE VISUALLY IMPAIRED
In the UK, meters for the visually impaired has been an area of neglect. After many years, when no speaking meters were available, there is now the new SensoCard Plus Meter from Cobolt Systems Ltd. The SensoCard speaks instructions for the user and also speaks the result. The meter has recently come down in price and strips are available on prescription. Control solution to check the meter is working properly and software to download results to a computer are also available from the company (www.cobolt.co.u)
Roche-Diagnostics also have a meter called Compact Plus which has an Acoustic Mode that can be activated for people who are visually impaired. The Acoustic Mode delivers a sequence of beeps which represent the results and the various error messages. Roche-Diagnostics are also expecting to launch the Voice Mate in the near future. This device obviously has a speaking mechanism to enable use by the visually impaired (www.roche-diagnostics.co.uk).
People with visual impairment can find it difficult to use finger-pricking devices and accurately apply a drop of blood to a test strip. However, in reality, they often find their own way to overcome these obstacles.
CONTINUOUS GLUCOSE MONITORING
Continuous monitoring of glucose levels over a 2–3 day period is a relatively new technique that is now available in some secondary care centres. Two systems are currently available, the Menarini Glucoday and the Roche MiniMed Glucose Monitoring System. Both involve passing a very fine catheter subcutaneously into the abdomen and measuring the interstitial fluid glucose levels. This can be a useful procedure for people with diabetes who have inexplicable patterns. It is particularly useful for detecting patterns of asymptomatic hypoglycaemia.
TESTING FOR KETONES
Whereas testing for glycosuria has been largely superseded by SMBG, there is still an important place for blood or urine testing for ketones.
All people taking sulphonylurea agents or insulin therapy should be advised to increase the frequency of their blood or urine monitoring during an illness. Those on insulin should also test their blood or urine for ketones 4-hourly, as they are at risk of developing diabetic ketoacidosis. They should seek help if any ketones appear in their blood or urine or if they do not feel better within 24 hours.
Growing children and teenagers are most prone to developing diabetic ketoacidosis because of high levels of growth hormone, which antagonises the action of insulin. As in all people with type 1 diabetes, a prompt and substantial increase in insulin dose might avert hospital admission.
As ketone testing strips are probably used infrequently, people need to be reminded to acquire new sticks before their old ones have gone beyond the expiry date. Some modern blood glucose meters are also able to measure blood ketones as well as blood glucose. This can be an advantage, as the people at most risk of diabetic ketoacidosis are most likely to have access to blood glucose monitoring already and so this eliminates the need to test for urine ketones.
Some people are meticulous in their monitoring and insulin dose adjustment yet never achieve optimal diabetic control. Polonsky (2002), in addressing the issue of burnout in people with diabetes, describes this as a situation where the person feels overwhelmed and defeated. People experience frustration and feelings of failure. Glycaemic control is worse and self-care deteriorates.
It is easy to understand that monitoring can contribute to these feelings, especially if the individual has not had the problem-solving education required to know how to act on suboptimal blood glucose levels. Some people need to take a break from diabetes by stopping monitoring for a while. Polonsky (2002) suggests that healthcare professionals need to be aware of burnout and suggests strategies to support people with diabetes if they have reached this stage. This includes establishing a strong collaborative relationship, negotiating person-centred goals and engaging the individual in active problem solving (see Chapter 3). A systematic review and meta-analysis of RCTs of psychological interventions in people with type 2 diabetes showed that these not only improved glycaemic control but also reduced psychological distress (Ismail et al 2004).
QUALITY ASSURANCE
Quality assurance is important because mistakes can lead to inappropriate treatment and management. It is the responsibility of the healthcare team to ensure that clinical measurements are as reliable and accurate as possible, as clinical decisions are made on this basis. Quality assurance relates not only to professionals but also to the individuals with diabetes who are self-monitoring (Seley & Quigley 2000).
Quality control of blood glucose monitoring in community practice is therefore of the utmost importance. It should assess two components of testing:
1. the testing skills of the personnel
2. the accuracy and performance of the testing equipment.
Most biochemistry departments have now implemented a quality assurance system for blood glucose meters and training of personnel within their areas. Training in technique and regular assessment of performance is essential. One way of assessing skill is by peer assessment. Asking a colleague to assess another coldleague’s technique according to the agreed standard will identify any areas of possible improvement.
Alternatively, solutions of known glucose concentration could be ‘tested’ to evaluate performance of either a meter or the individual performing the test. Each manufacturer gives different instructions for the use of their product. The healthcare team must be aware of the differences between the products and be competent in their practice.
The frequency of quality control can be determined by local practices but a weekly calibration and checking of meters used in practice is not uncommon. A record should be kept of the quality control results to assist with audit of this practice.
People with diabetes are encouraged to self-monitor either their blood or urine for glucose. Blood glucose testing gives a more precise result. The frequency of monitoring is variable but the timing depends on whether the person has type 1 diabetes or type 2 diabetes treated without insulin.
Despite self-monitoring, people with diabetes do not appear to be adjusting their therapy in the light of their results. Structured education programmes are widely recommended in guidelines and research. In these, self-monitoring is put into a context that enables people with diabetes to understand its value on a personal level. People require ongoing support and encouragement from all members of the healthcare team to build their confidence in therapy and lifestyle adjustments. Self-monitoring remains difficult for some individuals and the role of the healthcare professional is to assist with strategies that will help the individual overcome some of the barriers.
Some people will be dependent on others to monitor their diabetes for them. This will include those who are community nurse dependent and those with visual, literacy or language problems.
The healthcare team and the person with diabetes have various tests available to them to assess glycaemic control. These measure different parameters and should be used to acquire an overall picture of an individual’s glycaemic control.
REFERENCES
American Diabetes Association (ADA), Position statement. Tests of glycemia in diabetes, Diabetes Care 26 (2003) S106–S108.
Balance, Accurate glucose tests (2000 March—April) 66–69.
Bandolier, Glucose monitoring in type 2 diabetes, Online. Available: www.jr2.ox.ac.uk/bandolier/band134/b134-2.html (2005).
K Barraclough, There is no evidence that…, British Medical Journal 326 (7398) (2003) 1095.
JB Buse, Should postprandial glucose be routinely measured and treated to a particular target? No! Diabetes Care 26 (2003) 1615–1618.
Clinical Standards Board for Scotland (CSBS), Clinical standards for diabetes. (2001) CSBS, Edinburgh .
S Coster, MC Gulliford, PT Seed, et al., Self-monitoring in type 2 diabetes mellitus: a meta analysis, Diabetic Medicine 17 (2000) 755–761.
J Davidson, Should postprandial glucose be routinely measured and treated as a particular target? Yes! Diabetes Care 26 (2003) 1919–1921.
Department of Health (DH), National service framework for diabetes. (2003) DH, London ; Online. Available: www.publications.doh.gov.uk/nsf/diabetes/.
Diabetes Control and Complications Trial (DCCT) Research Group, The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus, New England Journal of Medicine 329 (1993) 977–986 .
Diabetes UK, What’s the point? Survey of attitudes among non-testers, Online. Available: www.diabetes.org.uk/infocentre/htm (2004).
European Diabetes Policy Group, A desktop guide to type 2 diabetes mellitus, Diabetic Medicine 16 September 1999 16 (9) (1999) 716–730.
European NIDDM Policy Group, A desktop guide of non-insulin dependent diabetes mellitus: an update, Diabetic Medicine 11 (1994) 899–909.
JMM Evans, RW Newton, DA Ruta, et al., Frequency of blood glucose monitoring in relation to glycaemic control: observational study with diabetes database, British Medical Journal 319 (1999) 83–86.
C Fox, G Wade, A Fox, What makes people with diabetes measure their blood glucose? Practical Diabetes International 11 (6) (1994) 74–76.
M Franciosi, F Pellegrini, G De Beradis, et al., The impact of blood glucose self monitoring on metabolic control and quality of life in type 2 diabetic patients, Diabetes Care 24 (2001) 1870–1977.
M Gallichan, Self-monitoring of glucose by people with diabetes: evidence based practice, British Medical Journal 314 (1997) 964–967.
P Home, A Chacra, J Chan, et al., Considerations on blood glucose management in type 2 diabetes mellitus, Diabetes Metabolism Research and Reviews 18 (4) (2002) 273–285.
K Ismail, K Winkley, S Rabe-Hesketh, Systematic review and meta-analysis of randomized controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes, Lancet 363 (2004) 1589–1597.
J Lawton, E Peel, M Douglas, O Parry, ‘Urine testing is a waste of time’: newly diagnosed type 2 diabetes patients’ perceptions of self-monitoring, Diabetic Medicine 21 (9) (2004) 1045–1048.
GP Leese, J Wag, J Broomhall, et al., Frequency of severe hypoglycaemia requiring emergency treatment in type 1 and type 2 diabetes, Diabetes Care 26 (2003) 1176–1180.
P Miles, J Everett, J Murphy, D Kerr, Comparison of blood or urine testing by patients with newly diagnosed non-insulin dependent diabetes: patient survey after randomised crossover trial, British Medical Journal 315 (7104) (1997) 348–352.
National Institute for Health and Clinical Excellence (NICE), Management of type 2 diabetes — managing blood glucose levels. Guideline G. (2002) NICE, London ; Online. Available: www.nice.org.uk/page.aspx?o=213902.
Northern Ireland Task Force, The Report of the Joint Clinical Resource Efficiency Support Team (CREST) Diabetes UK Taskforce on diabetes: a blueprint for diabetes care in Northern Ireland in the 21st century, Online. Available: www.diabetes.org.uk/frameworks/frameworks.htm (2003).
D Owens, J Pickup, AH Barnett, et al., The continuing debate on self-monitoring of blood glucose in diabetes, Diabetes and Primary Care 7 (2005) 9–21.
E Peel, O Parry, M Douglas, J Lawton, Blood glucose self-monitoring in non-insulin treated type 2 diabetes: qualitative study of patient’s perspectives, British Journal of General Practice 54 (2004) 183–188.
JC Pickup, Diabetic control and its measurement, In: (Editors: JC Pickup, G Williams) Textbook of diabetes 3rd edn. (2003) Blackwell Publishing, Oxford, pp. 34.1–34.17.
WH Polonsky, Understanding and treating patients with diabetes burnout, In: (Editors: BJ Anderson, RR Rubin) Practical psychology for diabetes clinicians (2002) American Diabetes Association, Alexandria, TX.
RM Reynolds, MWJ Strachan, Home blood glucose monitoring in type 2 diabetes, British Medical Journal 329 (2004) 754–755 .
RM Reynolds, DJ Webb, Recommendations and conclusions from a mini-symposium on self-blood glucose monitoring, Journal of the Royal College of Physicians Edinburgh 36 (2006) 155–158.
N Sachedina, JC Pickup, Performance assessment of the Meditronic-MiniMed Continuous Glucose Monitoring System and its use for measurement of glycaemic control in type 1 diabetic subjects, Diabetic Medicine 20 (2003) 1012–1015.
U Schwedes, M Siebolds, G Mertes, Meal related structured self-monitoring of blood glucose: effect on diabetes control in non-insulin treated type 2 diabetic patients, Diabetes Care 25 (2002) 1928–1932.
Scottish Executive Health Department (SEHD) NHS Scotland, Scottish diabetes framework. (2002) SEHD, Edinburgh .
JJ Seley, L Quigley, Blood glucose testing, American Journal of Nursing 100 (8) (2000) 24A–25E.
M Siebolds, O Gaedeke, U Schwedes, on behalf of the SMBG Study Group, Self-monitoring of blood glucose-psychological aspects relevant to changes in HbA1c in type 2 diabetic patients treated with diet or diet plus oral antidiabetic medication, Patient Education and Counseling 62 (1) (2006) 104–110.
The NHS National Prescribing Centre, When and how should patients with diabetes mellitus test blood glucose, MeReC Bulletin 13 (1) (2002); Online. Available: www.npc.co.uk.
United Kingdom Prospective Diabetes (UKPDS) Study Group, Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33), Lancet 352 (9131) (1998) 837–853.
LMC Welschen, E Bloemendal, G Nijpels, et al., Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin: a systematic review, Diabetes Care 28 (6) (2005) 1510–1517.
Welsh Department of Health, National service framework for diabetes standards in Wales, Online. Available: www.wales.nhs.uk/sites3/documents/334/diabetes-standards-wales.pdf (2003).