Case 7 Irritable bowel syndrome
Description of irritable bowel syndrome
Definition
Irritable bowel syndrome (IBS) is a chronic, functional bowel disorder characterised by abnormal defecation, visceral hypersensitivity and altered bowel motility. Depending on the prevailing stool pattern of the condition, IBS may be classified as IBS with constipation (IBS-C), IBS with diarrhoea (IBS-D), mixed IBS (IBS-M) or unclassified IBS (IBS-U).1
Epidemiology
Between four and thirty-five per cent of the world’s population is affected by IBS. Much of this variation can be explained by geographical variability, with higher prevalence rates observed in China and Western countries, and lower rates noted in South Africa and Thailand.1 Onset of IBS typically occurs in the teens or second decade of life; incidence peaks in the third and fourth decades of life and falls in the sixth and seventh decades.1 Race does not appear to be a factor in the incidence of IBS, though the condition does more commonly affect women than men, at a ratio of 3:1.2
Aetiology and pathophysiology
IBS appears to be a disease of multifactorial aetiology. While the primary cause is still not known, a number of factors have been shown to precipitate or aggravate the condition. These triggers include psychosocial stress (e.g. parental rejection, history of abuse, increased life stressors), anxiety, infectious gastroenteritis, diet (e.g. food intolerance), medication (e.g. antibiotics, hormone replacement therapy) and the act of eating.1–3
Even though the cause of IBS is not yet known, many theories have attempted to explain the pathophysiological basis of the disease. It is postulated that exposure to the triggers mentioned above, together with genetic predisposition, contributes to the development of chronic enteric inflammation and/or small intestinal bacterial overgrowth. These pathological changes may be responsible for local neuronal degeneration and immune dysfunction, and the subsequent development of visceral hypersensitivity (which may cause abdominal and/or rectal discomfort) and altered bowel motility (a possible cause of constipation, diarrhoea and nausea).1,3
Clinical manifestations
People with IBS often present with an array of gastrointestinal, psychological and/or systemic symptoms of varying intensity and frequency. Non-specific symptoms such as fatigue, chronic headache and sleep disturbances may be accompanied by psychological manifestations that include poor concentration, anxiety and depression. An individual may also complain of dyspepsia, flatulence, mucorrhoea, rectal sensitivity, nausea, abdominal bloating, left lower quadrant tenderness and periodic constipation and/or diarrhoea.2 According to the Rome III criteria for the diagnosis of IBS, the defining feature is the presence of colicky pain or continuous dull ache to the lower abdomen or left lower abdominal quadrant for at least 3 days a month in the past 3 months (with the onset of symptoms occurring at least 6 months prior), which is associated with at least two of the following: a change in stool consistency, a change in the frequency of defecation and/or improvement post defecation.1
Clinical case
32-year-old woman with irritable bowel syndrome
Rapport
Adopt the practitioner strategies and behaviours highlighted in Table 2.1 (chapter 2) to improve client trust, communication and rapport, as well as the accuracy and comprehensiveness of the clinical assessment.
Medical history
Family history
Mother has asthma and generalised anxiety disorder, father has hypertension.
Lifestyle history
Illicit drug use
| Diet and fluid intake | |
| Breakfast | Coffee, wheat biscuits (breakfast cereal) with full-cream milk. |
| Morning tea | Coffee, 2–3 sweet biscuits, muesli bar. |
| Lunch | Vegetarian pasty, white bread roll with lettuce, tomato and cheese, sandwich with white bread, ham, cheese and tomato, cola. |
| Afternoon tea | Coffee. |
| Dinner | White pasta with Neapolitan or carbonara sauce, chicken Kiev or cordon bleu with cauliflower, broccoli and green beans, omelette with ham and cheese. |
| Fluid intake | 3–4 cups of instant coffee a day, 2–3 cups of water a day, 375 mL cola 1–2 days a week. |
| Food frequency | |
| Fruit | 0–1 serve daily |
| Vegetables | 2–3 serves daily |
| Dairy | 2–3 serves daily |
| Cereals | 5–6 serves daily |
| Red meat | 3–4 serves a week |
| Chicken | 2 serves a week |
| Fish | 0–1 serve a week |
| Takeaway/fast food | once a week |
Physical examination
Olfaction
There is no evidence of halitosis or other abnormal body odours. According to the client, flatus can be foul-smelling, whereas stools generally are not.
Diagnostics
Pathology tests
Carbohydrate breath test
This test examines carbohydrate malabsorption (specifically, lactose and/or fructose malabsorption), orocecal transit time and small intestinal bacterial overgrowth.4 This test may be indicated if carbohydrate malabsorption is a suspected cause of IBS symptoms.
Faecal analysis
This test assesses a number of stool characteristics, including appearance, colour, occult blood, epithelial cells, leucocytes, carbohydrates, fat, meat fibres and trypsin.5 It may also help to exclude colorectal carcinoma, malabsorption, inflammatory bowel disease or intestinal infection as potential causes of IBS symptoms.
Functional tests
A comprehensive digestive stool analysis (CDSA) obtains data on enzymatic digestion, fatty acid absorption, microbiological balance and metabolic markers of disease. This test may be warranted if intestinal dysbiosis, intestinal candidiasis, malabsorption and/or indigestion cannot be excluded as potential causes of IBS symptoms.6
Invasive tests
Invasive tests are not usually required for a diagnosis of IBS. When serious underlying pathology is a suspected cause of the IBS symptoms, certain tests may be indicated, for example, small bowel biopsies can be performed to rule out coeliac disease and a colonoscopy and/or sigmoidoscopy may be indicated if colorectal carcinoma, inflammatory bowel disease or diverticulosis are suspected.
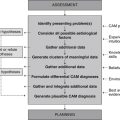
Diagnosis
Planning
Goals
Expected outcomes
Based on the degree of improvement reported in clinical studies that have used CAM interventions for the management of IBS,7–12 the following are anticipated.
Application
Diet
Elimination diet (Level I, Strength B, Direction +)
Food intolerance is implicated in the pathogenesis of IBS. The elimination of offending foods from the diet could potentially lead to an improvement in IBS symptoms and a reduction in symptom recurrence. In a systematic review of seven open label, single-arm studies (n = 386), between 12.5 per cent and 67 per cent of patients treated with an elimination diet demonstrated remission of IBS symptoms within 4 to 21 days.13 Double-blind challenges identified milk, wheat and eggs as the most frequent causes of IBS symptom exacerbation. While these studies have major methodological limitations, they are supported by data from three rigorously designed clinical trials (n = 562). These studies have shown the elimination diet to be superior to a sham elimination diet in reducing IBS symptom severity and global rating score at 12 weeks8 and as effective as disodium cromoglycate (1.5 g daily) at improving IBS symptoms at 4 weeks.14 When elimination diet and disodium cromoglycate (250 mg 4 times a day for 16 weeks) are used together, they are shown to be significantly more effective than diet alone in reducing IBS symptoms.15 Foods most frequently excluded from the elimination diets were milk, wheat, eggs, cashew nuts, tomato, yeast and potato. In spite of these favourable outcomes, the heterogeneity in diets across studies makes the clinical application of these findings difficult. Replicating studies using individualised elimination diets (i.e. by eliminating foods for which the patient has elevated IgG antibodies), such as that reported by Atkinson et al,7 would produce findings that are directly applicable to CAM practice and would help to improve consumer and practitioner confidence in this intervention.
High-fibre diet (Level I, Strength C, Direction + (for soluble fibre only))
Dietary fibre is essential for maintaining gastrointestinal (GI) tract function, specifically, fibre increases faecal volume, decreases GI transit time, decreases bowel lumen pressure, promotes the growth of beneficial bowel flora, generates lactate to acidify the colon lumen and generates short-chain fatty acids to stimulate mucosal cell proliferation and provide energy for colonic cells.16 Each of these functions can help to attenuate the pathophysiological processes and the symptoms of IBS. Evidence from a systematic review of 12 RCTs (n = 591) adds support to this claim, but only for certain types of fibre. When compared with placebo, for instance, soluble fibre (isphagula and psyllium) showed significant improvement in abdominal pain and global IBS symptoms, whereas insoluble fibre (10–30 g wheat bran daily) had no significant effect on IBS manifestations.8 It is probable, then, that IBS may be more responsive to soluble than insoluble fibre. Given that wheat intolerance is a common trigger of IBS,13 and most of the studies included in this systematic review neither listed wheat intolerance or coeliac disease as an exclusion criterion, nor investigated the presence of wheat intolerance, it is also possible that wheat intolerance could have confounded these results, which means that the comparative efficacy of different types of fibre warrants further investigation.
Lifestyle
Meditation (Level II, Strength B, Direction +)
Meditation is a mind–body therapy that uses a range of methods, such as mindfulness, breathing, concentration, visualisation, mantras and/or affirmations, to bring about inner tranquillity and/or improve self-awareness. Given that stress and anxiety aggravate IBS, meditation may be a suitable treatment option for people with the condition. This assumption was tested in a small RCT of 13 adults with IBS. The study found relaxation response meditation (15 minutes twice daily for 6 weeks) to be significantly superior to waiting list control at reducing the composite score of IBS abdominal symptoms.10 Even so, given the small size of the study, these findings should be interpreted with caution.
Relaxation therapy (Level I, Strength C, Direction + (for overall IBS symptom score only))
The relaxation response can be induced by a number of behavioural therapies. In so doing, these therapies may help to attenuate the effect of stress and anxiety on IBS. A recent Cochrane review of 10 RCTs examined the effect of relaxation therapy and stress management on the symptoms of IBS. The review found a small but statistically significant benefit from relaxation therapy and stress management on overall IBS symptom score when compared with usual care or waiting list control, although changes in abdominal pain and quality of life were not consistent across studies.17 The low methodological quality, small sample sizes and considerable heterogeneity of the trials prevents any firm conclusions being made about the effectiveness of relaxation therapy for IBS.
Yoga (Level II, Strength C, Direction + (for bowel symptoms only))
Yoga is an ancient Indian practice that integrates stretching, exercise, posture and breathing with meditation. As these techniques are likely to induce a relaxation response, yoga may be helpful in alleviating the symptoms of IBS. Two RCTs have examined the effectiveness of yoga in people with IBS. The first trial, which enrolled 22 adult males with diarrhoea-predominant IBS, found yoga (i.e. yogic poses and right-nostril breathing, twice a day for 8 weeks) to be as effective as the antidiarrhoeal loperamide (2–6 mg daily) at reducing bowel symptoms and state anxiety.18 In a trial of 25 adolescents with IBS, yoga (i.e. video-guided yogic poses and breathing, 10 minutes daily for 4 weeks) was found to be no more effective than waiting list control at reducing functional disability, anxiety and emotion-focused avoidance.11 The inconsistencies in these study findings, the uncertainty about the effect of meditation in adult females and the small size of these trials highlights the need for further research in this area.
Nutritional supplementation
Flaxseed (Level III-1, Strength C, Direction +)
The seeds of Linium usitatissimum, or flax, are a good source of dietary fibre, protein, lignans, alpha-linolenic acid (omega 3 fatty acid), linoleic acid (omega 6 fatty acid) and oleic acid (omega 9 fatty acid).19 The anti-inflammatory effects of these fatty acids, together with the mucilaginous effect of the seed, suggest flaxseed may be useful in the management of IBS. A single-blind RCT involving 55 patients with constipation-dominant IBS supports this claim. The trial found roughly ground, partly defatted flaxseed (6–24 g daily), when administered for 3 months, to be statistically significantly more effective than psyllium (6–24 g daily) at improving constipation, abdominal pain and bloating.20 Replication of these results may provide practitioners with the confidence to select this intervention as a treatment for IBS-C.
Prebiotics (Level II, Strength B, Direction o)
Prebiotics are non-digestible substances (typically, carbohydrates or soluble fibre) that stimulate the growth of bowel flora. In so doing, these substances help to maintain microbial balance in the GI tract. Few prebiotics have been investigated for their effectiveness in IBS. Fructo-oligosaccharides (FOS) are one exception. In a multicentre, double-blind RCT of 96 patients with IBS, FOS powder (20 g daily for 12 weeks) was found to improve abdominal distension, rumbling, flatulence and pain in fifty-eight per cent of patients; the difference between the FOS and placebo groups was not statistically significant.21
Probiotics (Level I, Strength A, Direction +)
Probiotics are live microbial dietary supplements. These agents are essential for minimising pathogen growth, synthesising vitamins, manufacturing short-chain fatty acids, modulating local immune and inflammatory responses, and maintaining intestinal epithelial integrity.22 Much of the research on probiotics and IBS has focused on the effectiveness of two microbial species – Lactobacillus spp. and Bifidobacterium spp. A systematic review and meta-analysis of 14 RCTs (n = 1225) found probiotic treatment for 4–26 weeks to be significantly more effective than placebo at improving abdominal pain (in seven trials), flatulence (in five trials), bloating (in four trials) and global IBS symptoms (in seven trials).9 Even though these outcomes are positive, the clinical application of these findings may be difficult due to the disparate dosages, treatment durations, types of microbial strains and combinations of strains used across studies.
Herbal medicine
Curcuma longa (Level III-1, Strength D, Direction +)
Turmeric is a culinary spice, an approved food colouring agent and a medicinal plant. In Ayurvedic, Chinese, Thai and Western herbal medicine the plant has been used for the treatment of pyrexia, menstrual irregularity, jaundice and pain. Turmeric has also been used as a treatment for digestive disorders, which, together with the anti-inflammatory,23 antispasmodic,24 immunomodulatory25 and antimicrobial activity of the plant (and its active constituents), highlight a potential role for turmeric in the management of IBS. While a partially blinded, randomised, two-dose, pilot study involving 192 otherwise healthy adults with IBS found turmeric extract (72 mg or 144 mg daily for 8 weeks) to be effective in reducing IBS prevalence, abdominal discomfort and IBS quality of life,26 a placebo effect cannot be ruled out given the absence of a placebo control and the similar efficacy of the two turmeric doses.
Cynara scolymus (Level III-1, Strength C, Direction +)
Globe artichoke, or artichoke leaf extract (ALE), is used in Western herbal medicine as a hepatoprotective, cholagogue, choleretic, hypolipidaemic and diuretic, primarily for the treatment of hepatic and biliary complaints. Recent studies suggest C. scolymus may also be useful for conditions affecting the GI tract, such as IBS. A postmarketing surveillance study, for instance, found ALE (1920 mg daily for 6 weeks) reduced IBS symptoms in 279 adults with IBS.27 A subanalysis of an open, two-dose clinical study found that ALE (320 mg or 640 mg for 8 weeks) was also effective at improving IBS incidence, bowel function and quality of life.28 Both of these studies have major methodological shortcomings and, as such, do not allow for any conclusions to be made about the efficacy of globe artichoke in IBS.
Mentha piperita (Level I, Strength A, Direction +)
Peppermint has a long history of use as an antiemetic, antimicrobial, carminative and antispasmodic. In fact, peppermint oil and its main constituent menthol have been shown in a number of different animal studies to reduce experimentally induced spasm of GI smooth muscle,29 an effect that has been attributed to the calcium antagonist effect of menthol. These findings are congruent with those reported in human studies, with a meta-analysis of four well-designed RCTs (n = 392) finding peppermint oil (450–748 mg daily for 4–12 weeks) to be significantly superior to placebo at improving abdominal pain and global IBS symptoms.8 The number needed to treat to prevent one patient having persistent symptoms was half that reported for conventional antispasmodics.
Plantago spp. (Level I, Strength A, Direction +)
The seed husks of psyllium and isphagula are classified as water-soluble fibres because they form a mucilagenous gel when exposed to water.30 This acts as an effective stool bulking agent that may be useful for the management of constipation and diarrhoea, both of which are characteristic symptoms of IBS. Evidence from a recent meta-analysis of six well-designed RCTs (n = 321) found psyllium (6.4 g daily for 8 weeks) and isphagula husk (unknown dose for 4–12 weeks) to be statistically significantly more effective than placebo at reducing abdominal pain and global IBS symptoms.9 The number needed to treat to prevent one patient having persistent symptoms was similar to that reported for conventional antispasmodics.
Other herbs
Filipendula ulmaria (meadowsweet), Foeniculum vulgare (fennel), Glycyrrhiza glabra (licorice), Matricaria recutita (chamomile), Melissa officinalis (lemon balm), Paeonia lactiflora (peony), Ulmus rubra (slippery elm) and Viburnum opulus (cramp bark) are traditionally used as carminative, spasmolytic and/or demulcent agents, but there is insufficient clinical evidence to support or refute the efficacy of these herbal monopreparations in humans with IBS.
Other
Acupuncture (Level I, Strength C, Direction o)
Acupuncture originated in China more than 4000 years ago.31 Since then, the therapy has established a large traditional evidence base; however, in the case of IBS, the best available evidence is not convincing. In a Cochrane review of six RCTs/quasi-RCTs (n = 464),32 as well as a more recent clinical trial (n = 43),33 acupuncture treatment for 4–24 weeks was found to be no more effective than sham acupuncture, psychotherapy, a Chinese polyherbal or orthodox medication at improving general wellbeing, global IBS symptoms, symptom recurrence, abdominal pain, defecation difficulties, diarrhoea or bloating. Yet when acupuncture was combined with psychotherapy, a significant improvement in IBS symptoms and symptom recurrence was observed when compared with psychotherapy alone.32 Similarly, significant improvements in IBS symptom severity scores were observed among patients receiving acupuncture and usual general practice (GP) care when compared to usual GP care alone.34 This suggests that an integrative approach to IBS may be more effective than acupuncture alone in the management of this condition.
Osteopathy (Level II, Strength B, Direction +)
Osteopathic medicine applies a range of manipulative techniques in order to facilitate recovery of neuromusculoskeletal complaints. Given that the aetiology of IBS has a neurological basis, osteopathic manipulation may have a place in the management of this disorder. Evidence from two RCTs supports this assumption. One RCT involving 39 IBS patients found 6 months of osteopathic treatment to be significantly more effective than standard care at reducing IBS severity and change in overall symptom improvement. Changes in mean symptom scores were not significantly different between groups.35 By contrast, an RCT of 61 IBS patients found 10 weeks of osteopathic manipulation to be superior to sham osteopathy in improving the incidence and intensity of abdominal pain, constipation, diarrhoea and abdominal distension.13 Even though these outcomes are promising they are not conclusive. Hence, larger, long-term studies are now required to strengthen the evidence in this area.
Reflexology (Level II, Strength C, Direction o)
Reflexology is a tactile therapy that induces neurophysiological reflexes or responses in distant glands, tissues and organs by stimulating specific zones of the feet, hands and/or ears. Several uncontrolled open-label studies have reported improvements in constipation following reflexology treatment,36,37 which suggests that reflexology could be effective in alleviating the symptoms of IBS. When foot reflexology (six 30-minute sessions over 10 weeks) was administered to patients with IBS under single-blind RCT conditions, it was found to be no more effective than light foot massage at reducing abdominal pain, constipation or diarrhoea or bloatedness.38 Given the small scale of the study (n = 34) and the potential confounding effect of the massage control, the value of reflexology in IBS should not be dismissed until larger, more rigorously designed trials are conducted in this area.
CAM prescription
The CAM interventions that are most appropriate for the management of the presenting case – that is, they target the planned goals, expected outcomes and CAM diagnoses, they are supported by the best available evidence, they are pertinent to the client’s needs and they are most relevant to CAM practice – are outlined below.
Primary treatments
Referral
1. Padovei M., Kuo B. Irritable bowel syndrome: a practical review. Southern Medical Journal. 2006;99(11):1235-1242.
2. Porter R., et al, editors. The Merck manual. Rahway: Merck Research Laboratories, 2008.
3. McQuaid K.R. Gastrointestinal disorders. In McPhee S.J., Papadakis M.A., editors: Current medical diagnosis and treatment 2009, 46th ed, New York: McGraw-Hill, 2008.
4. Saad R.J., Chey W.D. Breath tests for gastrointestinal disease: the real deal or just a lot of hot air? Gastroenterology. 2007;133(6):1763-1766.
5. Van Leeuwen A.M., Poelhuis-Leth D.J. Davis’s comprehensive handbook of laboratory and diagnostic tests with nursing implications, 3rd ed. Philadelphia: FA Davis Company; 2009.
6. Genova Diagnostics. Comprehensive digestive stool analysis. Asheville: Genova Diagnostics; 2008.
7. Atkinson W., et al. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut. 2004;53(10):1459-1464.
8. Ford A.C., et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. British Medical Journal. 2008;337:a2313.
9. Hoveyda N., et al. A systematic review and meta-analysis: probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterology. 2009;9:15.
10. Keefer L., Blanchard E.B. The effects of relaxation response meditation on the symptoms of irritable bowel syndrome: results of a controlled treatment study. Behaviour Research and Therapy. 2001;39(7):801-811.
11. Kuttner L., et al. A randomized trial of yoga for adolescents with irritable bowel syndrome. Pain Research and Management. 2006;11(4):217-223.
12. Muller A., et al. Osteopathy as a promising short-term strategy for irritable bowel syndrome: a randomized controlled trial. Osteopathische Medizin. 2006;7(3):20-21.
13. Niec A.M., Frankum B., Talley N.J. Are adverse food reactions linked to irritable bowel syndrome? American Journal of Gastroenterology. 1998;93(11):2184-2190.
14. Stefanini G.F., et al. Oral cromolyn sodium in comparison with elimination diet in the irritable bowel syndrome, diarrheic type. Multicenter study of 428 patients. Scandinavian Journal of Gastroenterology. 1995;30(6):535-541.
15. Leri O., et al. Management of diarrhoeic type of irritable bowel syndrome with exclusion diet and disodium cromoglycate. Inflammopharmacology. 1997;5(2):153-158.
16. Gropper S.S., Smith J.L., Groff J.L.. Advanced nutrition and human metabolism. Belmond, 5th ed.. Cengage Learning, 2008.
17. Zijdenbos I.L., et al. Psychological treatments for the management of irritable bowel syndrome. Cochrane Database of Systematic Reviews. 2009. (1): CD006442
18. Taneja I., et al. Yogic versus conventional treatment in diarrhea-predominant irritable bowel syndrome: a randomized control study. Applied Psychophysiology and Biofeedback. 29(1), 2004. 190–33
19. Braun L., Cohen M. Herbs and natural supplements: an evidence-based guide, 2nd ed. Sydney: Elsevier Australia; 2007.
20. Tarpila S., et al. Efficacy of ground flaxseed on constipation in patients with irritable bowel syndrome. Current Topics in Nutraceuticals Research. 2004;2(2):119-125.
21. Olesen M., Gudmand-Hoyer E. Efficacy, safety, and tolerability of fructooligosaccharides in the treatment of irritable bowel syndrome. American Journal of Clinical Nutrition. 2000;72(6):1570-1575.
22. Lee Y.K., Salminen S. Handbook of probiotics and probiotics, 2nd ed. Oxford: Wiley; 2009.
23. Lantz R.C., et al. The effect of turmeric extracts on inflammatory mediator production. Phytomedicine. 2005;12(6–7):445-452.
24. Itthipanichpong C., et al. Antispasmodic effects of curcuminoids on isolated guinea-pig ileum and rat uterus. Journal of the Medical Association of Thailand. 2003;86(Suppl 2):S299-S309.
25. Xia X., et al. Ethanolic extracts from Curcuma longa attenuates behavioral, immune, and neuroendocrine alterations in a rat chronic mild stress model. Biological and Pharmaceutical Bulletin. 2006;29(5):938-944.
26. Bundy R., et al. Turmeric extract may improve irritable bowel syndrome symptomology in otherwise healthy adults: a pilot study. Journal of Alternative and Complementary Medicine. 2004;10(6):1015-1018.
27. Walker A.F., Middleton R.W., Petrowicz O. Artichoke leaf extract reduces symptoms of irritable bowel syndrome in a post-marketing surveillance study. Phytotherapy Research. 2001;15(1):58-61.
28. Bundy R., et al. Artichoke leaf extract reduces symptoms of irritable bowel syndrome and improves quality of life in otherwise healthy volunteers suffering from concomitant dyspepsia: a subset analysis. Journal of Alternative and Complementary Medicine. 2004;10(4):667-669.
29. Grigoleit H.G., Grigoleit P. Pharmacology and preclinical pharmacokinetics of peppermint oil. Phytomedicine. 2005;12(8):612-616.
30. Jalili T., Medeiros D.M., Wildman R.E.C. Dietary fiber and coronary heart disease. In Wildman R.E.C., editor: Handbook of nutraceuticals and functional foods, 2nd ed, Boca Raton: CRC Press, 2006.
31. O’Brien K.A., Xue C.C. Acupuncture. In: Robson T., editor. An introduction to complementary medicine. Sydney: Allen & Unwin, 2003.
32. Lim B., et al. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database of Systematic Reviews. 2006. (4): CD005111
33. Schneider A., et al. Acupuncture treatment in irritable bowel syndrome. Gut. 2006;55(5):649-654.
34. Reynolds J.A., Bland J.M., MacPherson H. Acupuncture for irritable bowel syndrome an exploratory randomised controlled trial. Acupuncture in Medicine. 2008;26(1):8-16.
35. Hundscheid H.W., et al. Treatment of irritable bowel syndrome with osteopathy: results of a randomized controlled pilot study. Journal of Gastroenterology and Hepatology. 2007;22(9):1394-1398.
36. Bishop E., et al. Reflexology in the management of encopresis and chronic constipation. Paediatric Nursing. 2003;15(3):20-21.
37. Kunz B., Kunz K. Findings in research about safety, efficiency, mechanism of action, and cost effectiveness of reflexology (revised). Albuquerque: RRP Press; 2003.
38. Tovey P. A single-blind trial of reflexology for irritable bowel syndrome. British Journal of General Practice. 2002;52(474):19-23.
39. Wacher V.J., Wong S., Wong H.T. Peppermint oil enhances cyclosporine oral bioavailability in rats: Comparison with D-α-tocopheryl poly(ethylene glycol 1000) succinate (TPGS) and Ketoconazole. Journal of Pharmaceutical Sciences. 2001;91(1):77-90.