CHAPTER 65
Knee Chondral Injuries
Alison L. Cabrera, MD; Kurt Spindler, MD
Definition
Chondral injuries are any degree of loss of the normal thickness and structure of articular hyaline cartilage. Chondral damage can occur in any joint, but most of the literature has focused on the knee, which is the focus of this chapter. Outerbridge [1] classified cartilage lesions in 1961, and this remains the current classification used to date (Fig. 65.1). Partial-thickness articular cartilage injuries do not heal but are rarely associated with significant clinical symptoms [2]. Full-thickness cartilage injuries, in which the injury extends to the depth of subchondral bone, may heal in with fibrocartilage, but this type of cartilage has shown inferior biomechanical and biochemical properties compared with hyaline cartilage [2,3]. Large, full-thickness cartilage defects are less likely to benefit from the healing fibrocartilaginous response and frequently will lead to symptoms [2].
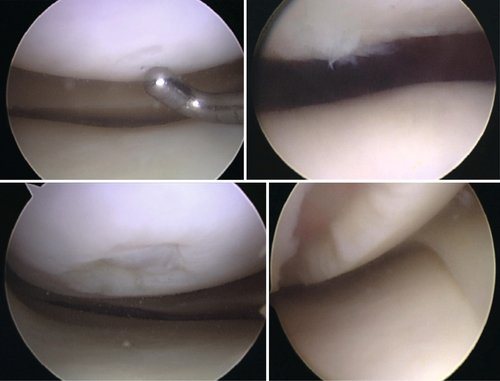
The exact incidence of acute cartilage injury is unknown. In young, active patients who present with a hemarthrosis of the knee after a traumatic event, 5% to 10% are found to have cartilage injury [4]. Many studies have retrospectively reviewed the incidence of cartilage injury after arthroscopy for other injury and found rates ranging from 5% to 11% for full-thickness lesions [5,6]. A study looking retrospectively at 25,124 arthroscopies found a 60% incidence of cartilage lesions [7]. Regarding age differences, the incidence of a localized grade III or grade IV cartilage lesion in patients undergoing arthroscopy ranged from 5% to 7% for patients younger than 40 years and 7% to 9% for patients younger than 50 years [6,7]. These studies included both acute and chronic cartilage lesions. Grade II was the most frequent lesion, seen in 42% [7]. The most common locations were the patellar articular surface (36%) and medial femoral condyle (34%) [7]. Seventy percent of the chondral lesions seen were associated with other injury, with medial meniscus tear (37%) and injury of the anterior cruciate ligament (36%) being the most common [7].
Most authors would agree that progression to osteoarthritis is a concern; however, there is little evidence to date that quantifies the incidence or severity of osteoarthritis after chondral injury. More natural history and outcomes research is needed to better predict a patient’s likelihood of progression to symptomatic osteoarthritis according to the characteristics of the cartilage injury.
Symptoms
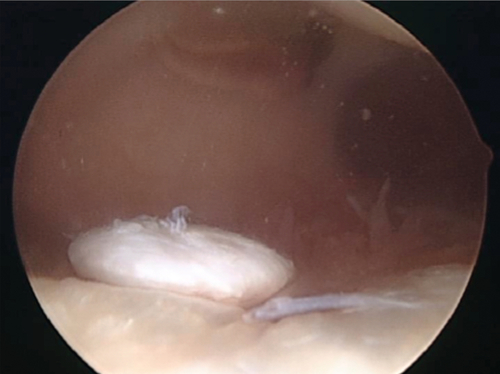
Patients may present with effusion, localized pain, and possible mechanical symptoms. If a full-thickness fragment has detached partially or completely, a patient may have locking or a “loose body” sensation as the fragment moves within the knee joint (Fig. 65.2). If the injury extends to subchondral bone, a hemarthrosis will be present. Some patients may have more vague symptoms without large effusion and a more generalized pain, making the diagnosis less clear.
Physical Examination
The practitioner should evaluate the patient for joint effusion and any tenderness to palpation. The patient will likely have tenderness to palpation over the anatomic region with chondral injury. The patient may have tenderness along the joint line, but more detail in examination will show that the area of maximal tenderness is over the site of chondral damage. Active and passive range of motion should be assessed. Crepitus or clunk may be present with range of motion with significant cartilage defects. It is important to perform a thorough ligamentous laxity examination to rule out any concomitant injuries [7].
Functional Limitations
The patient may have difficulty with ambulation and may walk with a limp. Activities such as climbing stairs and household chores may be limited by knee pain and swelling. Most often, patients will be unable to return to the activity they were participating in immediately after the acute injury.
Diagnostic Studies
Plain films including standing anteroposterior, lateral, and sunrise views are obtained initially to rule out osteochondral fracture, osseous loose body, osteoarthritis, osteochondritis dissecans, and Segond fracture. Magnetic resonance imaging (MRI) is currently the standard of care in evaluating the morphology of an articular cartilage injury and can also assess for ligamentous or meniscal injuries. Even with current MRI protocols, cartilage evaluation requires expertise to characterize lesions accurately. New biochemical imaging techniques including T2 mapping, T1rho imaging, sodium MRI, and delayed gadolinium-enhanced MRI of cartilage have been used primarily for research purposes but are gaining support for their use in the clinical setting to improve detection and management of cartilage lesions [8].
Treatment
Initial
Initial treatment is focused on pain control and resolving any effusion. Anti-inflammatories will usually suffice for pain relief. Aspiration of any effusion present will help alleviate pain and can be combined with injection of an anesthetic. Animal studies represent most of the literature on chondrolysis after intra-articular injection of anesthetic and primarily have evaluated bupivacaine toxicity [9]. However, a study using human chondrocytes has shown improved viability of chondrocytes after 0.5% ropivacaine versus 0.5% bupivacaine [9]. An intact matrix has been shown to offer a chondroprotective effect [9]. With our current knowledge of the chondrotoxicity of 0.5% bupivacaine, it should be used judiciously. A better alternative may be 0.5% ropivacaine, but longer term studies in humans are needed.
Rehabilitation
Early physical therapy is recommended to regain joint range of motion and muscle strength. Focus should be on quadriceps and hamstrings exercises. Most cartilage injuries will be associated with other injuries (e.g., anterior cruciate ligament injury), and therefore the rehabilitation protocol will vary. Numerous animal studies show an improved cartilage healing response with continuous passive motion after cartilage injury [10,11]. However, currently there are only few level III studies and no randomized controlled trials regarding this topic [12]. Nevertheless, most surgeons agree on using continuous passive motion as well as keeping the patient non–weight bearing after cartilage restoration surgery. Patients are kept non–weight bearing ranging from 6 to 12 weeks, depending on the surgeon’s protocol and any patient comorbidities.
Surgery
Most authors agree that cartilage defects to be considered for surgical treatment include full-thickness lesions (i.e., Outerbridge grade III or grade IV) larger than 2 cm2 in a patient younger than 40 years [13,14]. Without taking the grade of the chondral lesion into consideration, the incidence of chondral injuries larger than 2 cm2 was only 7% in one study [7]. Microfracture, osteochondral autograft transfer or transplantation (i.e., OAT), and autologous chondrocyte implantation (ACI) are the principal cartilage restoration procedures available that have been evaluated in randomized clinical trials.
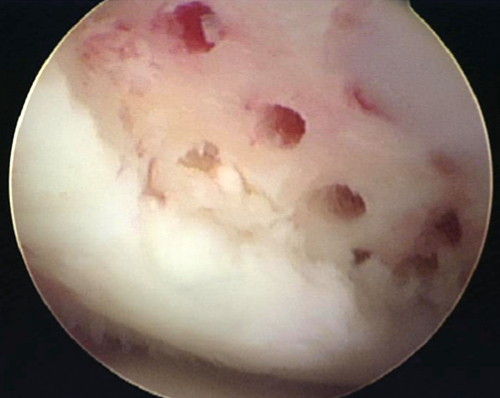
Microfracture involves arthroscopic débridement of the cartilage lesion to stable squared off edges. The zone of calcified cartilage is removed, and cortical penetration with an awl is performed, allowing medullary bleeding and clot formation [13] (Fig. 65.3). Osteochondral autograft transplantation or mosaicplasty involves harvesting of viable, structurally intact cartilage and bone plugs from a “dispensable” portion of a joint and transplanting the autograft to the chondral defect. ACI involves the harvest of chondrocytes from a nonessential portion of the knee during the first-stage surgery. The specimen undergoes expansion of chondrocytes in a laboratory. The chondrocytes are then placed in a medium, which is implanted on the defect with an overlying periosteal patch or collagen scaffold during the second-stage surgery. Given limitations of available comparative studies, no clear outcome benefit can be confirmed for either OAT or ACI over microfracture [13,15]. Only one level I study indicates better outcomes in athletes with initial arthroscopic OAT versus microfracture [16]. For cartilage lesions larger than 2 cm2, Gudas and colleagues [16] showed worse clinical outcomes with microfracture. Currently, the literature lacks any level I study with a natural history control group, which makes outcomes after current surgical procedures difficult to interpret. With lack of superiority of any one surgical treatment, microfracture has been considered the first-line therapy, given its technical ease, lack of donor site graft morbidity, and affordability [13,15].
Potential Disease Complications
Most clinicians assume that traumatic articular cartilage lesions will continue to progress to osteoarthritis. However, the true natural history of these lesions has yet to be formally studied and remains unknown. Messner and Maletius [17] looked at radiographs 14 years after diagnosis of severe chondral injury in 28 patients to find that 22 had joint space narrowing of less than 50% in the compartment with injury.
Potential Treatment Complications
Common complications encountered after cartilage restoration surgery include arthrofibrosis, superficial wound infections, and tissue hypertrophy [15]. Lower incidences of deep venous thrombosis, hemarthrosis, and graft malpositioning occur [15]. Arthrofibrosis appears to occur with equal frequency with microfracture, ACI, and the OAT procedure [15]. Tissue hypertrophy surrounding the lesion and reactive synovitis are associated more commonly with ACI [15]. Proud or recessed graft is limited to the OAT procedure [15]. Reoperation is another complication and appears to be higher with ACI versus microfracture [15,18]. Newer all-arthroscopic, second-generation ACI techniques with a collagen membrane patch may show lower reoperation rates and decreased arthrofibrosis over the first-generation periosteal ACI techniques [18].







