CHAPTER 63
Anterior Cruciate Ligament Tear
William Micheo, MD; Eduardo Amy, MD; Fernando Sepúlveda, MD
Definition
The anterior cruciate ligament (ACL) is an intra-articular structure essential for the normal function of the knee. It is commonly injured during activities that involve complex movements, such as cutting and pivoting. It is estimated that 1 in 3000 individuals sustains an ACL injury each year in the United States, corresponding to an overall injury rate of approximately 100,000 injuries annually [1]. The injury usually results from a sudden deceleration during high-velocity movements in which a forceful contraction of the quadriceps muscle is required. Other mechanisms of injury are valgus stress, hyperextension, and external rotation, as in landing from a jump, and severe internal rotation of the knee with varus or hyperextension [1]. Approximately 70% of the acute ACL injuries are sports related and affect women more than men, particularly in sports such as basketball and soccer [2]. Non–sports-related injuries might include a patient slipping on ice or falling from considerable height and landing with the knee in hyperextension and valgus. In the last two decades, there has also been an increase in the incidence and appropriate diagnosis of ACL injuries in children associated with more participation in high-demand contact and noncontact sports, increased awareness of the injury, and better imaging techniques [3]. Risk factors associated with ACL injuries can be classified as anatomic, hormonal, environmental, biomechanical, and neuromuscular. Some of the modifiable risk factors include proprioception, core strength, decreased hamstring strength (relative to quadriceps strength), conditioning, footwear, playing surface, weather conditions, training techniques, and biomechanical variations in landing from a jump or cutting. Nonmodifiable factors include gender, reduced femoral intercondylar notch size, increased slope of the tibial plateau, knee hyperextension, physiologic rotatory laxity, small ACL size, and familial predisposition [1,4–6].
The ACL may be partially or completely torn. It also may be injured in combination with other structures, most commonly tears of the medial collateral ligament and medial meniscus.
The ACL is a collagenous structure approximately 38 mm in length and 10 mm in width. The ligament arises from a wide base in the tibia anterolateral to the anterior tibial spine. It then traverses the knee in a posterolateral direction, attaching in a broad fan-like fashion at the posterolateral corner of the intercondylar notch of the femur. According to Fu and collaborators, it is organized in two major bundles named after their insertion sites on the tibia [7]. The anteromedial bundle, which tightens in flexion and is the longer of the two, controls anterior translation of the tibia on the femur. The posterolateral bundle, which tightens in extension and internal rotation, controls rotation [8–10].
Biomechanical studies, with use of cadaver specimens, have evaluated the forces that affect the ACL [11]. These forces are highest in the last 30 degrees of extension, in hyperextension, and under other load conditions, including anterior tibial translation, internal rotation, and varus. The ACL is a static stabilizer of the knee with a primary function of resisting hyperextension and anterior tibial translation in flexion and providing rotatory control. It is also a secondary restraint to valgus and varus forces in all degrees of flexion.
Symptoms
Individuals usually present with pain, immediate swelling, and limited range of motion. They may give a history of hearing a “pop.” In an acute injury, the individual will have severe pain and difficulty with walking. In a chronic injury, a patient may have a history of recurrent episodes of knee instability associated with swelling and limited motion. Patients may describe locking or a “giving way” phenomenon. They may also give a history of a remote injury to the knee that was not rehabilitated.
Physical Examination
The physical examination has been found to be sensitive and specific in the diagnosis of ACL tears and correlates with arthroscopically documented knee injuries [1]. The clinician should observe the knee for asymmetry, palpate for areas of tenderness, measure active and passive range of motion, and document muscle atrophy. The apprehension test to rule out patellar instability, valgus and varus testing with the knee in full extension and 30 degrees of flexion to evaluate the collateral ligaments, and joint line palpation as well as the McMurray test to evaluate the meniscus are all important tests to look for injury of associated structures.
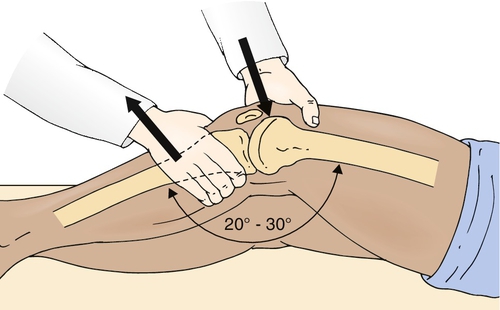
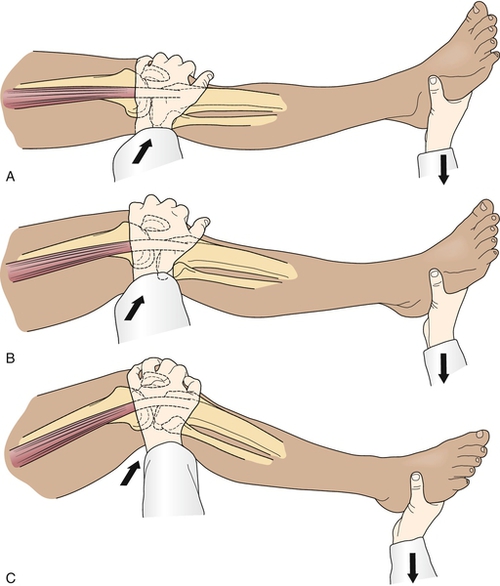
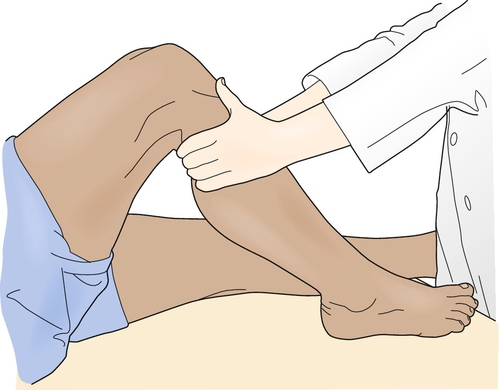
The key physical examination maneuver for evaluating the integrity of the ACL in the patient with an acute injury is the Lachman test, in which an anterior force is applied to the tibia with the knee in 30 degrees of flexion while the clinician tries to reproduce anterior migration of the tibia on the femur (Fig. 63.1). Another important test in the acute setting is the lateral pivot shift maneuver, in which the examiner attempts to reproduce anterolateral instability by internally rotating the leg, applying a valgus stress to the knee as it is flexed, and feeling for anterior migration of the tibia on the femur (Fig. 63.2). After injury, the Lachman test is most important for acute diagnosis, whereas the lateral pivot shift test has shown a better correlation to future sports participation and functional stability [1,12]. In the patient who is able to flex the knee to 90 degrees, particularly in chronic or recurrent injury, the anterior drawer test, in which an anterior force is applied to the tibia, should be performed (Fig. 63.3). In acute injury, this test may provide a false-negative result because the secondary stabilizers may reduce anterior tibial displacement with the knee in 90 degrees of flexion [13]. It is important to complete the examination by performing the posterior drawer test, which evaluates the posterior cruciate ligament; a torn posterior cruciate ligament with posterior tibial subluxation may give a false-positive result of the anterior drawer maneuver as the tibia is reduced. In general, findings should be normal on the neurologic examination, including muscle strength, sensation, and reflexes; however, there may be some associated weakness (particularly of the knee extensors) due to pain inhibition or disuse.
Functional Limitations
Limitations include reduced knee motion, muscle weakness, and pain that interferes with activities involving pivoting and jumping. Recurrent episodes of instability may limit participation in strenuous sports, such as basketball, soccer, tennis, and volleyball [14,15]. These episodes of the knee’s giving way may result in increased ligamentous laxity, leading to limitations with activities of daily living, such as going down stairs and changing directions while walking.
Diagnostic Studies
Diagnostic studies include plain radiographs to rule out intra-articular fractures (tibial spine avulsion, Segond fracture), loose bodies, and arthritic changes. These include the standing anteroposterior view, lateral view, tunnel view, standing posteroanterior 45-degree flexion view, and Merchant view of the patella. Magnetic resonance imaging may be indicated in the acute setting to evaluate associated pathologic changes, such as bone bruises, meniscal tears, and other ligamentous injuries, and to aid in treatment planning of combined injuries. In the pediatric and adolescent athlete, magnetic resonance imaging may also give information about physeal injuries that may otherwise go unnoticed.
Treatment
Initial
Immediately after injury, the management of an ACL tear includes relative rest, ice, compression, elevation, and analgesic or anti-inflammatory medication. Many patients will initially benefit from use of a knee immobilizer and crutches. If the knee is very swollen and painful with limited motion that restricts participation in treatment, arthrocentesis may be performed. It is important to establish an accurate diagnosis and the presence of associated injuries as these may necessitate prompt surgery. These include chondral or osteochondral fractures, meniscal tears, and other injured capsular structures. In general, in the absence of associated injuries, the acute management can be conservative with early protected rehabilitation.
Treatment of ACL injuries depends on a number of factors, including the patient’s age, level of activity, presence of associated injuries, and importance of returning to athletic activities that involve acceleration and deceleration and cutting moves. Surgery is the only definitive treatment of complete ACL injuries, but it is generally not necessary for older individuals who do not complain of knee instability with recreational activities or work.
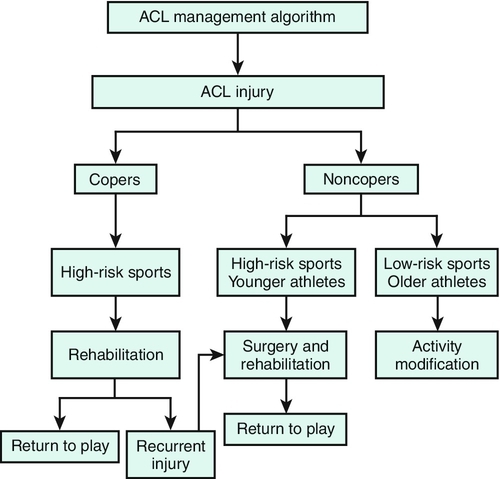
In general, younger patients and those with a high activity level should be considered for ACL reconstruction. Surgical referral is not necessary in the immediate postinjury period but should be facilitated as soon as it is clear that an individual desires surgery as a definite treatment measure. When associated injuries are present, especially if these cause mechanical symptoms, or in the case of the elite competitive athlete, surgical treatment should be considered as soon as the initial inflammatory phase has passed (Fig. 63.4) [16].
Rehabilitation
The rehabilitation of an ACL tear begins as soon as the injury occurs. Rehabilitation management focuses on reducing pain, restoring full motion, correcting muscle strength deficits, achieving muscle balance, and returning the patient to full activity free of symptoms [17]. The rehabilitation program consists of acute, recovery, and functional phases.
Anterior Cruciate Ligament Injury
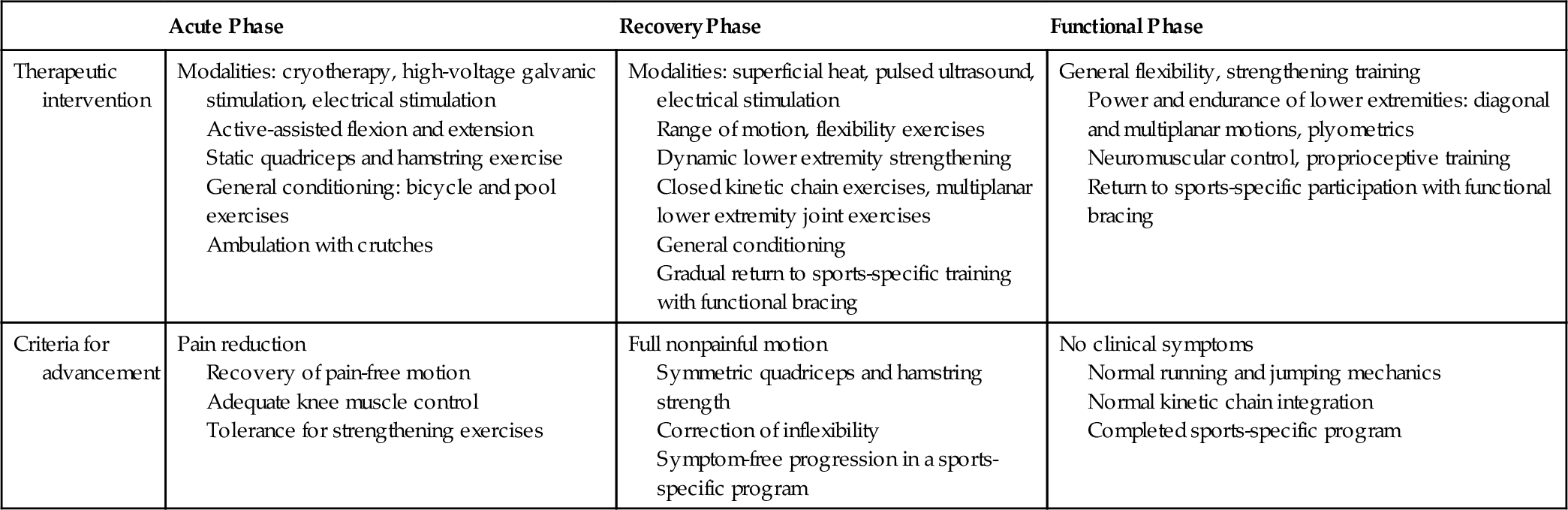
The patient with an ACL-deficient knee may present with an acute or a recurrent injury. In the acutely injured knee, protection of secondary structures is of paramount importance, and progression of rehabilitation will depend on the extent of damage to other knee structures. Early use of closed kinetic chain exercises, in which the distal segment of the extremity is fixed and the proximal segments are free to move, has allowed functional progression of strengthening. These exercises allow quadriceps strengthening with hamstring muscle co-contraction, which reduces the strain in the ACL and minimizes patellofemoral joint reaction forces (Table 63.1) [17,18].
The individual with a recurrently unstable knee will benefit from a trial of rehabilitation. Correction of muscle weakness and proprioceptive deficits and functional retraining in combination with activity modification could reduce episodes of instability and should be considered before surgery in the individual with low activity levels.
Acute Phase
This phase focuses on treatment of tissue injury, clinical signs, and symptoms. The goal in this stage is to allow tissue healing while reducing pain and inflammation. Reestablishment of nonpainful range of motion, prevention of muscle atrophy, and maintenance of general fitness should be addressed. This phase may last 1 to 2 weeks.
Recovery Phase
This phase focuses on obtaining normal passive and active knee motion, improving knee muscle function, achieving normal hamstrings and quadriceps muscle balance, and working on proprioception. Biomechanical and functional deficits, including inflexibilities and inability to run or jump, should begin to be addressed. This phase may last 2 to 8 weeks after the injury occurs.
Functional Phase
This phase focuses on increasing power and endurance of the lower extremities while improving neuromuscular control. Rehabilitation at this stage works on the entire kinetic chain, addressing specific functional deficits. This program should be continuous with the ultimate goal of prevention of recurrent injury and safe return to competition. The functional phase may last 8 weeks to 6 months after the injury occurs.
If the patient completes a rehabilitation program and is willing to modify the activity level, including the limitation of sports activity that involves cutting and pivoting maneuvers, the functional prognosis for daily living activities is good [11,19]. In this group of patients, functional braces may be used for sports participation that involves changes of direction. These braces may reduce symptoms of instability in individuals who use them, improve proprioception, and appear to reduce some strain in the ACL in low-demand activities.
Postsurgical Rehabilitation
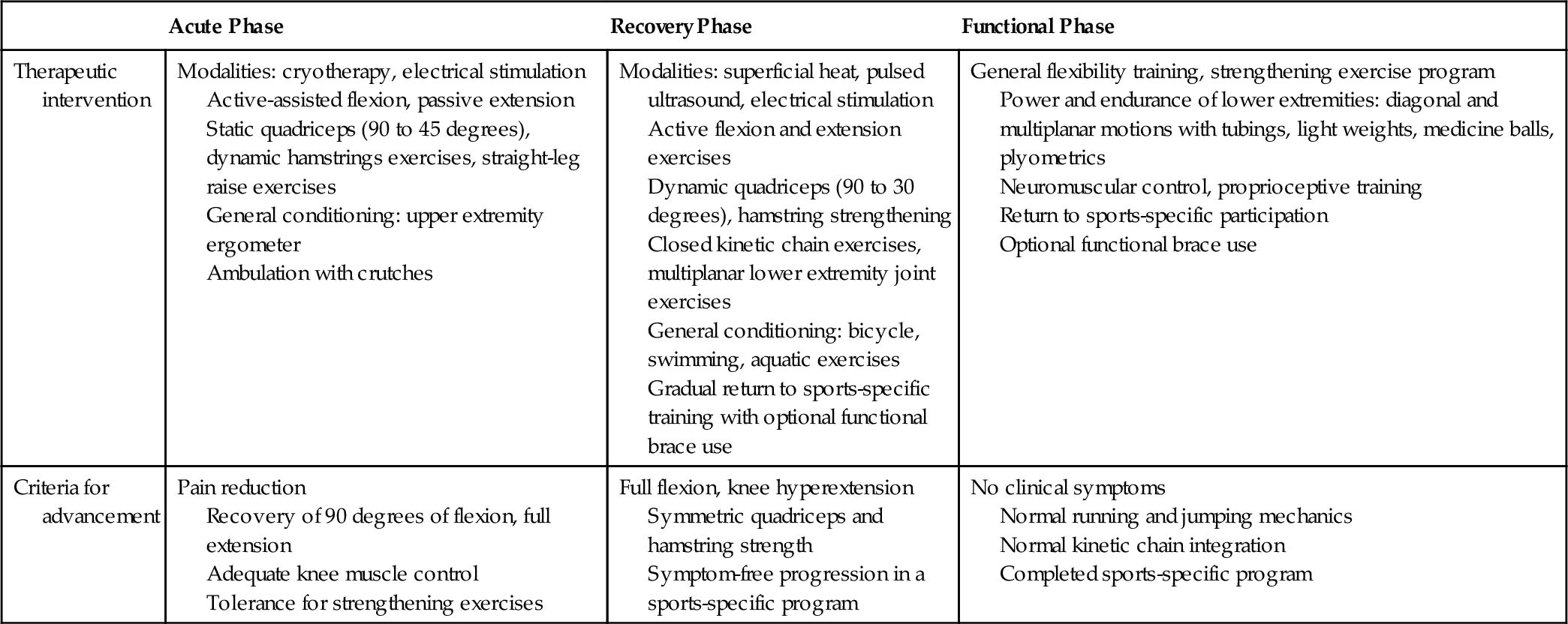
In the patient who is a candidate for ACL reconstruction, rehabilitation should start before surgery. Reduction of pain and swelling, achievement of full range of motion, voluntary muscle activation, and finally achievement of normal muscle strength should be attempted before reconstruction. After surgery, rehabilitation should begin on the first postoperative day. Early use of cryotherapy, compression, and elevation has been shown to reduce swelling postoperatively. It is important to achieve full extension and to initiate early active flexion in the first few days after surgery. Weight bearing with crutches is usually started immediately after the operation [20].
Rapid progression of the rehabilitation program has reduced complications usually associated with ACL knee surgery, which included stiffness, muscle atrophy, muscle weakness, and patellofemoral pain. In the early rehabilitation period, special precautions need to be taken to avoid excessive strain of the reconstructed ligament with terminal (0 to 30 degrees) extension-resisted quadriceps exercises. Early use of closed kinetic chain exercises, such as mini-squats, steps, and leg press, has allowed quadriceps strengthening with tolerable shear forces to the graft [17,18,20] (Table 63.2). Aquatic exercises that allow progressive weight bearing with benefit from the effects of buoyancy can be started as soon as the sutures are removed.
Individuals will vary in the rate in which they achieve full motion, normal strength, normal proprioception, and adequate sports-specific skills. Achievement of these goals should be accomplished before the individual is allowed to return to sports activity. With accelerated rehabilitation programs, patients usually return to activity in 6 to 8 months after surgery. These accelerated rehabilitation programs do not lead to an increase in anterior knee laxity compared with nonaccelerated rehabilitation, and both accelerated and nonaccelerated rehabilitation appear to have the same effect in terms of clinical assessment, satisfaction of patients, functional performance, proprioception, and isokinetic thigh muscle strength [21,22]. The use of postoperative functional bracing does not improve outcome compared with no bracing [23].
Procedures
Knee joint aspiration may be attempted in the first 24 to 48 hours after the injury to document hemarthrosis and to assist in the diagnosis. If the injured individual is not progressing in treatment because of swelling and significant limitation of motion, aspiration may be performed at later stages of treatment for symptom relief. Under sterile conditions, with a 25-gauge, 1-inch sterile disposable needle, infiltrate the skin with a local anesthetic approximately 2 cm proximal and lateral (or medial) to the patella. Follow this injection with a second injection by use of an 18-gauge, 11⁄2-inch needle into the joint capsule. Aspirate any fluid and note the color and consistency. Without withdrawal of the needle, take off the syringe and empty it. Repeat this until all of the fluid is aspirated. Use one hand to compress the suprapatellar area to be sure all of the fluid is out before the procedure is completed.
After aspiration, icing of the knee for 15 minutes after the procedure and then for 20 minutes two or three times daily for several days is recommended.
Surgery
Surgery is indicated in patients with recurrent episodes of instability in activities of daily living and in active recreational athletes who are symptomatic and do not wish to modify their activities. Surgery is definitely indicated in the high-demand competitive athlete.
The surgical procedure of choice is the arthroscopically assisted autograft by full endoscopic or two-incision techniques, with use of either patellar bone–tendon–bone graft or four strands of hamstring tendon graft. A debate exists between the proponents of each graft source and fixation, although both seem to be fairly equal in long-term studies [24,25]. The hamstring tendon group tends to recover faster initially and to have less pain and swelling. However, although the graft strength is good, the fixation of the graft as well as the incorporation of the graft to bone seems to be weaker than with the patellar tendon [15,26,27].
The patellar tendon group, on the other hand, has better fixation and incorporation because it heals bone to bone and behaves biomechanically like a single unit; the sum of these gives it a more reproducible result, making it the preferred graft for the high-demand athlete [3,28]. The downside of its use is that it tends to produce more morbidity, such as swelling, pain, and difficulty in gaining motion initially. It also has a higher incidence of postoperative patellofemoral pain. Other grafts used are the quadriceps tendon and contralateral patellar tendon. Allograft tendon material, such as patellar tendon and tibialis anterior tendon, has also gained popularity. The advantages of these are clear and include less donor site morbidity. The disadvantages are the possibility of disease transmission, slower graft incorporation, potential for stretching of the graft, and higher cost.
A new modality of reconstruction, the anatomic ACL reconstruction, has been popularized by Fu and coworkers [15]. This procedure tries to reconstruct both the anteromedial and the posterolateral bundles of the ACL. Fu and others have shown in vitro that this double-bundle anatomic ACL reconstruction better controls the important aspect of rotational instability of ACL deficiency. A study showed that the anatomic single- and double-bundle technique resulted in better anteroposterior and rotational stability than conventional single-bundle reconstruction [15,29,30]. The use of the ligament augmentation reconstruction system (LARS) synthetic ligament has gained popularity, with fewer graft failures and cases of synovitis compared with previous generations of synthetic grafts. However, longer follow-up studies should be undertaken to evaluate for possible complications [31].
Increased participation in competitive sports has caused an upsurge in pediatric ACL tears. The management of such injuries in the skeletally immature population is controversial. Whereas some advocate conservative management, this could cause damage to secondary structures and subsequent early osteoarthritis. Therefore surgical management has been advocated in patients with ACL deficiency returning to high-risk activities. Multiple techniques have been described in this population, including physeal-sparing, partial transphyseal, and complete transphyseal procedures. Good functional outcomes have been obtained with all techniques; however, the physeal-sparing approach has been promoted as a way to reduce the risk of growth retardation [32].
Potential Disease Complications
An untreated ACL injury can produce changes in the knee joint that may lead to significant alterations in the patient’s lifestyle. The patients who continue participation in strenuous sports have recurrent episodes of pain and giving out secondary to anterior laxity and rotatory instability. These may lead to damage of associated structures, such as the menisci and other secondary restraints. A significant number of patients develop joint space narrowing with evidence of osteoarthritis. Risk factors associated with osteoarthritis after initial injury and surgical treatment include injury to the articular cartilage, meniscal tears, loss of range of motion (particularly in extension), high body mass index, and possibly arthrogenic muscle inhibition resulting in quadriceps weakness. The prevalence of radiographic knee osteoarthritis after surgical reconstruction ranges from 29% to 51%, with no statistically significant difference compared with conservative management (24%-48%). However, the prevalence of tibiofemoral osteoarthritis in isolated ACL injury has been reported to be lower (0%-13%) than in combined ACL and meniscal injuries (21%-48%), which highlights the role of meniscal injury in associated morbidity [1,11,19,33,34].
After surgical reconstruction, the incidence of ipsilateral graft tear is 5.8%; the risk of contralateral rupture is 11.8% [35]. Attention has recently been given to prevention of initial or recurrent ACL injury by modifying neuromuscular risk factors, such as muscle weakness and muscle imbalance and proprioceptive deficits, and working on sports-specific techniques, such as cutting and jumping. These programs combine strengthening, balance, and plyometric exercises with apparent improved dynamic stability and a reduction in the incidence of injury [35–37].
Potential Treatment Complications
Medication complications include gastric, cardiovascular, and renal toxicity with nonsteroidal anti-inflammatory drugs. Injections carry the risk of infection (in approximately 1% to 2% of cases). Surgery has the risks of venous thrombosis and complications from the anesthesia. Fibrous ankylosis and significant loss of motion can be seen in the early rehabilitation stages secondary to poor progression of therapy or compliance of the patient [35].
Poor graft placement and fixation can lead to loss of motion and subsequent graft failure with recurrent instability. In patients in whom chondral lesions or meniscal tears are identified at the time of surgery, long-term sequelae include the development of arthritis even after the reconstruction [4,6,30,33,34,38].