CASE 51
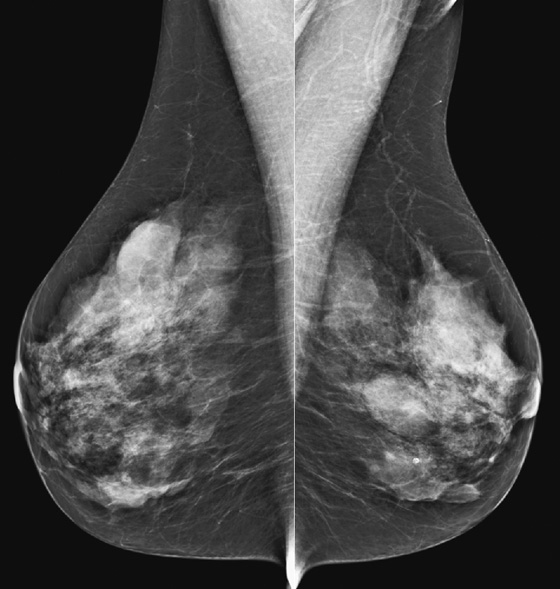
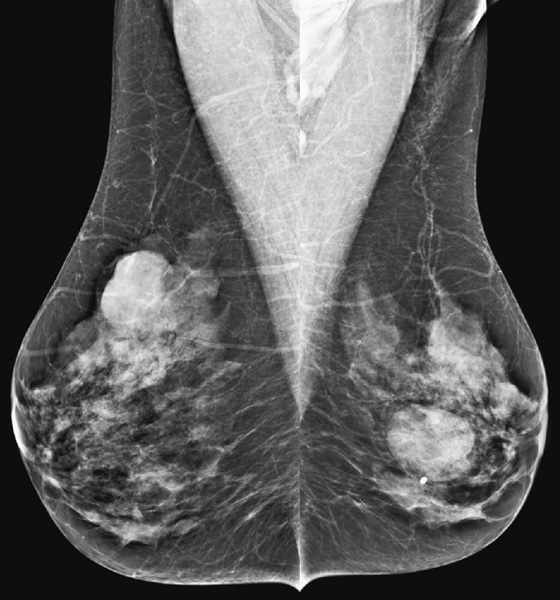
History: An asymptomatic woman presents for routine screening mammogram.
1. What should be included in the differential diagnosis based on the images shown? (Choose all that apply.)
A. Multiple bilateral fibroadenoma
B. Multiple bilateral cysts, with a suspicious mass in the upper left breast
D. Multiple bilateral complicated cysts
2. What is the next step in management of this patient?
A. Recall for bilateral ultrasound
C. Routine screening mammogram in 1 year
3. What is the cancer rate in women with bilateral circumscribed masses?
A. Slightly higher than the national average because of proliferative change
B. Approximately double the normal rate
C. Approximately the same as the normal population
D. Slightly higher, unless the cysts are palpable
4. What is the BI-RADS (Breast Imaging Reporting and Data System) for this finding on a baseline mammogram?
ANSWERS
CASE 51
Changing Pattern of Multiple Bilateral Masses
1. A, C, and D
2. C
3. C
4. A
References
Berg WA, Campassi CI, Ioffe OB. Cystic lesions of the breast: sonographic-pathologic correlation. Radiology. 2003;227(1):183–191.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:137. 381
Comment
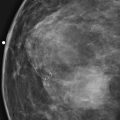
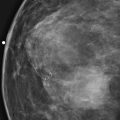
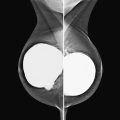
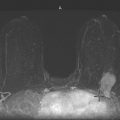
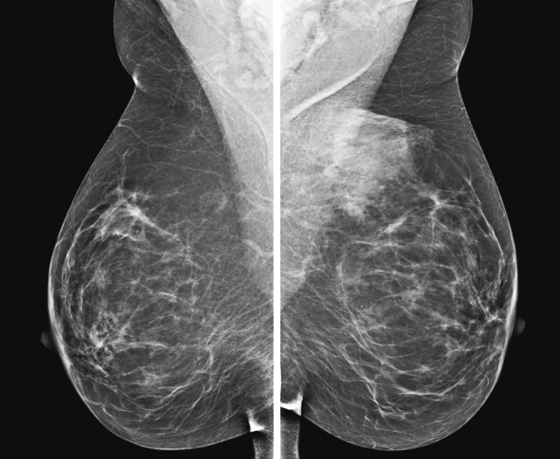
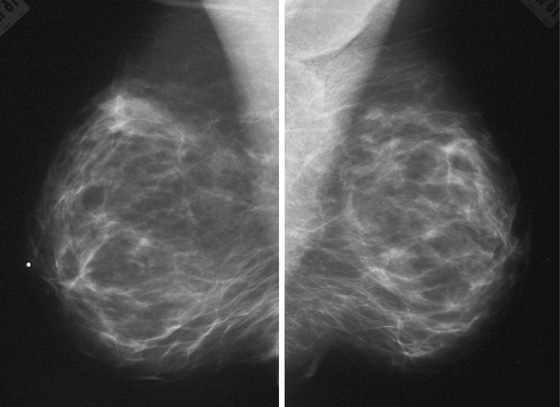
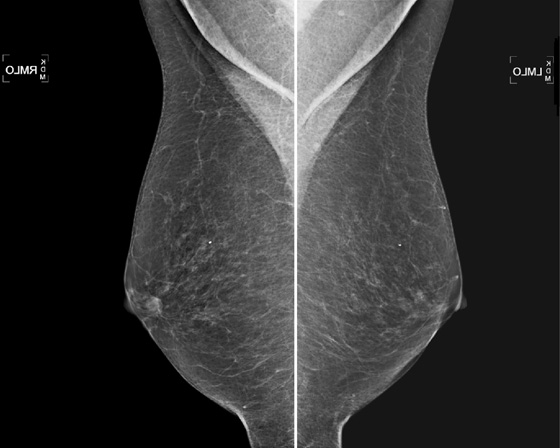
The simple cyst is a round or oval space, filled with fluid and lined by epithelium. It is the most common mass in the female breast and has no malignant potential. It develops and regresses spontaneously, and cysts may increase when estrogens are taken.
On mammography, simple cysts are usually round or oval but may be lobulated and are typically water density, the same as the surrounding parenchyma. They are often multiple and bilateral. Although a cyst cannot be differentiated from a solid mass on mammography, the presence of multiple bilateral circumscribed masses can be considered benign. There should be at least three circumscribed or partially circumscribed masses, with at least one in each breast, to be considered a benign finding. There should be no suspicious findings, such as distortion or suspicious microcalcifications. The masses are most commonly cysts or fibroadenomas. When the masses change in size, location, and number on successive mammograms, they can be considered cysts (see the figures). Ultrasound is not needed if the patient is asymptomatic and all of the above-mentioned conditions are met. The rate of malignancy in a large study of women with bilateral circumscribed masses was approximately 0.1%, lower than the incident rate of breast cancer in the normal age-matched population. If one mass is disproportionately larger or denser or has poorly defined margins, the patient should be recalled.
This patient with bilateral, similar, circumscribed masses is at no increased risk of malignancy, and her mammogram is benign, BI-RADS 2. Assigning the patient to a BI-RADS 3 category, with short-interval follow-up, is unnecessary.
CASE 52
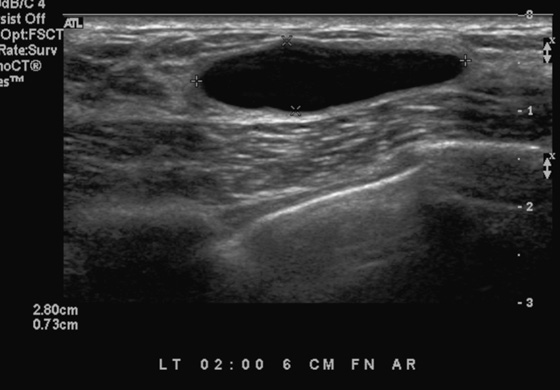
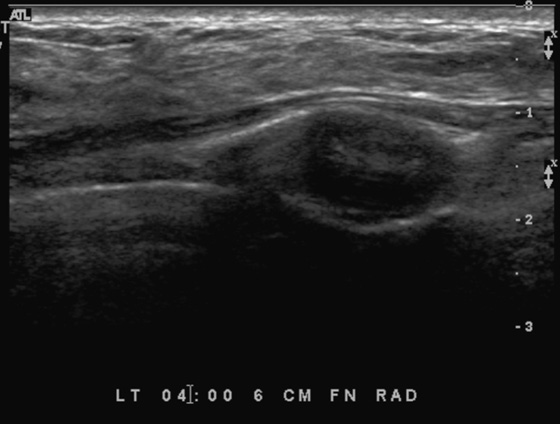
History: A 21-year-old woman presents with two palpable masses in her left breast—one in the upper outer quadrant and one in the lower outer quadrant.
1. What should be included in the differential diagnosis for the two ultrasound images shown? (Choose all that apply.)
A. Suspicious mass in the lower outer breast
B. Simple cyst in the upper outer breast
C. Rib in the lower outer breast
D. Fibroadenoma in the lower outer breast
2. How is a rib distinguished from a breast mass on ultrasound?
A. The rib is posterior to the chest muscle.
B. The breast mass should be posterior to the chest muscle.
C. The rib never looks oval, and masses are often oval.
D. Masses are typically more hypoechoic than the rib.
3. What is the BI-RADS (Breast Imaging Reporting and Data System) code for the ultrasound examination of this patient?
4. How reliable is the negative ultrasound examination when evaluating a palpable mass?
A. Not reliable—a mammogram must be performed for more complete evaluation.
B. Not reliable—the patient must be seen by a surgeon for consideration for biopsy.
C. Relatively reliable—the radiologist may correlate the palpable finding with the ultrasound.
D. Somewhat reliable—the patient should return for a short-interval follow-up.
ANSWERS
CASE 52
Ultrasound of Rib
1. B and C
2. A
3. C
4. C
References
Dennis MA, Parker SH, Klaus AJ, et al. Breast biopsy avoidance: the value of normal mammograms and normal sonograms in the setting of a palpable lump. Radiology. 2001;219(1):186–191.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:100. 150, 175
Comment
Patients often present with a palpable finding. They may note cysts (see the figures), solid masses, or normal parenchyma. Patients may confuse normal anatomy with pathology; the normal rib may be perceived as a mass. Ultrasound is an important tool in the evaluation of a breast lump because the area of concern can be addressed in a precise manner. One method of evaluating the lump is to place your finger or a cotton-tipped swab over the lump, as pointed out by the patient. Apply the ultrasound transducer to the finger or swab, then withdraw the finger and scan directly underneath.
The beginner ultrasonographer may confuse the rib in cross section with a breast mass (see the figures). To differentiate the rib from a breast mass, note the position of the finding relative to the pectoralis muscle, note the periodicity of the ribs, and note that turning the transducer 90 degrees elongates the rib.
CASE 53
History: A 55-year-old woman presents for a screening mammogram. Her last mammogram was obtained 4 years ago and is available for comparison.
1. What should be included in the differential diagnosis for the finding in the right breast? (Choose all that apply.)
B. Pseudoangiomatous stromal hyperplasia
D. Asymmetric glandular tissue
2. What is the BI-RADS (Breast Imaging Reporting and Data System) category?
3. Which of the following imaging tools is inadequate for further work-up?
A. Additional mammographic views
4. Which of the following statements regarding focal asymmetry is false?
B. It is not a very rare finding on screening mammogram.
C. A new focal asymmetry requires further work-up.
D. Biopsy is useful in differentiating a benign from a malignant process.
ANSWERS
CASE 53
Developing Asymmetry
1. A, B, and D
2. A
3. D
4. A
References
Leung JW, Sickles EA. Developing asymmetry identified on mammography: correlation with imaging outcome and pathologic findings. AJR Am J Roentgenol. 2007;188(3):667–675.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:410.
Comment
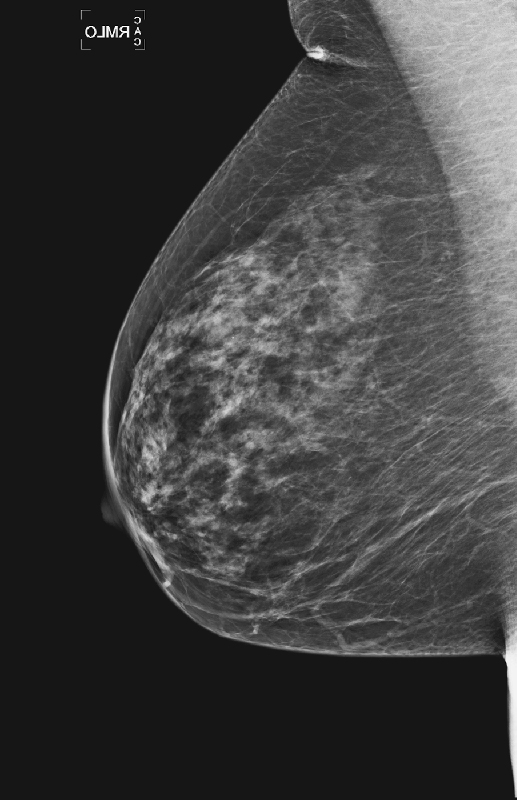
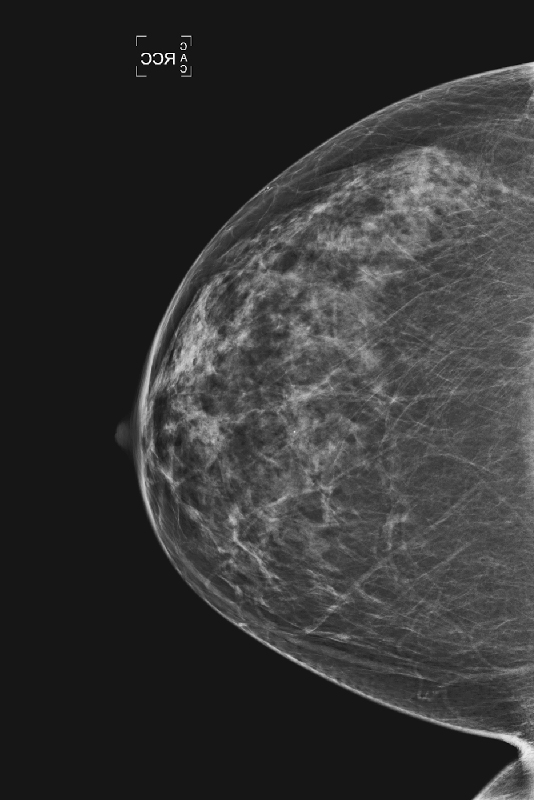
The breasts are usually fairly symmetric in density and architecture. Nevertheless, asymmetries are not an unusual finding on routine mammogram (see the figures), reportedly seen in 3% of healthy women. According to the BI-RADS lexicon, asymmetric findings constitute an area of tissue with fibroglandular density that is more extensive in one breast compared with the corresponding region of the contralateral breast. Asymmetries are planar, lack convex borders, usually contain interspersed fat, and lack the conspicuity of a three-dimensional mass.
There are three types of asymmetric findings: global asymmetry, focal asymmetry, and developing asymmetry. Global asymmetry involves a large portion of the breast (at least a quadrant), focal asymmetry corresponds to a density with similar shape in two views but lacking convex outward borders, and developing asymmetry is focal asymmetry that is new or increasing. A developing asymmetry may be a normal variant but can also constitute a sign of malignancy and must be viewed with caution and evaluated thoroughly.
It is important to review all previous mammograms, with at least a 2-year interval, because an area of increasing density may not be apparent over a shorter interval (see the figures). If the previous mammograms are at a different site, there is value in obtaining them. If the area is stable compared with prior films, no further work-up is needed. However, a new, larger or denser asymmetry and a palpable abnormality require additional evaluation. Additional mammographic views help to determine if the tissue spreads out or if there is interspersed fat, which is a benign feature. Additional views may include spot compression views, rolled craniocaudal views, and a true lateral view.
Ultrasound is a very good tool for evaluating focal asymmetries. The classic ultrasound appearance of an island of normal breast tissue is that of a hyperechoic area with ducts coursing through. There should be no mass, distortion, or abnormal shadowing.
A core biopsy can be performed if concern is expressed by the patient or if the asymmetry is palpable. The histology is often benign breast tissue, stromal fibrosis, or pseudoangiomatous stromal hyperplasia. MRI can be done instead of needle biopsy, although the role of MRI for assessment of asymmetric breast findings has not yet been established. With administration of contrast material, the enhancement of the asymmetric tissue should be the same as that of the rest of the glandular tissue, with no abnormal enhancement.
CASE 54
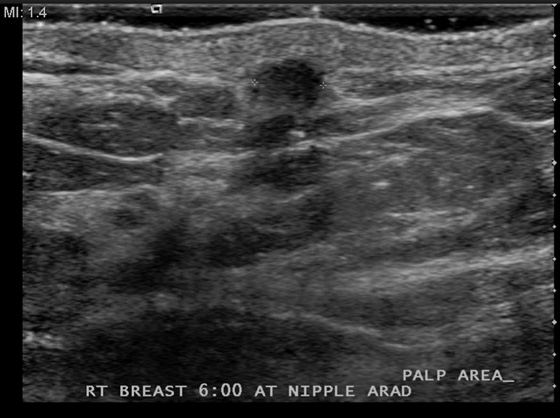
History: A 61-year-old woman of normal risk palpated a tiny mass just below her right nipple. Mammogram views of the right breast are shown.
1. What is the differential diagnosis of the mammogram of the right breast? (Choose all that apply.)
A. There is a suspicious mass near the nipple at the 6 o’clock position.
B. There is no suspicious finding.
D. Suspicious clustered microcalcifications are seen near the nipple.
2. What is the next step in the management of this patient?
A. The mammogram is normal, so she can return in 1 year.
B. The patient should seek a surgical consultation, because the mammogram is abnormal.
C. The patient should be evaluated with ultrasound of the palpable mass.
D. The patient should be asked to return in 6 months.
3. How accurate is the normal mammogram in the setting of a palpable mass?
B. Not very accurate: 20% to 40%
C. The accuracy depends on the glandular density.
D. The mammogram is nearly 100% accurate in the patient with a dense breast.
4. What should be the next step if ultrasound reveals a simple cyst at the palpable area?
A. All palpable cysts must be aspirated.
B. All simple cysts should be referred to a surgeon for excision or aspiration.
C. All simple cysts should be followed with an ultrasound exam in 6 months.
ANSWERS
CASE 54
Importance of Ultrasound in a Palpable Mass
1. B
2. C
3. C
4. D
References
Berg WA, Gutierrez L, Ness Aiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233(3):830–849.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:100. 137
Comment
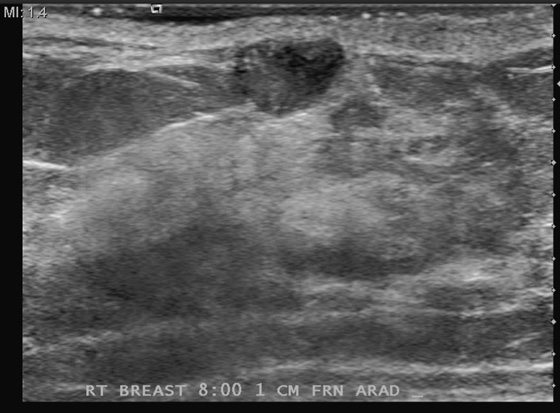
Patients often present with a palpable mass noted either on self-exam or on a clinician’s exam. The mammogram is not a perfect study for the detection of cancer. A 10% false-negative rate is published, based on early studies (Seidman et al, 1987). Depending on the density of the breast tissue, the false-negative rate may be higher. Conversely, when the breast is entirely fatty, the mammogram has a very low false-negative rate. If a patient presents with a palpable finding, and the mammogram shows glandular density in the area (see the figures), then a mass may be obscured by the surrounding gland tissue, and ultrasound should be performed. In this case, the patient failed to mention the palpable concern to the technologist before the mammogram views were performed, so no marker was placed to locate the site of concern. The patient decided to mention the concern after the views, and the exam was converted from screening to diagnostic because of the concern.
Ultrasound was performed (see the figures) and demonstrates a tiny mass in the subcutaneous tissues inferior to the nipple. The mass is indeterminate for the presence of malignancy. Although it is roughly oval and wider than tall (both benign features), the borders are microlobulated. This is a suspicious feature, and the presence of one suspicious feature means that biopsy should be performed. Needle core biopsy was performed with ultrasound guidance, and histology was invasive mammary carcinoma with duct and lobular features.
Ultrasound should be performed in the setting of the normal mammogram and a palpable mass. The ultrasound should be correlated with a physical exam, while scanning, to ensure that the palpable concern is evaluated. If ultrasound demonstrates a simple cyst, no further work-up is needed. A simple cyst on ultrasound is anechoic, with imperceptible walls, a sharp back wall, and increased through transmission posteriorly. If a complex cyst is seen (mass within the cyst, or cyst with irregular, thickened wall), then biopsy should be performed. If a solid mass is seen, biopsy is typically performed, particularly if there is a suspicious feature on ultrasound, such as in this patient.
CASE 55
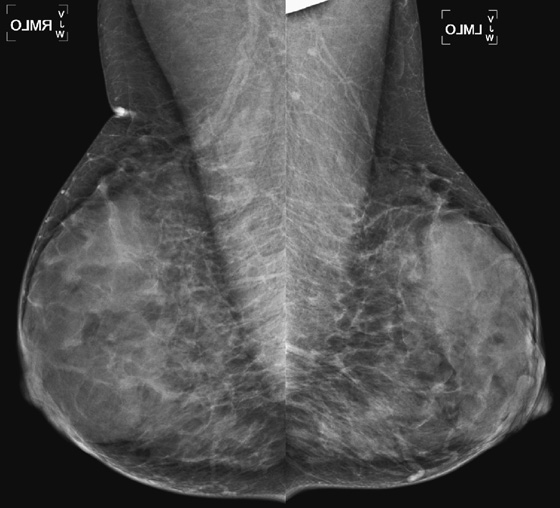
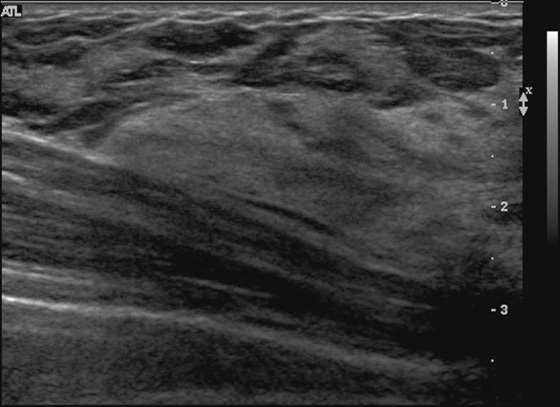
History: A 38-year-old woman who feels a lump in the right upper outer breast undergoes a baseline mammogram.
1. What should be included in the differential diagnosis for the images shown? (Choose all that apply.)
A. Focal masslike density in the right breast on mammogram and normal ultrasound
B. Normal mammogram and ultrasound
C. Normal mammogram and dense, focal fibrosis on ultrasound
D. Dense glandular tissue on mammogram with fibrous ridge on ultrasound
2. What is the negative predictive value of a normal mammogram and normal ultrasound in the setting of a palpable mass?
A. The negative predictive value is less than 50%.
3. What is the management of this patient after normal standard imaging?
B. The patient should be referred for mandatory surgical evaluation.
C. Needle biopsy of the echogenic tissue seen on ultrasound should be offered.
4. What is the BI-RADS (Breast Imaging Reporting and Data System) of this study?
D. BI-RADS 5—highly suspicious
ANSWERS
CASE 55
Predictive Value of Negative Imaging
1. B, C, and D
2. C
3. A
4. A
References
Dennis MA. Breast biopsy avoidance: the value of normal mammograms and normal sonograms in the setting of a palpable lump. Radiology. 2001;219(1):186–191.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:174.
Comment
When a patient older than 30 years presents with a palpable finding, a mammogram should be performed as the initial study. Ultrasound is particularly helpful in the setting of a normal dense mammogram, with no evidence of a mass or suspicious microcalcifications (see the figures). Ultrasound may reveal an occult mass or cyst or may be normal. A normal mammogram and normal ultrasound in a patient who feels a lump are quite common. Often the reason for the palpable finding can be seen on ultrasound as an isolated dense, echogenic area surrounded by fat or a dense ridge (see the figures). The dense tissue is adenosis or fibrosis but is a normal variation and should be communicated as such to the patient and the referring physician.
Several studies have shown that the positive predictive value of a negative mammogram and negative ultrasound in the setting of a palpable finding is nearly 100%. The normal findings are reliable, the report is given a BI-RADS 1, and no imaging follow-up is needed. The patient and the physician may choose to follow up with a surgical consultation based on clinical grounds alone.
It is important that the radiologist personally correlate the clinical and imaging findings in this situation. The technologist performing the ultrasound may miss a subtle finding or may believe that the clinically apparent lump is quite suspicious on palpation. If the clinical finding is suspicious, and no abnormality is seen on standard imaging, MRI can be performed to evaluate further.
CASE 56
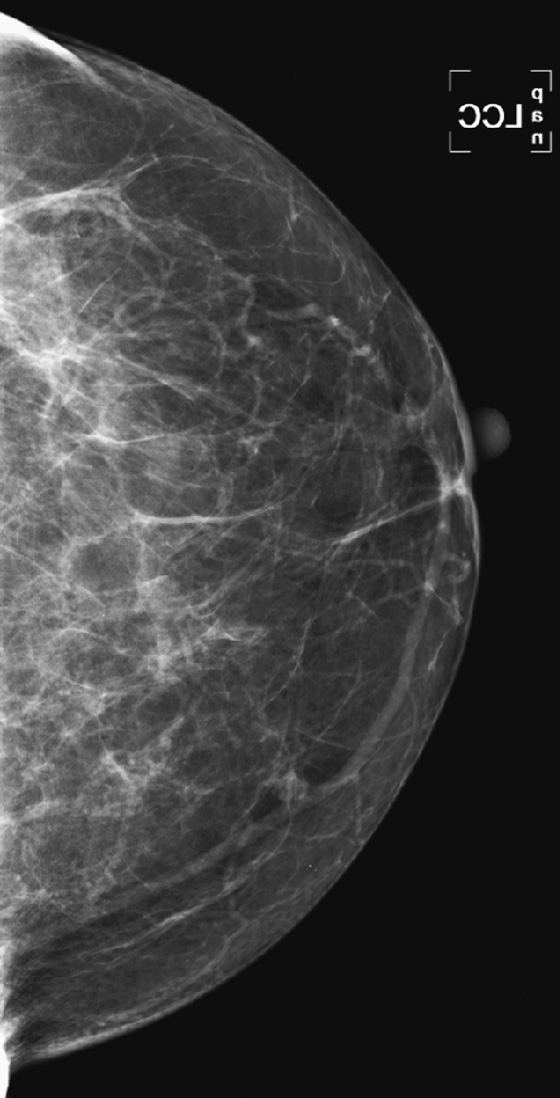
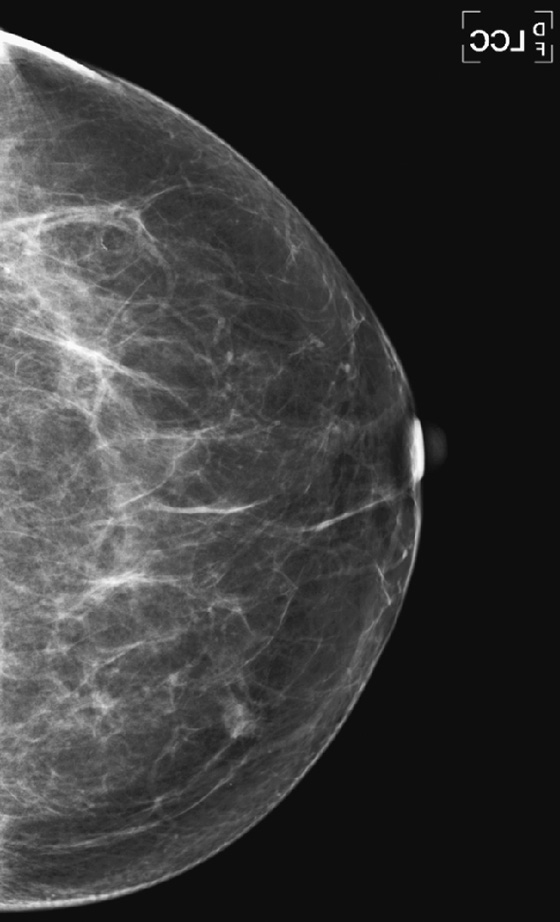
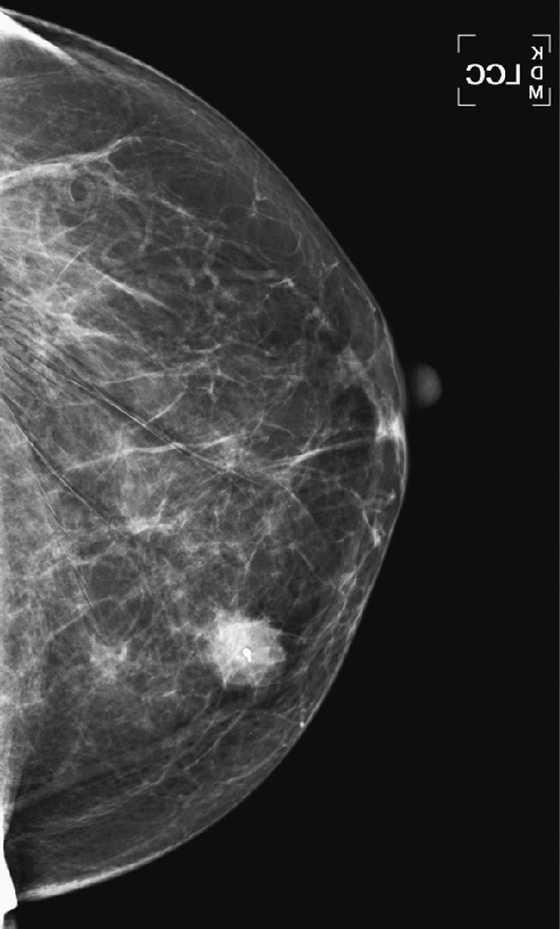
History: A 66-year-old woman presents with a palpable mass in her left breast, 6 weeks after a screening mammogram that was interpreted as normal.
1. What should be included in the differential diagnosis for the three mammogram images shown? (Choose all that apply.)
A. Rapid growth of a breast malignancy
B. Abscess developing in left breast
C. Benign mass was excised; mass represents palpable surgical site
D. Rapid growth of a benign fibroadenoma
2. Why are cancers missed at screening mammography?
A. The breast tissue is fatty.
B. The missed cancer is spiculated and associated with calcifications.
C. The radiologist incorrectly interpreted the mammogram.
D. There are prior mammograms for comparison.
3. What is an interval cancer?
A. A cancer that develops between routine screening examinations
B. A cancer that is intermediate grade
C. A cancer that develops in a woman who has a family history of breast cancer in her daughter
D. A cancer that develops in the same breast as cancer was diagnosed earlier
4. What are “minimal signs” or “nonspecific signs” on mammography?
A. A visible lesion on mammogram, not worrisome enough to warrant recall
B. Tissue density that is 25% to 50% dense
C. Lesions that are suspicious for malignancy
D. Secondary signs of malignancy, such as skin thickening
ANSWERS
CASE 56
Interval Cancer
1. A, B, and C
2. C
3. A
4. A
References
Cho N, Kim SJ, Choi HY, et al. Features of prospectively overlooked computer-aided detection marks on prior screening digital mammograms in women with breast cancer. AJR Am J Roentgenol. 2010;195(5):1276–1282.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:408.
Comment
An interval cancer is one that is detected between routine screening mammograms. It is typically found on clinical examination by the patient’s physician at a routine clinical visit or felt by the patient on breast self-examination. The incidence of interval cancer relates to the sensitivity of mammography, which is variably reported as 80% to 90%—that is, some cancers are missed on mammography, no matter how skilled the radiologist.
When the patient presents with a clinical finding, and the previous screening mammogram is reviewed, a suspicious lesion may be seen that was clearly overlooked by the interpreting radiologist. This situation may be due to a perception error or a misinterpretation error. A smaller percentage of findings on the previous screen are termed “minimal” or “nonspecific,” meaning that although a lesion is present, it does not meet the threshold for recall. It may be low density, with smooth margins, as in the patient in this case (see the figures). Other cases are truly occult on the previous screening mammogram and are typically aggressive, rapidly growing tumors (see the figures).
Several studies have shown that women with interval cancers have higher stage disease and more often have positive lymph nodes compared with women with cancers detected on screening examination. Interval cancers are more often well-defined masses, which are uncommon and which may have a nonspecific appearance, such as the special types of infiltrating ductal carcinoma, including medullary, mucinous, invasive papillary, and tubular cancers. This patient had the much more common infiltrating ductal carcinoma, not otherwise specified, grade II/III. She had a positive axillary lymph node.
To attempt to reduce the number of overlooked cancers, the radiologist should be alert to subtle changes in lesions, including change in size or density (see the figures), and should judge a lesion by its most suspicious feature.
CASE 57
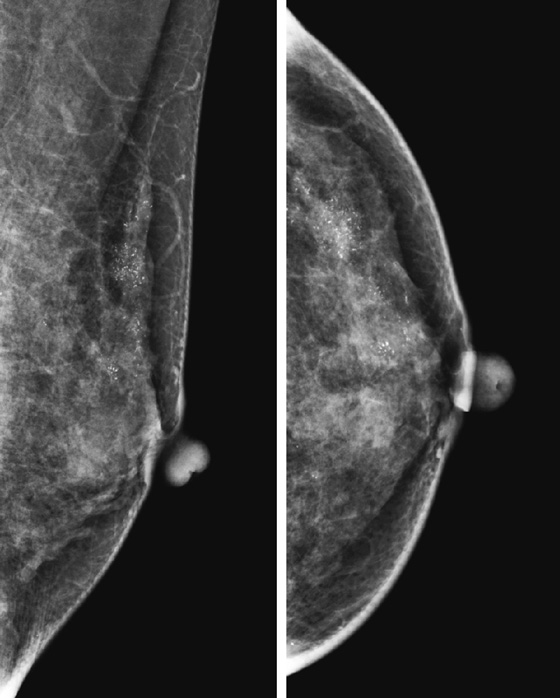
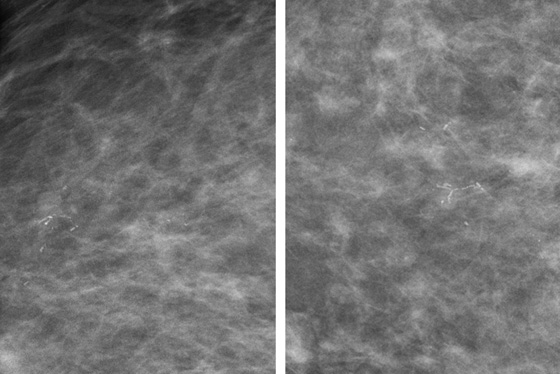
History: Two patients present for routine screening mammography. The first figure shows the left mammogram in a 40-year-old asymptomatic woman. The second figure shows the magnification views performed as additional views after routine mammogram in a 58-year-old asymptomatic woman.
1. What should be included in the differential diagnosis for the two patients shown? (Choose all that apply.)
A. Lobular carcinoma in situ (LCIS)
D. Ductal carcinoma in situ (DCIS)
2. What are the descriptive terms used for the calcifications in patient 1?
A. Coarse, heterogeneous, and segmental
C. Coarse, linear, and rodlike
D. Clusters of punctate calcifications
3. What are the descriptive terms used for the calcifications in patient 2?
A. Snakeskin, filling the ducts
C. Fine, linear, and branching
4. Why is it important to recognize the location of calcifications within the breast?
A. Calcifications not in ducts, lobules, or masses are typically benign.
B. Calcifications in ducts are almost always malignant.
C. Calcifications in masses are typical of fibroadenoma.
D. Calcifications in lobules are typical of LCIS.
ANSWERS
CASE 57
Ductal Carcinoma In Situ
1. C and D
2. A
3. C
4. A
References
Evans A. The diagnosis and management of pre-invasive breast disease: radiological diagnosis. Breast Cancer Res. 2003;5(5):250–253.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:64.
Comment
Mammography is the primary tool for the detection and biopsy of DCIS. Calcifications develop in ducts and have characteristic appearances. Calcifications are present in 80% to 90% of cases of DCIS that have a mammographic abnormality (see the figures). The remaining 10% to 20% of cases manifest as a noncalcified mass or developing asymmetric density.
DCIS is classified as low grade, intermediate grade, and high grade. The histopathologist uses the terms comedocarcinoma, micropapillary, solid, and cribriform to describe the DCIS architecture on histology. The individual calcifications form in the necrotic portions of the intraductal tumor. The shapes of calcifications are termed pleomorphic, fine linear, and branching (see the figures); coarse and fine heterogeneous or granular (see the figures); amorphous; and indistinct. The term pleomorphic means the calcifications within the group vary in shape, size, and density, which is common in DCIS. The distribution of the calcifications is very important. Terms for DCIS distribution include clustered, linear, and segmental. The first figure shows segmental distribution, and the second shows clustered distribution.
In the assessment of breast calcifications, it is important to consider the worst-appearing calcifications within the group. In the second figure, there are some punctate forms, which are typical of benign fibrocystic change. This patient’s histology report described cancerization of the lobules. In cancerization, tumor spreads from the duct into the adjacent lobules and forms calcifications in lobules, which are typically punctate. In this cluster, the fine linear branching calcification is highly suspicious for DCIS. The presence of the punctate calcifications should not rule out classifying this cluster as suspicious.
It is impossible to predict definitely the subtype of DCIS based on the shape and distribution of the calcifications; however, the coarse heterogeneous pattern seen in patient 1 is often seen in micropapillary type with necrosis, which was her histology. Patient 2 had a high–nuclear grade solid and cribriform pattern, with extension into lobules, which is commonly seen in the fine linear branching form of DCIS.
CASE 58
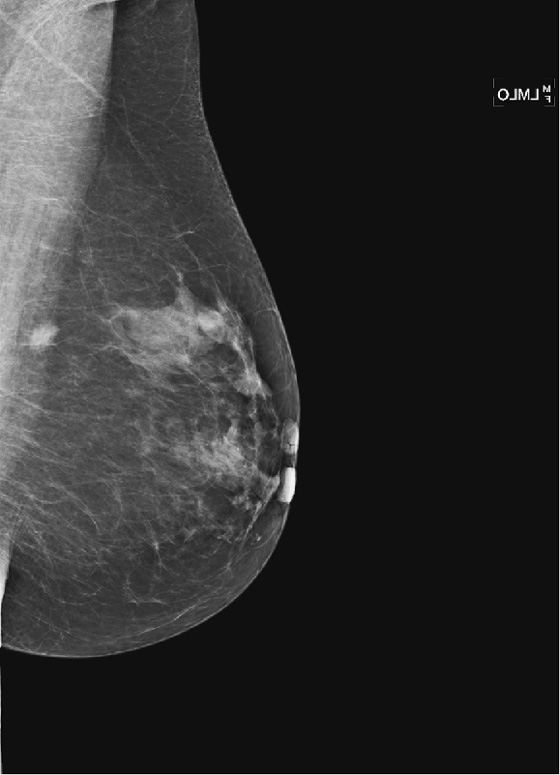
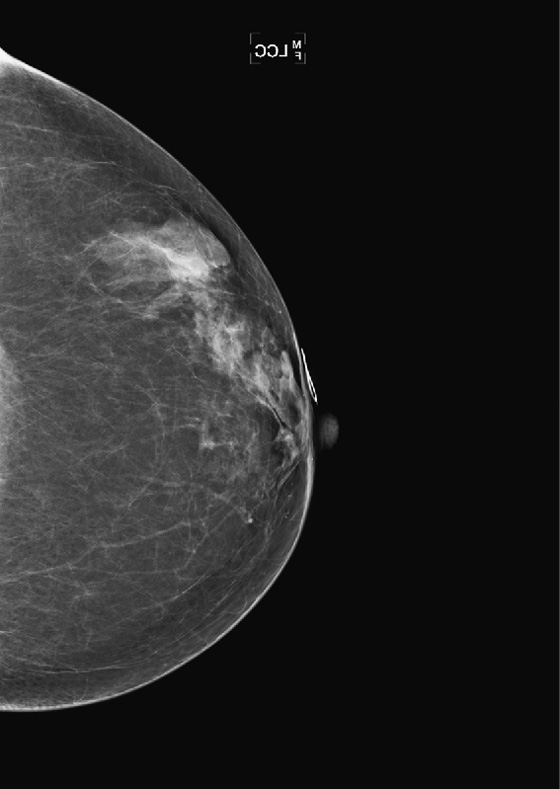
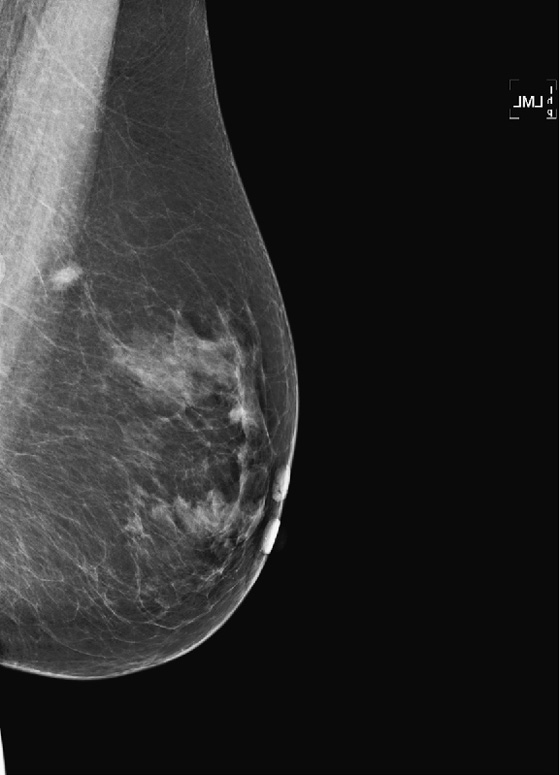
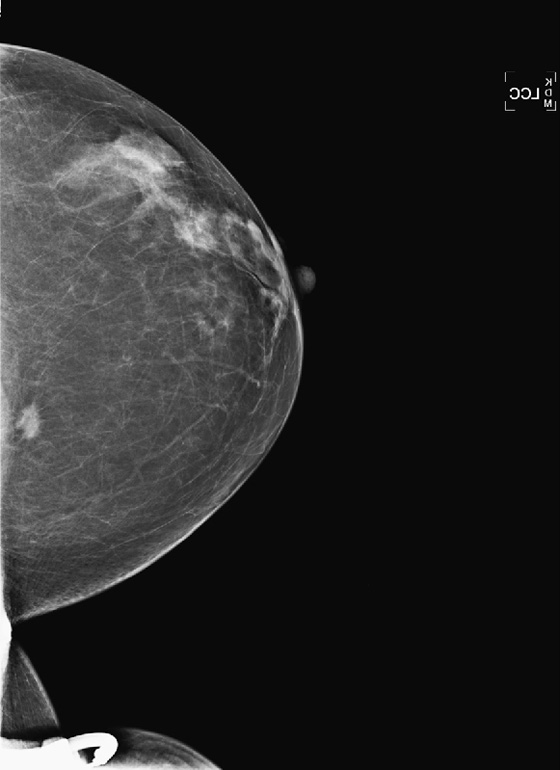
History: A 58-year-old woman presents for screening mammogram.
1. What is the differential diagnosis, based on the images presented? (Choose all that apply.)
C. Focal density on MLO view only, possible mass
D. Without prior films, there is no way to give a diagnosis
2. What is the next step of the work-up?
A. Do a 90-degree lateral mediolateral (ML) view.
B. Do a rolled craniocaudal (CC) view.
C. Do an ultrasound of the entire breast, looking for a mass.
D. Do an MRI, looking for an enhancing lesion.
3. You do a 90-degree mediolateral (ML) view next. Why?
A. To see if the image is a real lesion or is caused by overlapping normal structures
B. To identify the location of the density in the medial or lateral breast
C. To include more lateral breast tissue in the image
D. To determine if the density is more likely a lymph node
4. What is “triangulation”?
A. The process of eliminating an image you think is not a real lesion
C. Finding a mass in the lateral aspect of the breast not included on the CC view
D. Using ultrasound to help find a mass seen only on one view
ANSWERS
CASE 58
Mass Seen on One View
1. B and C
2. A
3. B
4. B
References
Sickles EA. Practical solutions to common mammographic problems: tailoring the examination. AJR Am J Roentgenol. 1988;151:31–39.
Sickles EA. Breast imaging: from 1965 to the present. Radiology. 2000;215:1–16.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:49.
Comment
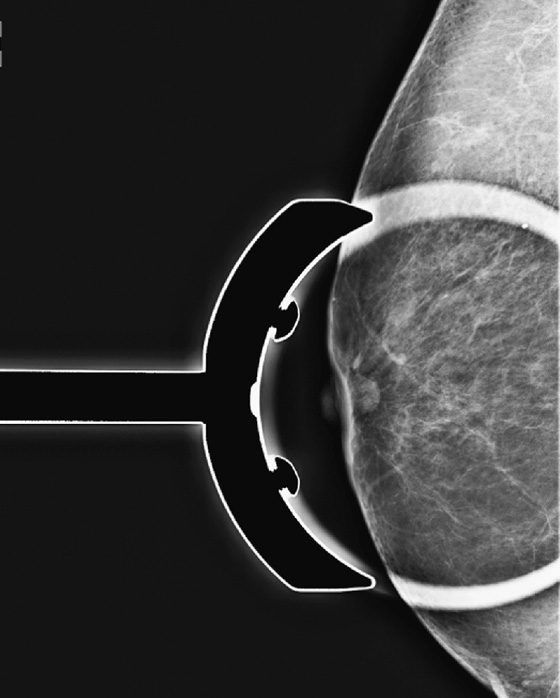
This asymptomatic patient presented for a routine mammogram. Not shown are her prior mammograms, which are negative. The focal density in the upper posterior left breast is a new finding. However, even in the absence of old films, this focal asymmetric density must be viewed with suspicion. Even though it is seen on only one view, it is no less suspicious. Its location in the posterior breast means that it might not have been included on the CC view, even though the CC view appears adequate for interpretation.
The way to determine the location of the lesion is to find it on two orthogonal views. The true lateral view is orthogonal to the CC view. The location of the density on the true lateral view is helpful in locating the lesion in two ways: It gives the distance from the nipple in the vertical plane, and by comparing the two views, the MLO and the true lateral, you can determine the location of the lesion in the breast, either medial or lateral to the nipple. This is well illustrated in the article by Dr. Sickles in 1988. If the lesion is more cephalad on the ML view, compared to the MLO view (goes up), it is in the medial breast. If it is more caudal on the ML view compared to the MLO view, it is in the lateral breast. This process is termed triangulation and can be visually demonstrated by lining up three views of the same breast in the order ML, MLO, CC, with the nipples at the same level and pointing the same way. A line drawn between the lesion on the two views predicts the location in the third view.
In this patient, the lesion is in the upper breast on the MLO view (see the figures) and is located more cephalad in the breast on the ML view (see the figures). Thus, you can determine that it is a medial lesion and instruct the technologist to perform a CC view, exaggerated to the medial aspect. This specialized view is shown in the fourth figure. The lesion is seen, and it can now be determined to be a true mass, located in the upper inner aspect of the left breast, at about the 10 o’clock position. The distance from the nipple in the vertical plane can be determined from the ML view, and the distance from the nipple in the radial plane can be determined from the exaggerated medial CC (XCCM) view. The next step would be to perform an ultrasound of the left 10 o’clock location to find the mass, assess its characteristics, and target the lesion for biopsy. MRI is typically used after a diagnosis of cancer has been made, in order to evaluate extent of disease, because additional masses may be missed on the mammogram.
CASE 59
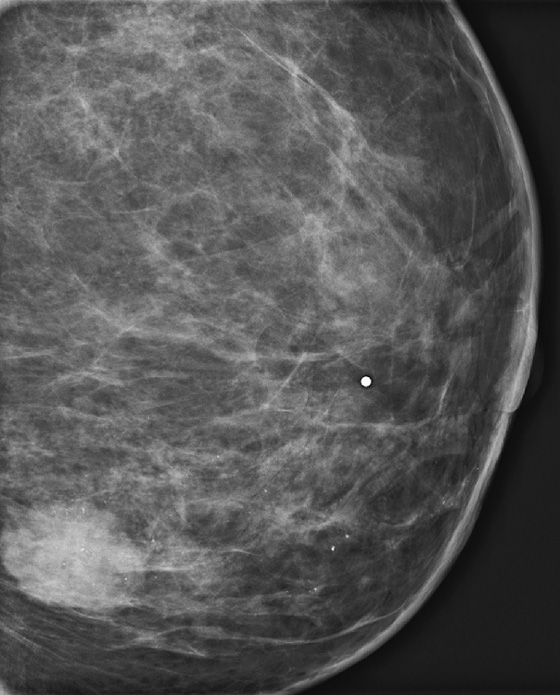
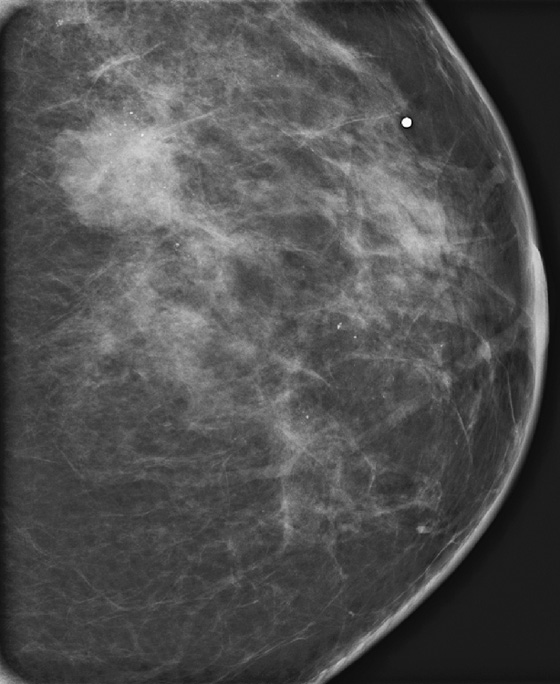
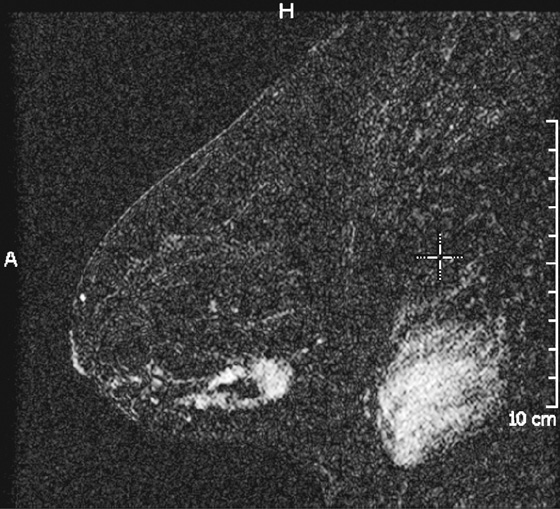
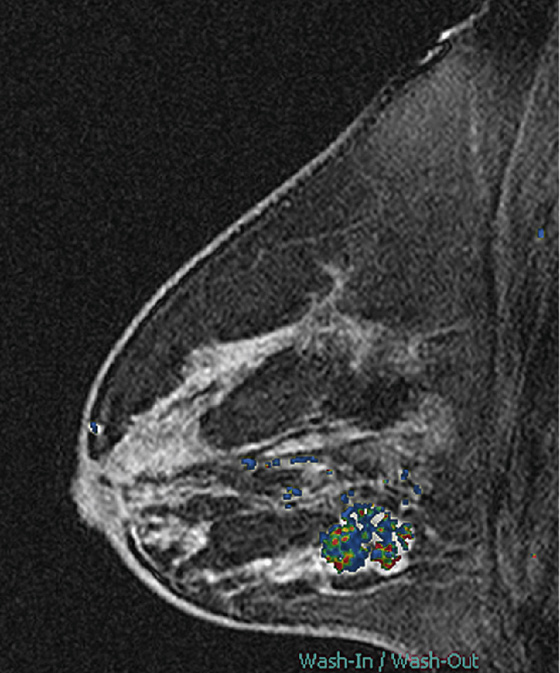
History: A 42-year-old woman presents with a palpable mass in her left breast.
1. What should be included in the differential diagnosis, based on the images provided? (Choose all that apply.)
A. Malignant mass with ductal carcinoma in situ (DCIS) extending toward nipple
B. Phyllodes tumor with adjacent DCIS
C. Simple cyst and benign calcifications
D. Malignant mass with benign calcifications
2. What is an extensive intraductal component?
A. Malignancy that contains a large proportion of DCIS
B. Purely intraductal carcinoma
C. DCIS and atypical ductal hyperplasia
D. A long segment of DCIS involving the entire quadrant of the breast
3. What mammographic features suggest an extensive intraductal component?
B. A cluster of microcalcifications less than 1 cm in diameter
D. Mass with microcalcifications in an area greater than 3 cm
4. Which of the following statements is not true about calcifications adjacent to a suspicious mass?
A. Unless the calcifications are recognized and removed, the patient’s risk of recurrence is higher.
C. The size of the tumor in the breast affects staging.
ANSWERS
CASE 59
Extensive Intraductal Component
1. A, B, and D
2. A
3. D
4. B
References
Stomper PC, Connolly JL. Mammographic features predicting an extensive intraductal component in early-stage infiltrating ductal carcinoma. AJR Am J Roentgenol. 1992;158(2):269–272.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:95.
Comment
An extensive intraductal component is a histologic feature that is associated with a higher recurrence rate after breast conservation therapy. In studies in which the tumor was excised, without regard to evidence of DCIS at the margins, 24% of patients had recurrence of their cancer after treatment compared with 6% of patients who had histologically benign margins. Radiation therapy at the accepted dose may be inadequate to treat the residual tumor. It is important to recognize when an extensive intraductal component may be present to guide the treating physician to excise all the malignant tissue before radiation.
Microcalcifications, in particular, when they extend from the tumor in linear distribution at least 3 cm, are the mammographic feature most commonly associated with an extensive intraductal component. Studies show that the proportion of infiltrating ductal carcinomas with an extensive intraductal component is approximately 25% to 35%, so evaluation of the mammogram with a suspicious mass for adjacent calcifications is prudent (see the figures).
MRI is also useful for evaluation of an extensive intraductal component. Linear enhancement, long spicules, a regional enhancing area, and nodules adjacent to a mass are suspicious MRI findings for an extensive intraductal component (see the figures). In one more recent study, MRI was shown to be superior to the mammogram in predicting an extensive intraductal component and assessing total tumor size.
This patient presented for baseline mammogram at age 42 with a palpable mass in the left breast. Microcalcifications were recognized in the same quadrant as the mass and extending toward the nipple in segmental distribution. She underwent image-guided biopsy of both the mass and the calcifications that were seen farthest away from the mass to establish the extent of disease. The mass was invasive ductal carcinoma, grade III, and the calcifications were DCIS, grade III.
If breast conservation therapy is desired in patients with an extensive intraductal component, bracketing the location of calcifications is necessary at the time of needle localization to include all of the suspected and known disease in the lumpectomy specimen. A mammogram after surgery is helpful to assess the complete excision of all suspicious calcifications before radiation therapy.
CASE 60
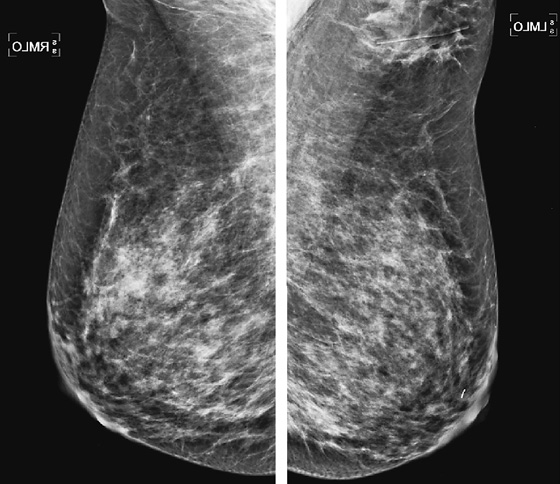
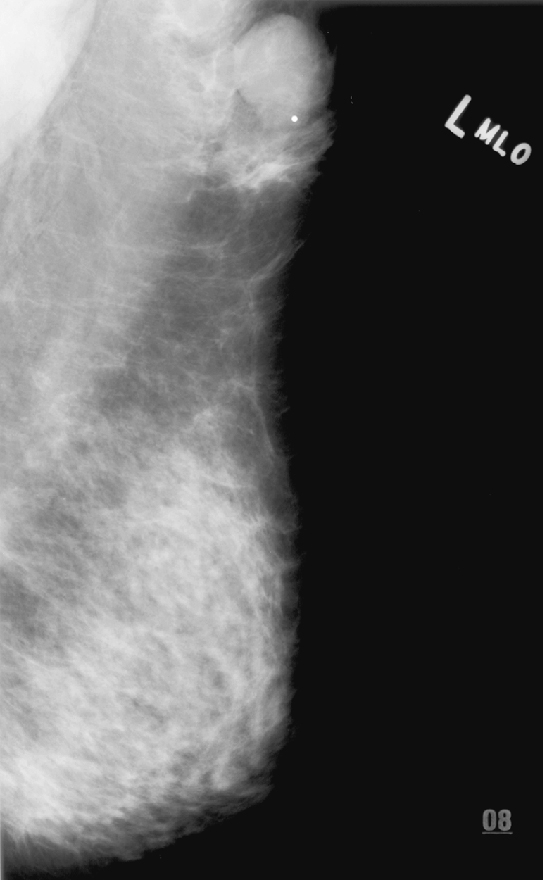
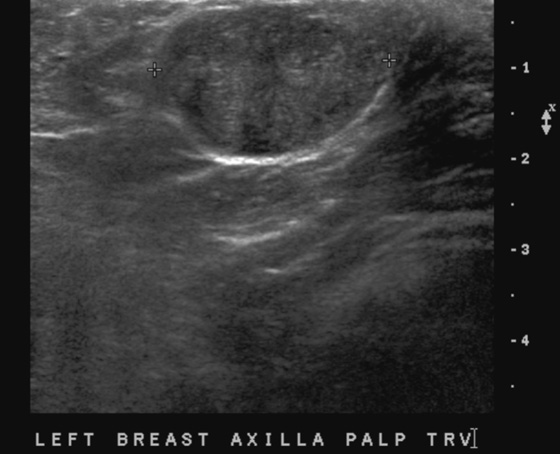
History: A 43-year-old woman presents for routine screening mammogram. She had an excisional biopsy in her left axilla 3 years previously with benign findings.
1. What should be included in the differential diagnosis of the more recent mammogram? (Choose all that apply.)
A. Ectopic breast tissue in the left axilla
B. Spiculated suspicious mass in the left axilla
C. Supernumerary breast in the left axilla
2. What is the clinical concern regarding ectopic gland tissue?
A. Malignancy is more common in ectopic locations.
B. Malignancy can occur in ectopic breast tissue.
C. The concern is primarily cosmetic.
D. Malignancy in the axilla is much more likely to become metastatic.
3. Which of the following statements is not true about the “milk line”?
A. It is an area where primordial rests of breast tissue are present.
B. It relates to animals, not humans.
D. Accessory nipples occur along this line.
4. What is the one best diagnosis of the mammogram and ultrasound from 3 years previously?
D. Left axillary complex cystic mass
ANSWERS
CASE 60
Ectopic Tissue
1. A and C
2. B
3. B
4. C
References
Ciralik H, Bulbuloglu E, Arican O, et al. Fibroadenoma of the ectopic breast of the axilla—a case report. Pol J Pathol. 2006;57(4):209–211.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:36–37.
Comment
Ectopic breast tissue refers to the presence of breast tissue outside the normal confines of the central breast cone and typically occurs along the “Hughes line,” or “milk line,” which extends from the axilla inferomedially into the abdomen. It is seen in approximately 2% to 6% of women. The ectopic tissue can be associated with an extra nipple (polythelia) and can form into a cone of breast tissue, as an accessory breast (polymastia). Although most common along the milk line, ectopic tissue can also occur anywhere in the body and can occur in men, although it is much more common in women. Along the milk line, ectopic tissue is most commonly seen in the axilla (see the figures).
The ectopic tissue arises from quiescent primordial rests of breast tissue. It may remain subclinical and then become manifest during pregnancy and lactation. Because the tissue is normal glandular tissue, albeit located in an aberrant spot, it can develop any change that can occur in the central breast tissue; breast cysts, fibroadenomas, and malignancy can develop.
Masses in the axilla that are of breast origin must be differentiated from axillary adenopathy. Ultrasound is useful in the evaluation of the axilla. Solid benign masses such as fibroadenoma in the axilla should have the same appearance as fibroadenoma anywhere in the breast (see the figures). Adenopathy may appear as a node with a central fatty hilum and a thickened eccentric cortex, or the node may be completely involved with tumor, and the fatty hilum is not seen. In this instance, the abnormal node can appear anechoic and cystic. Color flow Doppler of the axillary mass should always be performed so that adenopathy is not confused with a cyst in axillary ectopic gland tissue.
CASE 61
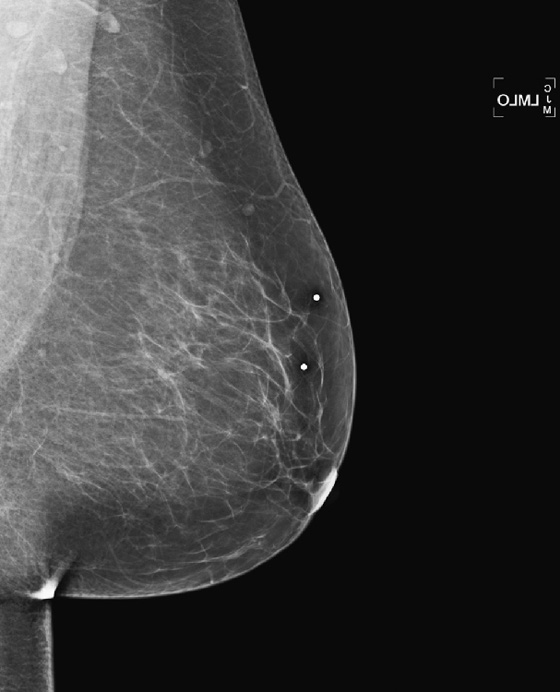
History: A 38-year-old woman presents with two adjacent palpable masses in her left breast.
1. What is the differential diagnosis? (Choose all that apply.)
2. How would you proceed with the work-up after mammography and ultrasound?
B. Perform an MRI to check for abnormal blood supply.
C. No further work-up is needed.
D. Perform a clinical history.
3. After a 6-week delay, the masses are unchanged. Should biopsy be the next step?
A. Yes, the patient must be seen by a surgeon because the lesions are palpable.
B. Yes, image-guided needle biopsy should be performed using ultrasound guidance.
C. Yes, a stereotactic biopsy should be performed.
D. No, follow-up ultrasound in 3 months is recommended.
4. What is the ultrasound finding that is not typical of benign lesions?
C. Location in the subcutaneous tissue
ANSWERS
CASE 61
Angiolipoma
1. A, B, and C
2. D
3. B
4. B
References
Noel JC, Geertruyden JV, Engohan-Aloghe C. Angiolipoma of the breast in a male: a case report and review of the literature. Int J Surg Pathol. 2009. Dec 24
Weinstein SP, Conant EF, Acs G. Case 59: angiolipoma of the breast. Radiology. 2003;227:773–775.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:139.
Comment
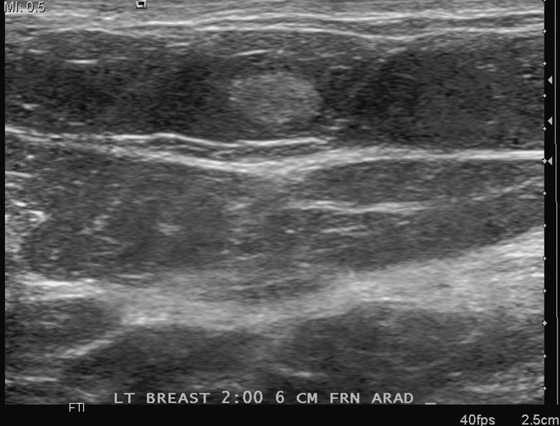
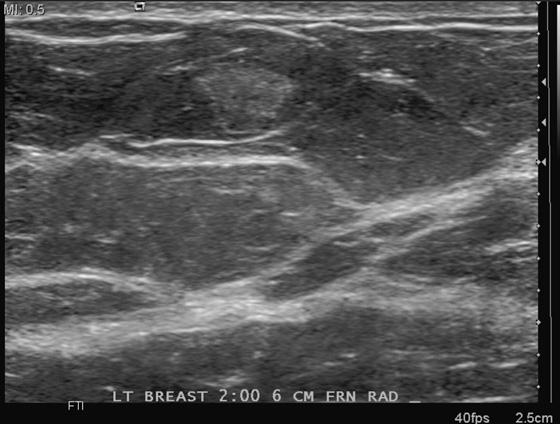
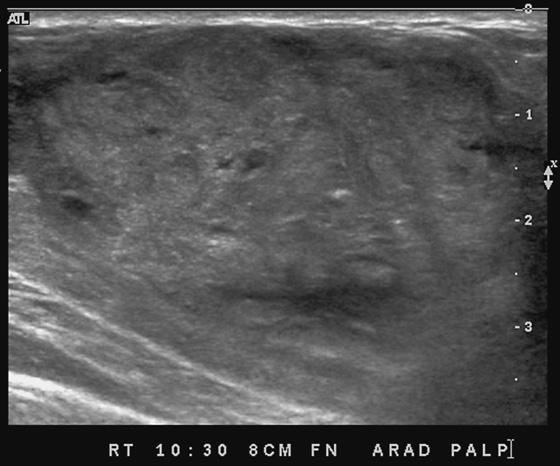
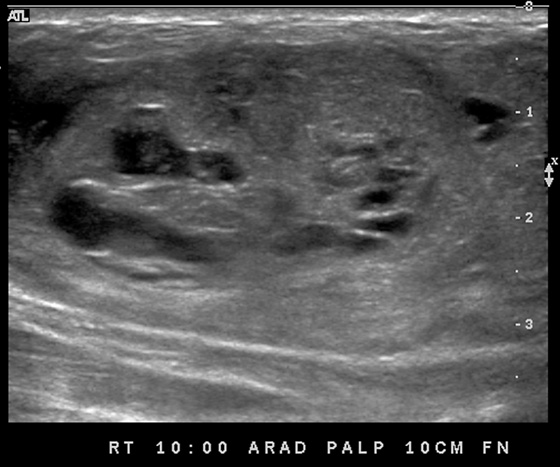
This patient presents with two adjacent palpable, superficial masses. On the mammogram, there is no evidence of a mass (see the figures). Ultrasound shows two adjacent masses in the subcutaneous tissues, corresponding to the palpable masses (see the figures). The margin of one of the masses is microlobulated, and the masses are almost purely echogenic. These features are indeterminate for the presence of malignancy, although they favor a benign diagnosis. The differential diagnosis includes angiolipoma, angiosarcoma, hemangioma, and hematoma.
The patient related no history of trauma. However, a common presentation after trauma is a palpable mass, a hematoma, that can have an identical appearance on mammography and ultrasound. Careful questioning of the patient for any history of recent trauma is helpful, as is examination of the breast for signs of bruising. If the clinical presentation is consistent with trauma, no biopsy is necessary. The patient can be asked to return for follow-up in 6 to 8 weeks, and if the mass resolves completely, no further evaluation is needed. Trauma can cause an occult breast malignancy to manifest as a palpable concern if the mass bleeds and enlarges.
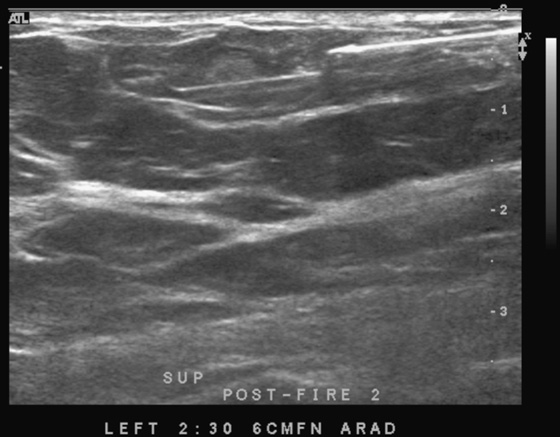
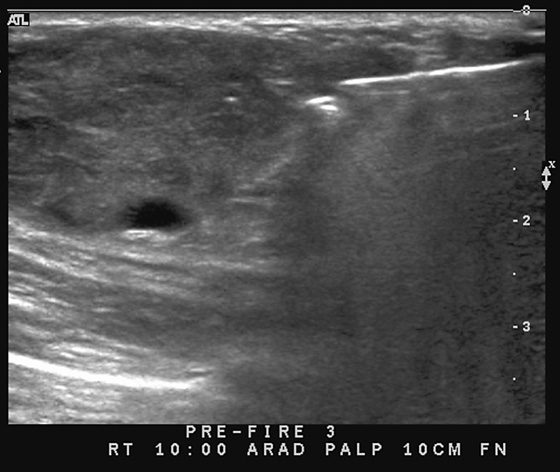
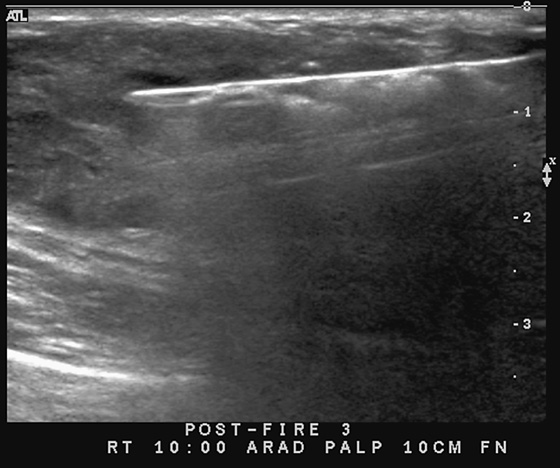
This patient underwent a needle core biopsy using vacuum technique (see the figures), and the histology report was that of an angiolipoma. Excision of the remainder of the mass was advised to exclude angiosarcoma. The histology of the excised specimen was two cellular angiolipomas, and no malignant transformation was seen. This is a relatively unusual tumor of the breast; it is more typically seen in the back, neck, and shoulder.
CASE 62
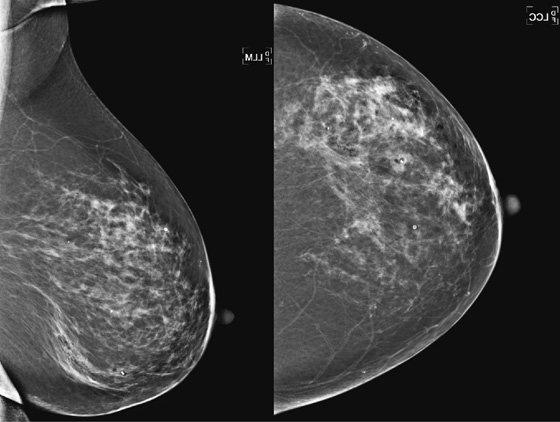
History: A 53-year-old woman has a developing mass, seen best on mediolateral oblique (MLO) view, in the left lower breast. A stereotactic biopsy was performed.
1. What should be included in the differential diagnosis for the left mammogram presented? (Choose all that apply.)
A. Infiltrating lobular carcinoma
C. Pseudoangiomatous stromal hyperplasia (PASH)
2. What is PASH?
C. Always an incidental finding on a biopsy specimen of another finding
3. Which of the following is not in the management scheme of PASH, when found on histology of core needle biopsy?
4. Is this patient at increased risk of future breast cancer?
A. No, but short-interval follow-up is necessary.
B. No, she is at no increased risk.
D. Yes, her risk is increased threefold.
ANSWERS
CASE 62
Pseudoangiomatous Stromal Hyperplasia
1. A, B, and C
2. B
3. C
4. B
References
Polger MR, Denison CM, Lester S, et al. Pseudoangiomatous stromal hyperplasia: mammographic and sonographic appearances. AJR Am J Roentgenol. 1996;166(2):349–352.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:134. 231
Comment
PASH is a benign, relatively rare condition. It was initially described in 1986 in premenopausal women or in postmenopausal women taking hormone supplements but more recently has been found in girls as young as 12 years old and in men. The histology is a proliferation of stromal and epithelial elements. These form slitlike spaces that appear similar to vascular channels, which give this lesion its name of pseudoangiomatous.
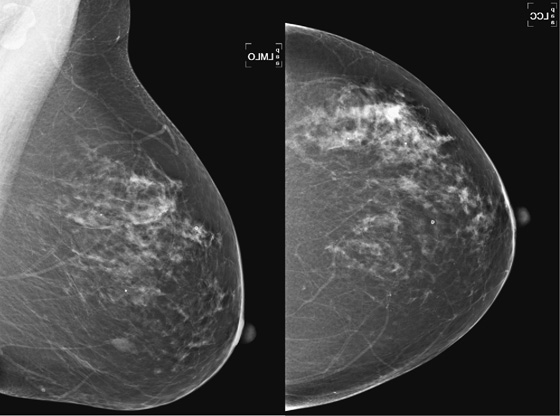
PASH can manifest radiographically as an incidental finding on a biopsy specimen of an adjacent lesion, such as a mass or calcifications; it can manifest as a palpable mass because the stromal overgrowth may coalesce similar to a tumor, such as a fibroadenoma; or it may manifest as a nonpalpable developing density on mammogram, not visible on ultrasound, as in this patient (see the figures). On mammography, PASH is commonly ill defined, as in this patient. It characteristically is not associated with calcification. If a mass is seen on mammography, ultrasound should be performed. Ultrasound features are similar to fibroadenoma or phyllodes tumor; cystic spaces may be present within an oval mass that is wider than tall. However, PASH may also have features overlapping with malignancy on ultrasound; it may be irregular and vertically oriented. In this patient, the focal density was seen best on MLO view and could not be identified on ultrasound.
When PASH is the predominant lesion determined after a needle core biopsy, correlation of clinical and ultrasound findings is crucial to ensure that the targeted area was sampled. Correlation can be obtained by placing a clip after the biopsy and repeating the mammogram and comparing with the initial study.
Management can be routine follow-up if the patient is asymptomatic. However, there are cases of very large (9 cm) palpable masses that are PASH, and these may be best treated with surgical excision, after needle core biopsy establishes the benign diagnosis of PASH. The lesions can recur and can be multiple.
CASE 63
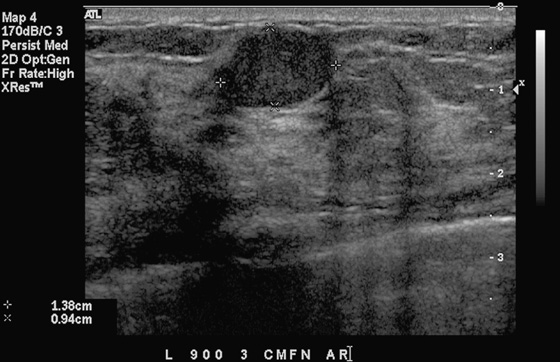
History: A 15-year-old girl presents with a solitary breast mass.
1. What is the differential diagnosis for this ultrasound image of the palpable mass? (Choose all that apply.)
2. How should imaging of this patient proceed?
A. Mammogram as initial work-up, then ultrasound if needed
B. Mammogram as initial work-up, then MRI to assess for additional masses
3. What is the management for this lesion?
B. Perform stereotactic biopsy.
C. No management is needed. Have the patient return for routine mammogram at age 40 years.
D. Follow with mammogram in 1 year.
4. What are the ultrasound features that predict benign histology?
A. Taller-than-wide orientation
C. Heterogeneous internal echotexture
ANSWERS
CASE 63
Palpable Mass in a Teenager
1. B and C
2. C
3. A
4. D
References
Kronemer KA. Gray scale sonography of breast masses in adolescent girls. J Ultrasound Med. 2001;20(8):881.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:117.
Comment
Ultrasound is the imaging method of choice in the young, since it uses no ionizing radiation. Ultrasound is used to determine if the palpable finding is cystic or solid. A solid mass may be hyperechoic, hypoechoic, or isoechoic to neighboring fat. When evaluating a solid mass, look for features that help characterize the lesion as benign or malignant. These characteristics include margin analysis, acoustic shadowing, and echotexture. Benign masses are oval, gently lobulated, parallel to the skin, and sharply circumscribed, with no acoustic shadowing.
In this patient, there is a hypoechoic mass with gentle lobulations and sharply circumscribed borders, corresponding to the palpable finding. It is most consistent with a fibroadenoma, although other etiologies cannot be excluded, such as papilloma, hamartoma, or hematoma (see the figure).
The fibroadenoma is a common benign mass of the breast occurring in 10% to 25% of women. The mass arises from the breast lobule and is most likely stimulated by estrogen. Fibroadenomas occur as multiple masses in approximately 20% of cases. Histologically, there is a fibrous stroma without cytologic atypia, and there is also an epithelial component. Morphologically, fibroadenomas are circumscribed round, oval, or bilobed, and they can occur anywhere in the breast.
In a study of 57 masses in adolescent girls (median age, 15.4 years), 36 masses were fibroadenoma. All masses were benign. Malignancy is extremely rare in this age group.
Because malignancy is unusual, there are options in the management of the solid mass in the breast of an adolescent. Traditionally, excisional biopsy was performed. It is now acceptable to follow the mass, once benign characteristics have been established with ultrasound evaluation. If there is growth, excision is recommended. If knowledge of the histology is desired but surgery is not, needle core biopsy gives a reliable histologic diagnosis. In this patient, excision was performed and the mass was a fibroadenoma.
CASE 64
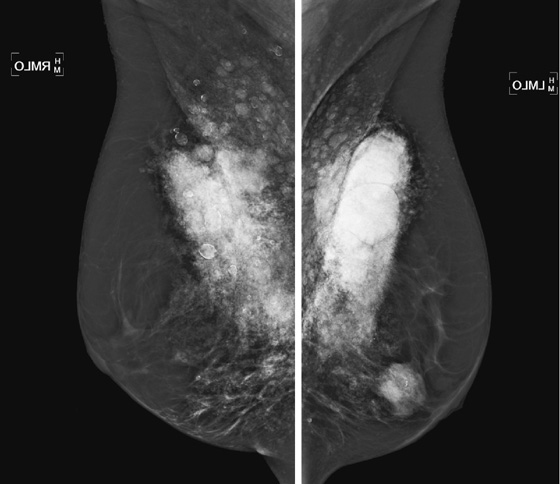
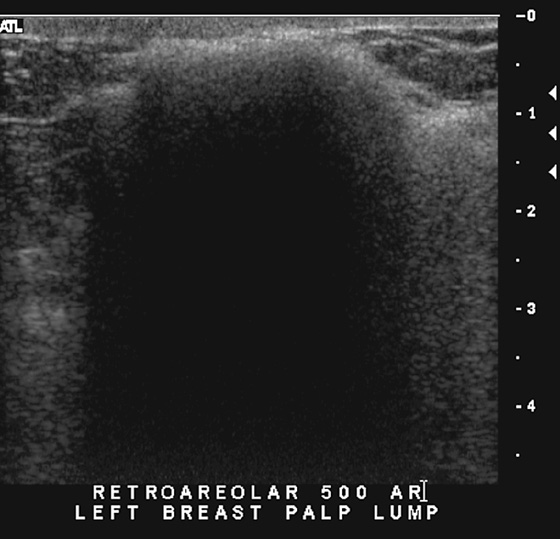
History: A 56-year-old woman presents for routine screening mammogram. Ultrasound images are also shown from a previous diagnostic examination performed because of a palpable lump.
1. What should be included in the differential diagnosis for the images shown? (Choose all that apply.)
A. Patient had liquid silicone injected into the breasts
B. Previous bilateral silicone implant rupture
C. Previous bilateral saline implant rupture
2. Which breast evaluation modality is least affected by injected silicone?
3. What is the next step in management for this patient?
A. MRI is necessary because of the limited mammogram.
B. Routine screening mammogram should be performed.
C. Ultrasound should be performed as a routine adjunct to mammogram.
D. Breast-specific gamma imaging should be performed annually.
4. Which of the following is not a complication of free silicone?
A. Delayed discovery of breast cancer
C. Increased risk of breast cancer
D. Migration of silicone into the arm
ANSWERS
CASE 64
Silicone Injections
1. A, B, and D
2. B
3. B
4. C
References
Caskey CI, Berg WA, Hamper UM, et al. Imaging spectrum of extracapsular silicone: correlation of US, MR imaging, mammographic, and histopathologic findings. Radiographics. 1999;19:S39–S51. (Spec No):
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:349.
Comment
Liquid silicone is used as a method for augmenting the size of the breasts. It was used in the United States until the 1970s, when its use was banned by the U.S. Food and Drug Administration (FDA). It is still used in Asia. When seen on the mammogram, liquid silicone has a characteristic appearance of dense material, typically in rounded masses, which often have a calcified rim. It can also cause a spiculated mass (see the figures). The rounded masses are granulomas, which is a natural response of the host to the foreign material.
On ultrasound, the appearance is also characteristic, with a highly echogenic surface and dense shadowing, with loss of information deep to the surface, caused by scatter of the sound beam (see the figures). This has been termed “snowstorm” appearance. Because of the dense shadowing and scatter of the beam, very little information about adjacent breast abnormalities can be gained when free silicone is present.
Silicone is inert and in a study published in 1994 was not found to cause connective tissue disease. However, it can have many adverse effects, including adenopathy, infection, granuloma formation, granulomatous hepatitis, and embolism. Patients with free silicone may present with a palpable mass, as this patient did in the past (see the figures). The silicone granulomas, or “siliconomas,” are hard masses that may feel like a cancerous mass. Physical examination of the breast is limited when silicone has been injected into the breasts. MRI can be used to obtain more complete information in a patient with free silicone because the silicone is hyperintense on T2-weighted images. If water suppression is also used, the silicone should be the only hyperintense material on the image.
CASE 65
History: A 23-year-old woman noticed a mass in her right breast during pregnancy; she described the mass as growing during the course of the pregnancy. She presents 4 weeks after delivery for imaging evaluation and biopsy.
1. What should be included in the differential diagnosis for the mass in the right breast? (Choose all that apply.)
D. Highly suspicious for invasive ductal carcinoma
2. Which of the following is not a complication of ultrasound-guided core biopsy during pregnancy?
3. Should biopsy of a breast mass be postponed until after parturition?
A. Yes, it is not safe to perform a biopsy of a breast mass during pregnancy.
B. Yes, nearly all breast masses found during pregnancy are benign.
4. What is the most common reason for a palpable solid mass during pregnancy?
D. Infiltrating ductal carcinoma
ANSWERS
CASE 65
Mass Developing during Pregnancy
1. A, B, and C
2. A
3. D
4. C
References
Darling ML, Smith DN, Rhei E, et al. Lactating adenoma: sonographic features. Breast J. 2000;6(4):252–256.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:376.
Comment
Benign masses are the most common palpable masses that develop during pregnancy. Pregnancy-associated breast cancer can occur and should always be considered. The incidence of breast cancer in pregnant women is 1:3000 to 1:10,000. Benign masses include fibroadenoma and lactating adenoma. Abscess and galactocele also manifest as palpable masses and should be considered. These lesions can appear as complex or complicated cysts and can be mistaken for solid masses. Ultrasound is the initial method of imaging used in pregnant women (and in women <30 years old), although if a mass looks suspicious on ultrasound, mammography should be performed, even in a pregnant patient. Scatter radiation to the fetus is minimal (about 0.4 mrad to the fetus from two mammographic views), and shielding can be used to limit the scatter radiation.
This mass has many benign features (see the figures), including oval shape, wider-than-tall appearance, and thin smooth margin. However, the cystic spaces in the mass are unusual for a fibroadenoma or lactating adenoma; these can be seen in phyllodes tumor, which can be locally aggressive and needs excision. The patient in this case expressed concern because of the large size of the mass (approximately 7 cm), and core biopsy was performed. It is important to determine the histology of the mass because a phyllodes tumor needs to be widely excised, whereas a lactating adenoma or fibroadenoma can be excised without a wide margin or can be followed. Performing core needle biopsy in women who are lactating or pregnant can cause the complication of milk fistula. The present patient was postpartum and was not breastfeeding.
Ultrasound-guided core needle biopsy is an inexpensive diagnostic tool to obtain the histology of a breast mass. The technique involves cleansing and numbing the skin and subcutaneous tissues and inserting a needle into the mass under ultrasound direct visualization. This technique allows real-time monitoring of the needle in the breast, which is impossible when performing stereotactic or MRI-guided biopsies. The last two figures illustrate the placement of the needle, parallel to the skin and adjacent to the mass; the core needle is “fired” into the breast, using a spring device built into the handle of the needle. At least five samples are taken; more may be necessary to sample the lesion adequately. In this patient, the mass was a fibroadenoma, with lactational change, explaining the cystic spaces. There was no evidence of malignancy.
CASE 66
History: A 67-year-old woman presents for a screening mammogram. She has a family history of breast cancer in her sister at age 50, mother at age 65, and three maternal aunts.
1. What should be included in the differential diagnosis of the mammogram and ultrasound images shown? (Choose all that apply.)
2. What is the next step in management?
A. Clinical examination—if this superficial mass is not palpable, it can be followed
B. MRI to check for enhancement
D. PET/CT to check for enhancement
3. If the mass is a papilloma, what is the management?
A. Routine mammogram in 1 year because papilloma is benign
B. Referral to a surgeon for excision
C. MRI to check for abnormal enhancement
D. Short-interval follow-up with ultrasound
4. If the patient had bloody nipple discharge, does that change management?
A. Yes, the patient would go directly to surgical consultation instead of imaging evaluation.
B. Yes, the mass is more likely to be malignant if bloody discharge is present.
C. No, management is the same.
D. Yes, the patient would have a ductogram in her work-up.
ANSWERS
CASE 66
Subareolar Cancer
1. A, B, and D
2. C
3. B
4. C
References
Chang JM, Moon WK, Cho N, et al. Management of ultrasonographically detected benign papillomas of the breast at core needle biopsy. AJR Am J Roentgenol. 2011;196(3):723–729.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:120.
Comment
This patient has a developing mass with benign features on mammography and ultrasound: relatively low density and round on mammography and sharply circumscribed, oval shape, and wider than tall on ultrasound (see the figures). Its subcutaneous location in the subareolar breast suggests a diagnosis of papilloma, complicated or complex cyst, or sebaceous cyst. However, any developing mass in a postmenopausal woman with a strong family history should be viewed with concern.
This woman underwent core needle biopsy, and the histology was consistent with malignant cells in a papillary lesion. The area of the mass was completely removed at core biopsy; when the patient had surgery, no malignancy was seen in the breast. It had been entirely removed at core biopsy. Her sentinel node was negative.
Clinical examination of the subareolar area is difficult because of the presence of the nipple, and masses in this location may escape clinical notice. This area should be carefully examined on mammography. The round shape and low density of this mass on mammography suggest a benign etiology; however, there is overlap in the appearance of benign and malignant processes in the breast on mammography and ultrasound, so care must be taken not to dismiss a potentially malignant developing mass. If the histopathology on core biopsy had been benign (e.g., a benign papilloma), surgical excision would still be recommended because there is a small but significant risk of malignant histology in papillomas (3% to 14% in several series).
CASE 67
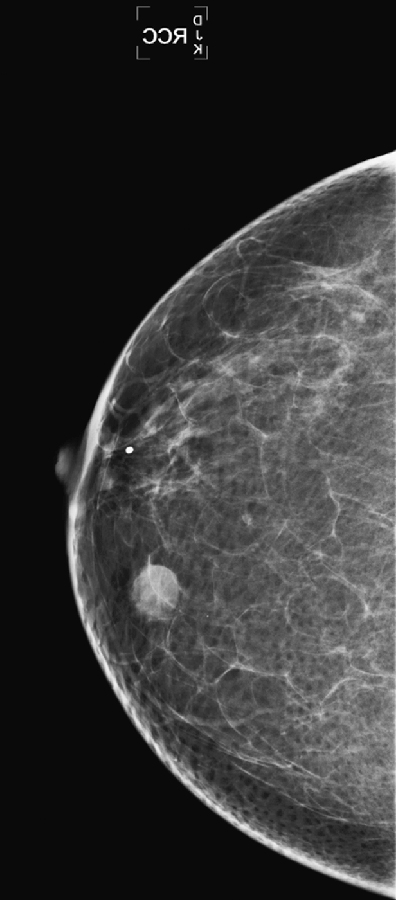
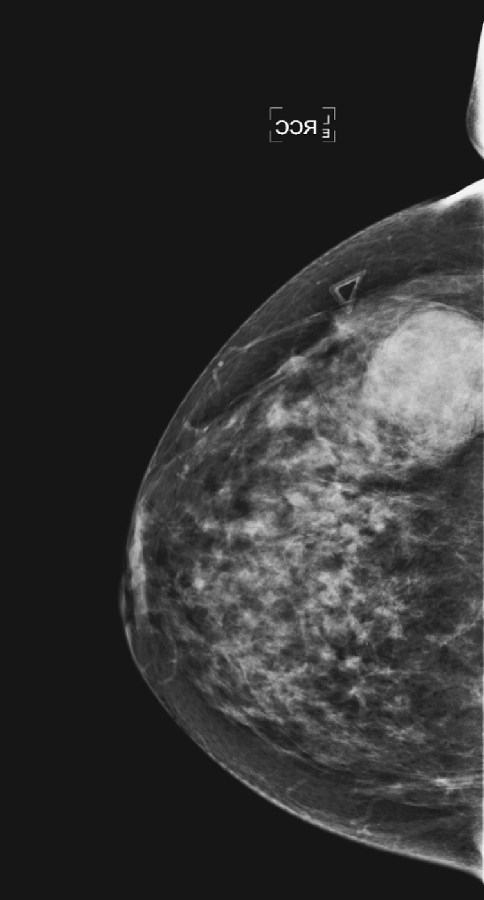
History: An asymptomatic 51-year-old woman presents for a screening mammogram.
1. What is the differential diagnosis for the round mass in the lower right breast on the mammogram? (Choose all that apply.)
2. If the patient presents with a palpable mass and this mammogram, what would the next step be?
A. Spot compression views of the mass
C. Ultrasound of the palpable finding
3. What Breast Imaging Reporting and Data System (BI-RADS) score would you give when reporting this mammogram, if it is seen as a screening exam?
A. BI-RADs 0—incomplete, needs further evaluation
C. BI-RADs 4—suspicious, biopsy should be considered
D. BI-RADS 3—probably benign, recommend short-interval follow-up
4. Where in the right breast is the lesion located?
A. Right breast at 2:00 o’clock position
B. Right breast at 10:00 o’clock position
C. Right breast at 8:00 o’clock position
D. Right breast at 5:00 o’clock position
ANSWERS
CASE 67
Sebaceous Cyst
1. A, B, and D
2. C
3. B
4. D
References
Baker JA, Soo MS. Breast US: assessment of technical quality and image interpretation. Radiology. 2002;223:229–238.
Stavros AT. Breast Ultrasound. Philadelphia: Lippincott Williams & Wilkins; 2004. pp 325–333
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:141.
Comment
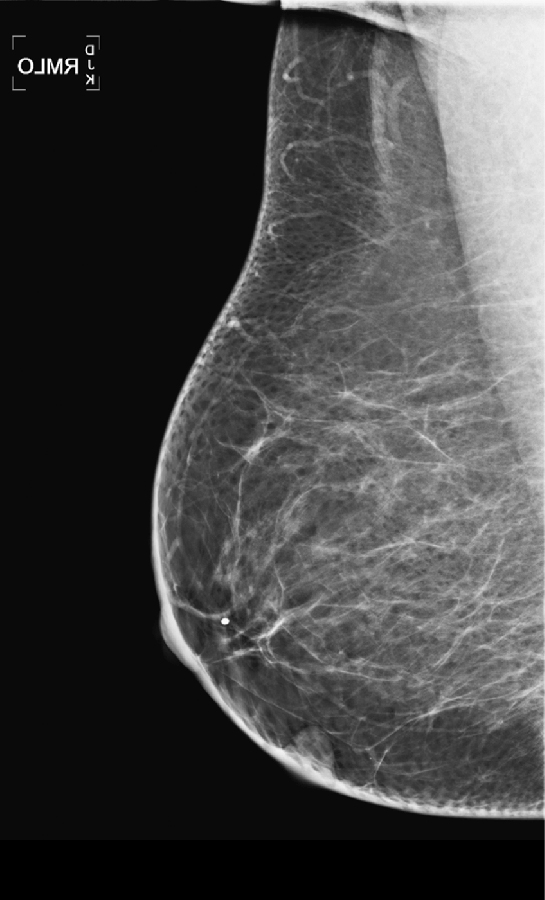
The mammographic views show a round mass that triangulates to the lower inner aspect of the breast (see the figures). On the craniocaudal (CC) view, the mass is in the medial breast. On the mediolateral oblique (MLO) view, the mass is seen in the skin, projecting in toward the breast tissue, rather than outward into the air. This is pathognomonic for a dermal mass. The three most likely dermal masses are sebaceous cyst, which is a retention cyst of a hair follicle in the dermis; an epidermal inclusion cyst, which arises as a result of traumatic skin disruption, such as biopsy; and a Montgomery gland cyst, which arises from the superficial Montgomery gland of the areola. Questioning (and/or examination) of the patient might reveal an inflamed skin lesion consistent with a dermal cyst; however, with the mammographic appearance seen in this case (see the figures), such examination is not necessary.
On the film-screen mammogram, rather than on the digital mammogram as in this case, the skin is not as well seen, and there may be some doubt about the location of the mass. In these cases, physical examination of the skin often is diagnostic of a skin mass. If you are unsure, ultrasound can be used to help make the diagnosis. Ultrasound reveals a well-defined oval mass contained within the dermal layer, or just deep to the dermal layer with a thin neck, which is the hair follicle extending through the echogenic skin layer to the skin surface. To image the follicle, a standoff pad and a high-frequency transducer may be necessary.
Once the etiology has been determined, no further evaluation is needed. Routine screening mammography is recommended.
CASE 68
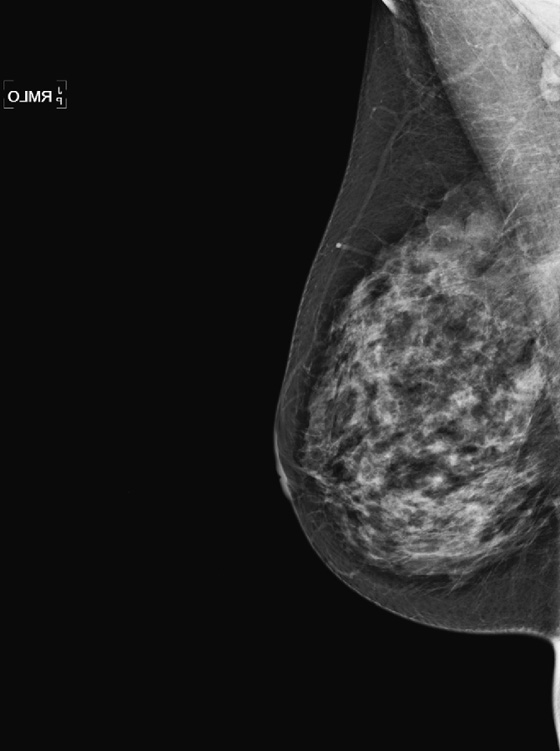
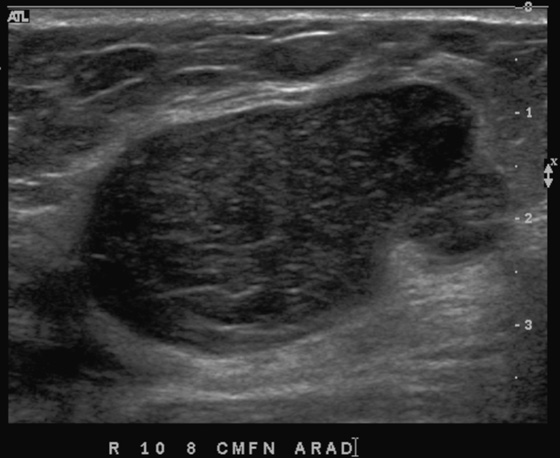
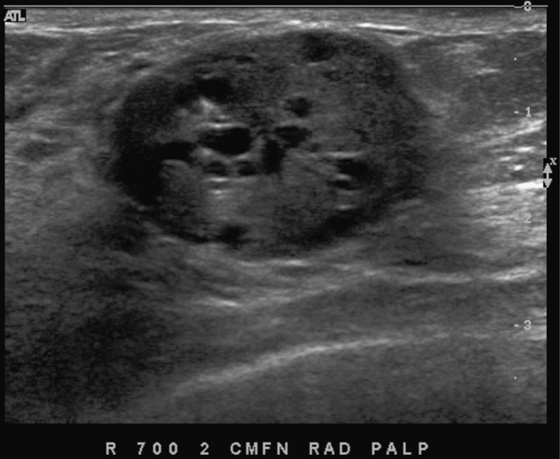
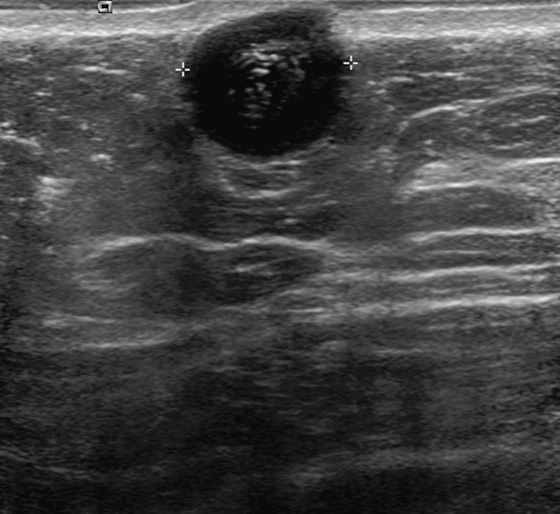
History: A 56-year-old woman presents with a palpable right breast mass, within 1 year of her previous normal screening mammogram.
1. What should be included in the differential diagnosis for the developing right breast mass? (Choose all that apply.)
2. What is the next step in imaging?
A. MRI to differentiate benign from malignant
C. Positron emission tomography (PET) scan to check for additional masses throughout the body
3. Ultrasound is performed, and the mass is solid, with benign features. What is the next step?
A. Ultrasound-guided needle biopsy
B. Following of benign-appearing masses; ultrasound examination in 6 months recommended
C. MRI to assess enhancement characteristics to decide whether biopsy is needed
4. The biopsy histology is benign phyllodes tumor. What is the next step?
A. Follow-up of the benign lesion in 6 months with ultrasound
D. MRI to determine if mass needs excision
ANSWERS
CASE 68
Phyllodes Tumor
1. A, C, and D
2. B
3. A
4. C
References
Liberman L, Bonaccio E, Hamele-Bena D, et al. Benign and malignant phyllodes tumors: mammographic and sonographic findings. Radiology. 1996;198(1):121–124.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:120.
Comment
Phyllodes tumor is rare, constituting approximately 1% of breast masses, and can be either benign or malignant. Approximately 25% are malignant. The older term was cystosarcoma phyllodes, which implied malignancy, and this term is no longer used. Growth is characteristically rapid; benign phyllodes tumors can grow faster than infiltrating ductal carcinomas.
Histologically, there is stromal overgrowth, which is markedly hypercellular, and the stroma forms leaflike patterns that protrude into cystic spaces microscopically. The name phyllodes is derived from these leaflike patterns. The malignant form can metastasize hematogenously to the lungs and liver and can be fatal. The benign type can recur after excision but does not spread outside the breast.
The benign and malignant forms can be identical on imaging. Larger masses (>3 cm) are statistically more likely to be malignant, according to one report. On mammography, phyllodes tumor is a circumscribed mass, with lobulated contours, and is relatively dense. On ultrasound, the mass is circumscribed, with a hypoechoic internal texture that can show through transmission. Cystic spaces are common, as seen in the last figure, from a different patient. The cystic spaces are characteristic of a phyllodes lesion. When cystic spaces are seen in a mass on ultrasound, phyllodes tumor should be considered, and biopsy should be performed.
CASE 69
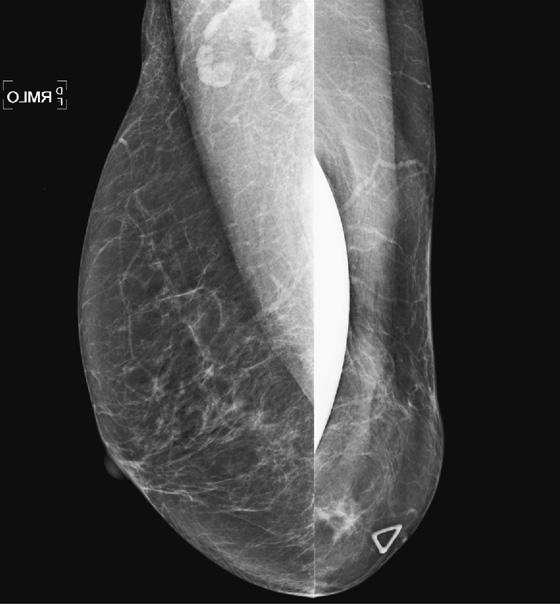
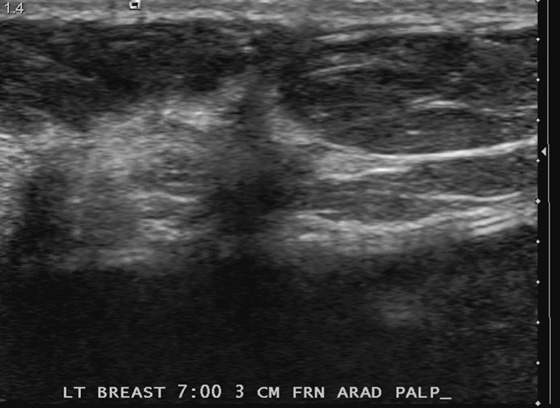
History: A 63-year-old woman who had left breast cancer diagnosed in 1998 and treated with mastectomy has a palpable finding on her mastectomy side. She has a silicone implant reconstruction.
1. What should be included in the differential diagnosis of the bilateral mammogram and ultrasound image shown? (Choose all that apply.)
A. Fat necrosis on the mastectomy side
B. Recurrent cancer on the mastectomy side
C. Distortion from surgical biopsy
D. Lipoma on the mastectomy side
2. What is the incidence of recurrent tumor on the ipsilateral side after mastectomy?
A. Relatively common, about 50%
C. Relatively uncommon, about 25%
D. Extremely rare, less than 1%
3. Is ipsilateral mammography required after mastectomy?
A. Yes, because of the risk of recurrence, mammography should be performed annually.
B. There is no requirement, but it is strongly recommended.
C. No, ipsilateral mammogram is usually not performed after mastectomy.
4. Which of the following statements is true regarding recurrence after mastectomy?
A. Recurrence is typically found on physical examination.
C. Recurrence usually takes the form of a metastatic axillary node.
D. Postoperative radiation therapy to the mastectomy site does not affect local recurrence rate.
ANSWERS
CASE 69
Recurrent Cancer after Mastectomy
1. A, B, and C
2. B
3. C
4. A
References
Fajardo LL, Roberts CC, Hunt KR. Mammographic surveillance of breast cancer patients: should the mastectomy site be imaged?. AJR Am J Roentgenol. 1993;161(5):953–955.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:328.
Comment
Mastectomy is a surgical procedure used to treat breast cancer. Mastectomy is often performed when the tumor occupies a large portion of the breast. It is also preferred by some women to treat small cancers that could be treated with local excision and radiation. Recurrence can occur after mastectomy because of incomplete local removal of tumor or as an early sign of disseminated disease. Tumors that are more likely to recur include larger tumors, tumors of higher stage, and tumors with negative hormone receptors. Local recurrence decreases survival. Radiation therapy has been shown to reduce recurrence by two thirds. It is important to be vigilant about the possibility of recurrence after mastectomy because it has been reported in various studies to occur in approximately 5% to 10% of patients. Most recurrence (70%) occurs in the first 3 years after surgery.
Mammography has been shown to be of limited usefulness in screening for recurrence after mastectomy because the chest wall is not included in the image and that is the primary site of local recurrence. The sensitivity of clinical examination is greater than that of mammography, so patients who have undergone mastectomy should be clinically evaluated routinely for any sign of palpable mass in the chest wall, supraclavicular region, or axilla. If a palpable mass is found, mammography may be useful, as in this patient (see the figures). Ultrasound is very useful because it can evaluate the chest wall better than mammography. Recurrent tumors have the same appearance as primary tumors and can be circumscribed, irregular, or spiculated masses. They can also manifest as microcalcification with or without a mass. Treatment of recurrence includes surgical excision, radiation therapy, systemic hormone therapy, or systemic chemotherapy.
CASE 70
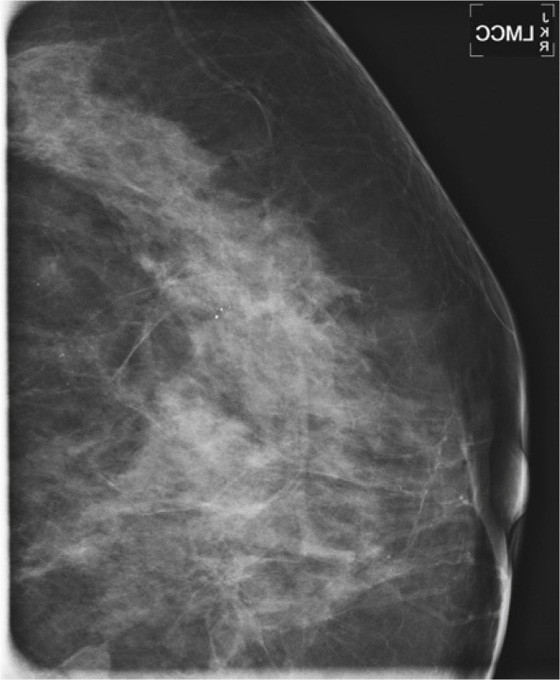
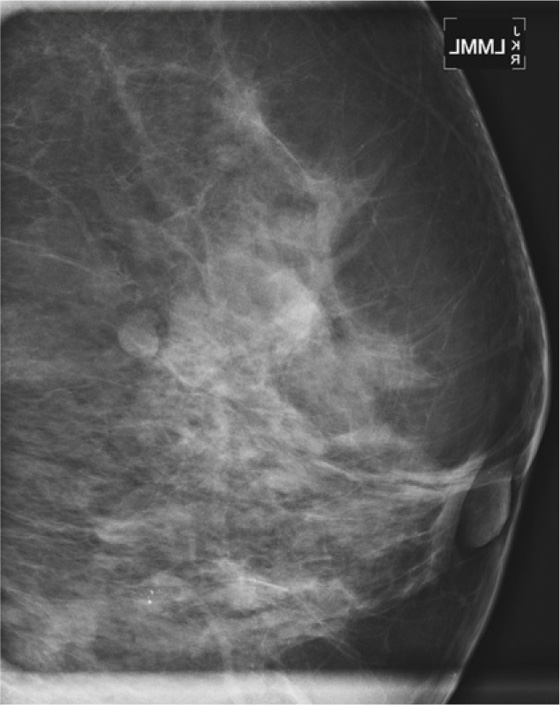
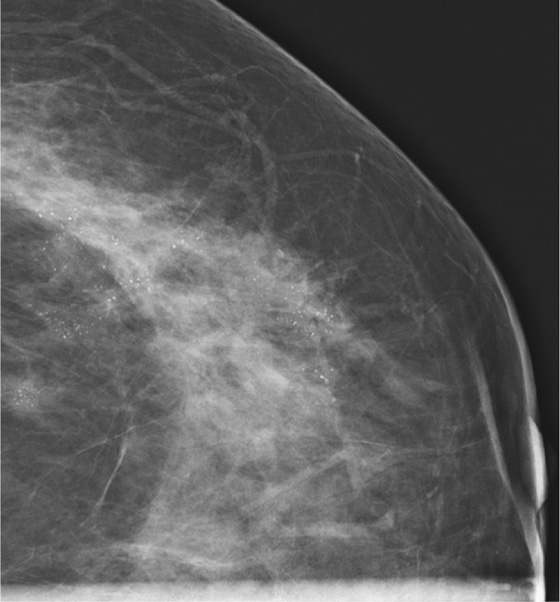
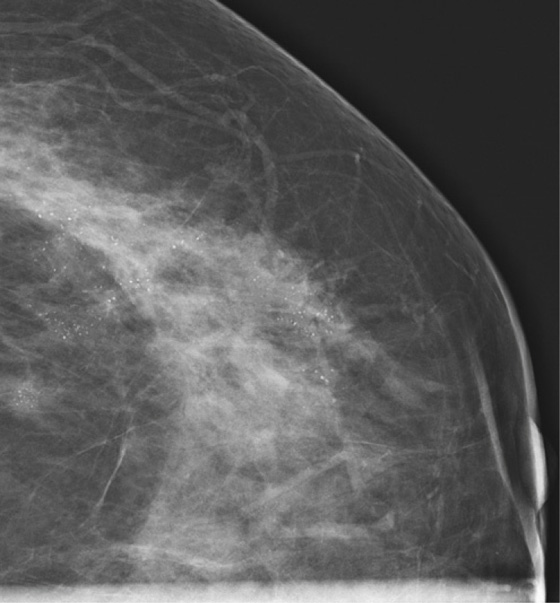
History: An asymptomatic patient who had developing microcalcifications on routine screening mammogram in her left breast had magnification views and returned for follow-up magnification views 2 years later.
1. What should be included in the differential diagnosis for the change between the two examinations shown? (Choose all that apply.)
A. Fibrocystic change, with increasing calcifications
B. Increasing calcifications, suspicious for DCIS
C. Atypical ductal hyperplasia
2. What is the distribution of the calcifications in the second figure?
3. Why is it important to identify the extent of suspicious calcifications on the mammogram?
A. It is important to establish all sites of disease before surgery.
B. There is no cancer present where there are no calcifications.
C. Biopsy is not needed if calcifications are seen in another quadrant.
D. It is of academic interest and does not affect the surgical management.
4. What is the next step in management?
B. Stereotactic biopsy of one site in the center of the calcifications
C. Biopsy of at least two areas of calcifications that are farthest apart
ANSWERS
CASE 70
Developing Calcifications and Ductal Carcinoma In Situ
1. B, C, and D
2. C
3. A
4. C
References
Majid AS, de Paredes ES, Doherty RD, et al. Missed breast carcinoma: pitfalls and pearls. Radiographics. 2003;23(4):881–895.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:70.
Comment
DCIS develops in the duct and its branches. Distinguishing DCIS calcifications from benign calcifications can be difficult. The shape of each individual calcification is important. Benign calcifications are round (punctate) or cigar-shaped (secretory type). Suspicious calcifications are linear, branching, and pleomorphic (irregularly shaped particles that look like shards of broken glass).
In the patient in this case, the initial magnification views were interpreted as probably benign because the shape of the calcifications was thought to be punctate. However, the distribution of calcifications is also important, and clustered or grouped calcifications are suspicious. On the initial magnification views, this patient had calcifications that were mostly punctate but that were grouped together (see the figures).
This patient did not return for follow-up for 2 years. In the second set of magnification views, the calcifications have increased considerably, and now their suspicious appearance is more obvious (see the figures). The distribution is segmental, which indicates that the calcifications are filling a duct and its branches. One way to identify a segmental distribution is to look for a triangle-shaped area of calcifications, with the apex pointing toward the nipple (see the figures).
When calcifications are newly developing, care should be taken to identify the most aggressive-appearing feature seen. Biopsy should be performed if there are any suspicious features. The benefit of early diagnosis is clear: DCIS is a precursor to invasive ductal carcinoma. Cancer that is diagnosed by mammography as DCIS has a higher cure rate than invasive ductal carcinoma and is less likely to need aggressive local and general therapy.
CASE 71
History: A 57-year-old woman had infiltrating ductal carcinoma, grade I, detected in her left breast in 2007. It was treated with lumpectomy and partial breast irradiation.
1. What should be included in the differential diagnosis for the mammographic images shown? (Choose all that apply.)
A. Fat necrosis in the left breast
B. Changes caused by partial breast irradiation
C. Sequelae of lumpectomy without radiation
D. Complicated cyst in the left breast
2. What mammographic findings are unique to accelerated partial breast irradiation (APBI) compared with external beam radiotherapy?
A. The mammographic findings are similar.
B. APBI causes much more distortion at the lumpectomy site.
C. Fat necrosis is much more common with APBI.
D. The changes associated with APBI are less focal.
3. Why may APBI be preferred instead of external beam radiotherapy?
A. The long-term data show lower recurrence compared with external beam radiotherapy.
B. Shorter duration of therapy improves patient compliance.
C. Fewer side effects occur with APBI compared with traditional therapy.
D. Larger tumors can be treated better with APBI.
4. What is the appropriate management in this case?
A. Biopsy of the spiculated mass at the lumpectomy site
B. Short-interval mammogram follow-up of the left breast in 6 months
ANSWERS
CASE 71
Partial Breast Irradiation
1. A, B, and C
2. A
3. B
4. D
References
Ahmed HM, Dipiro PJ, Devlin PM, et al. Mammographic appearance following accelerated partial breast irradiation by using MammoSite brachytherapy. Radiology. 2010;255(2):362–368.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:313.
Comment
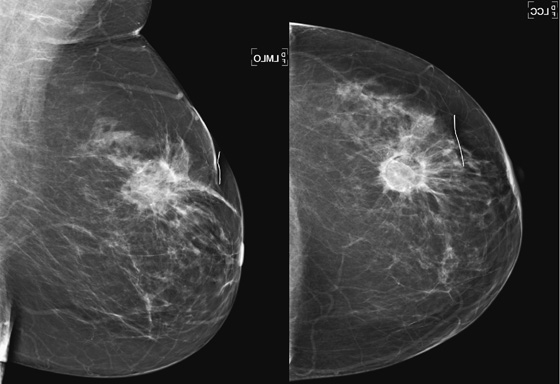
Radiation therapy is administered to reduce the risk of recurrence in patients treated with lumpectomy. This treatment allows preservation of the breast. Traditionally, external beam radiation was used. More recently, brachytherapy has been used in a selected group of patients. Brachytherapy is the term given to the technique that inserts radioactive materials into the breast. Brachytherapy may take the form of multiple catheters or a balloon inserted into the lumpectomy cavity after excision of the malignant mass; this is termed accelerated partial breast irradiation (APBI).
Dosing may be twice a day for 5 to 7 days. In contrast, external beam radiation therapy is administered 5 days a week for 5 to 7 weeks. In brachytherapy, only the tumor site is treated. Several studies have shown that 90% of recurrence occurs at the lumpectomy site. APBI targets most potential recurrences, without exposing the entire breast, chest wall, and internal organs to radiation. However, recurrence or residual disease at a distance from the lumpectomy site would not be treated. Patient selection for APBI is important; patients with a relatively small, single tumor mass are appropriate candidates.
Mammographic findings after APBI are similar to the effects of external beam radiation, including skin thickening, diffuse and focal increased density, seroma, and fat necrosis (see the figures). However, with APBI, the changes of fibrosis and scarring may occur earlier than with external beam therapy and may be more severe, owing to the higher dose of APBI per treatment. In the patient in this case, the mammogram performed 6 months after surgery and APBI shows a focal mass, with a fluid-fluid level on mediolateral view, consistent with fat necrosis and scarring (see the figures). At 4 years after treatment, the mass has decreased in size, and the fibrosis has diminished. The patient did not develop calcifications after treatment. However, calcification is not unusual; it is seen in about 25% of patients after external beam treatment. This percentage may be higher with APBI.
CASE 72
Prior examination

History: A 68-year-old woman underwent a screening mammogram. A mammogram from 2 years ago was available for comparison. She is called back for further evaluation of the left breast finding.
1. What should be included in the differential diagnosis? (Choose all that apply.)
D. Asymmetric glandular tissue
2. What imaging test should be performed next?
D. No further imaging is needed
3. Which of the following is the least useful biopsy procedure for diagnosis?
C. Vacuum-assisted core biopsy
4. Which of the following is not a reason for placing a clip after core biopsy?
A. To document the location of the original finding
B. To avoid additional unnecessary biopsies
ANSWERS
CASE 72
Lymphoma in Intramammary Node
1. A, B, and C
2. A
3. A
4. C
References
Cyrlak D, Carpenter PM. Breast imaging case of the day: intramammary and axillary lymph node metastases from infiltrating lobular carcinoma of the breast. Radiographics. 1999;19:S73–S79. Spec No:
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:395.
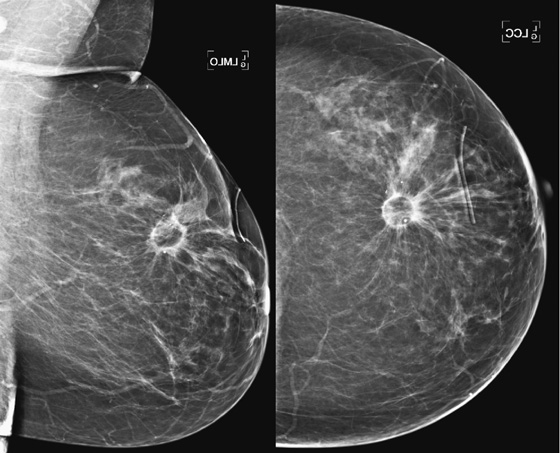
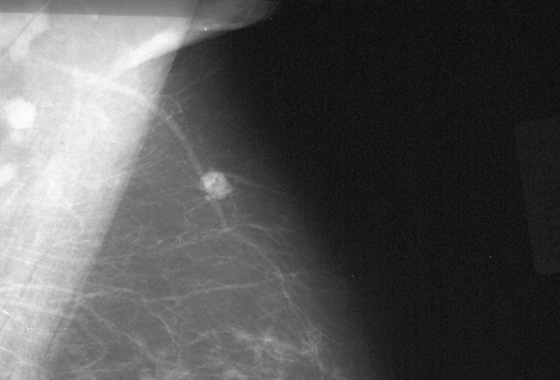
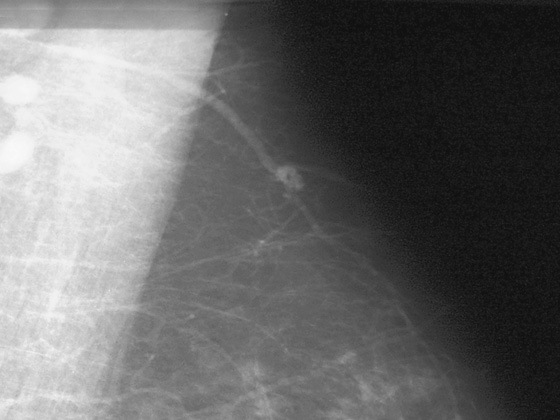
Comment
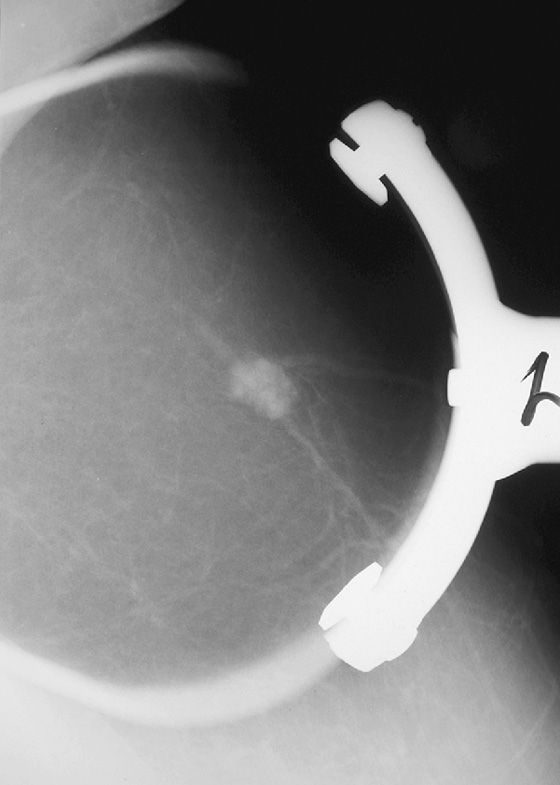
In this 68-year-old woman, a left intramammary node was noted to have enlarged on her screening mammogram when it was compared with the examination from 2 years earlier (see the figures). On the previous examination, the lymph node appeared oval, had a low density and symmetric cortex, and contained a fatty hilum. These are all features of a normal lymph node, so no further imaging was needed at that point. However, on the current mammogram, the same node appears larger and denser with irregular margins and loss of its fatty hilum. The previously normal architecture is no longer seen (see the figures).
This change in a solitary, unilateral node may be due to benign causes, such as granulomatous disease, collagen vascular disease, inflammation or infection, or hyperplasia, but metastatic disease and lymphoma must be considered first. Metastatic disease could develop in a node from a breast, lung, or melanoma primary.
After initial diagnostic mammography, ultrasound is the next step in evaluation, helping to characterize the lesion further and guide future biopsy. Core needle biopsy of the node gives the histology of the disease causing the enlargement, and it can help tailor the remaining work-up. The biopsy can be performed with or without vacuum assistance. Surgical excision is also helpful because it provides the entire node for histologic analysis; nevertheless, it constitutes a more invasive procedure. Fine-needle aspiration biopsy is not as useful in determining the exact etiology of the lesion because it may not provide sufficient information (only cells, not tissue). If the node contains metastatic breast cancer and no primary malignancy is seen on mammography, MRI is performed to evaluate for occult cancer because it has a higher sensitivity for invasive tumor than mammography and ultrasound.
This patient underwent an ultrasound-guided core needle biopsy that showed grade I follicular lymphoma. A clip was placed after the biopsy to document the location (see the figures).
CASE 73
History: A 25-year-old woman presents with a palpable mass.
1. What is the differential diagnosis for the ultrasound images? (Choose all that apply.)
C. Cyst with two distinct fluid densities, with layering
D. Debris within a complicated cyst
2. What is the first step in the imaging evaluation of a 25-year-old woman with a palpable breast lump?
A. Bilateral mammogram with a marker over the area of concern
B. Unilateral mammogram of the side of interest, with a marker over the area of concern
C. MRI of the side of interest
D. Ultrasound of the palpable concern
3. What is the definition of a simple cyst?
A. A cyst that is anechoic, has imperceptible margins, and has enhanced through-transmission
C. Any cyst that is anechoic; margins can be irregular, lobulated, or circumscribed
D. All anechoic structures in the breast
4. What is the echogenic intracystic fluid in this case?
A. The echogenic fluid is debris.
B. The echogenic fluid is an intracystic mass.
C. The echogenic fluid contains fat.
D. The echogenic fluid is milk of calcium.
ANSWERS
CASE 73
Cyst with Layering Fluid
1. C and D
2. D
3. A
4. C
References
Berg WA, Sechtin AG, Marques H, Zhang Z. Cystic breast masses and the ACRIN 6666 experience. Radiol Clin North Am. 2010;48(5):931–987.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:159.
Comment
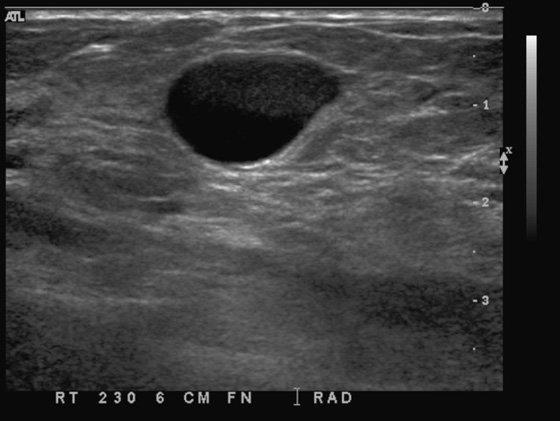
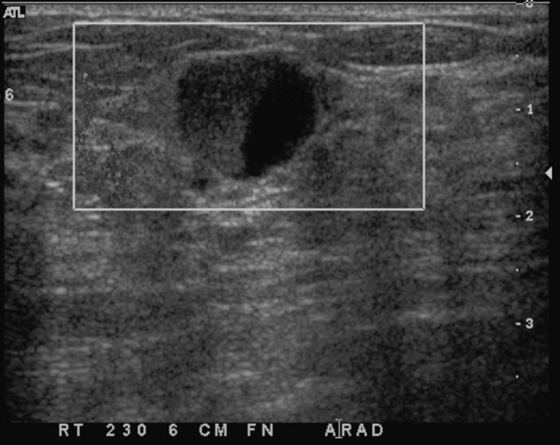
This young patient presented with a palpable lump. Patients younger than 30 years should be evaluated with imaging that does not use ionizing radiation, and ultrasound is often diagnostic. MRI of the breast is not the first imaging method in the case of a palpable lump. The palpable finding may be a cyst, a solid mass, or normal glandular tissue. The radiologist should search for any suspicious features that might suggest malignancy, even in young women.
The simple cyst is completely anechoic, has a well-circumscribed, nearly imperceptible margin, and is enhanced through transmission. A complicated cyst may contain a fluid-debris level or fat-fluid level in the cyst, as in this case (see the figures). If the dependent layering fluid is more echogenic, this suggests debris, might indicate infection or inflammation, and is often seen in fibrocystic change. If the fluid-fluid level is not a straight level line, the presence of an intracystic mass must be excluded. The patient can be turned to a decubitus position while the cyst is observed under ultrasound, to watch the fluid level. It should change position within the cyst if it is layering fluid or debris; this change can take up to 10 minutes to occur. A mass will stay the same and will not change its position within the cyst when the patient’s position is changed.
In this case, the echogenic fluid is nondependent, or floating (see the figures). This is consistent with fat. Fat may be seen in galactocele or in fat necrosis, but a cyst with a fat-fluid level is most commonly seen in fibrocystic change, as in this case. This type of cyst, with floating fat, is invariably benign, Breast Imaging Reporting and Data System (BI-RADS) 2, and aspiration is not necessary.
CASE 74
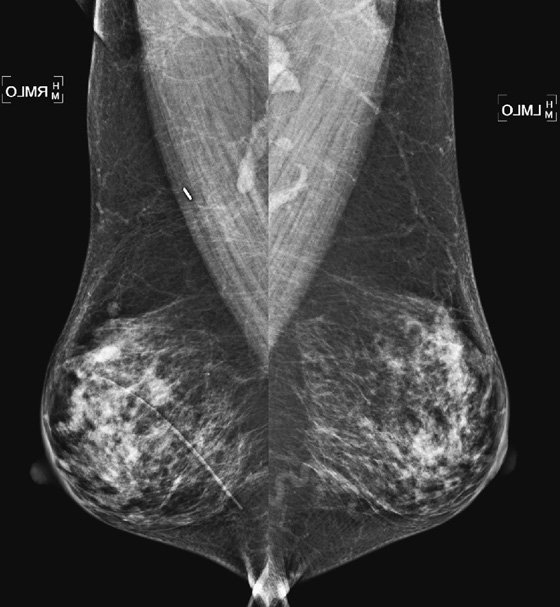
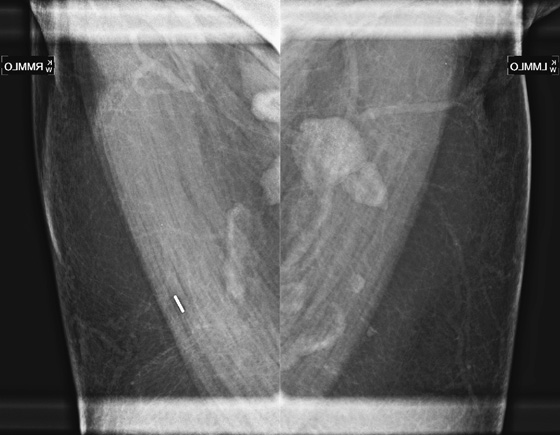
History: A 58-year-old woman presents for a routine screening mammogram. She is recalled for additional views to evaluate calcifications in the axillary nodes.
1. What should be included in the differential diagnosis for the mammographic views shown? (Choose all that apply.)
A. Patient with history of granulomatous disease
B. Patient who received gold therapy for rheumatoid arthritis
C. Patient with history of prior bilateral saline implant rupture
D. Patient with past history of bilateral silicone implant rupture
2. What is the next step in the work-up?
A. Obtaining a thorough patient history
B. Biopsy of an axillary node using ultrasound guidance
3. Can breast cancer manifest with calcifications in the axillary nodes?
A. No, only ovarian cancer metastases can produce calcifications in axillary nodes.
B. Yes, but only calcified ductal carcinoma in situ can produce calcifications.
C. Yes, but the breast tumor must contain calcifications.
D. Yes, infiltrating mammary carcinoma can cause metastases containing calcifications.
4. What is the management of a patient with history of gold therapy and with gold deposits in the axillary nodes?
A. Biopsy of a node using ultrasound guidance
D. Comparison with prior films; this is mandatory
ANSWERS
CASE 74
Gold Therapy
1. A, B, and D
2. A
3. D
4. C
References
Bruwer A, Nelson GW, Spark RP. Punctate intranodal gold deposits simulating microcalcifications on mammograms. AJR Am J Roentgenol. 1987;163(1):87–88.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:396.
Comment
The axillary lymph nodes should be carefully assessed on each mammogram. Radiodense particles can deposit in the nodes from various sources, including calcifications from granulomatous disease, tattoo pigment, metal fragments from gunshot, metal fragments sheared off during needle localization, and gold therapy (also called chrysotherapy) for rheumatoid arthritis (see the figures). Artifacts on the skin can simulate radiodense materials in the nodes and are more common. Deodorant, lotion, and powder should be searched for, and if these materials are present, the skin should be cleaned and the images retaken.
Granulomatous disease typically has coarse calcifications, and gold therapy typically has very fine, almost imperceptible metal density particles, as in this patient (see the figures). These findings should be easily differentiated. Magnification views may be helpful to evaluate the material better. If the nodal material is due to a benign condition, the mammogram is benign (BI-RADS [Breast Imaging Reporting and Data System] 2), and routine screening mammogram is recommended.
Breast cancer and, rarely, ovarian cancer can cause calcifications in axillary lymph nodes and may be the first presentation of an occult cancer. This is a relatively rare presentation of breast cancer; one report found that 3% of breast cancers may cause microcalcifications in the axillary nodes. If there is no history of gold therapy, granulomatous disease, tattoo, needle localization, silicone implant, or gunshot wound, the material should be suspected to be malignant calcification, and biopsy of the node should be performed.
CASE 75
One month later.
History: A 60-year-old woman, who has a history of bilateral breast cancer, has a new area of pain and lump in her left breast. She had breast conservation therapy in the left breast 4 years earlier.
1. What should be included in the differential diagnosis of the mammogram and ultrasound images shown? (Choose all that apply.)
2. What is the next step in management?
A. Biopsy must be performed because there is a chance of malignancy.
B. This possible hematoma can be followed with ultrasound in about 1 month.
C. The patient can return for her regular mammogram in 1 year.
D. The patient can monitor the palpable finding and return only if it gets larger.
3. Which of the following is not an ultrasound appearance of a hematoma?
A. Oval echogenic mass with cystic center
B. Anechoic round mass with through-transmission and thin wall
C. Hypoechoic, with thick and thin weblike echoes
D. Oval, with fluid-debris level
4. What imaging study would not help differentiate hematoma from malignancy?
C. Standard mammographic images
D. Spot compression magnification views
ANSWERS
CASE 75
Hematoma
1. A, B, and D
2. B
3. B
4. C
References
Pojchamarnwiputh S, Muttarak M, Na-Chiangmai W, et al. Benign breast lesions mimicking carcinoma at mammography. Singapore Med J. 2007;48(10):958–968.
Stavros AT. Breast Ultrasound. Philadelphia: Lippincott Williams & Wilkins; 2004.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:139.
Comment
A developing mass in the breast of a patient who has been diagnosed with cancer in the past is suspicious. Additional views and ultrasound can help in differentiating malignancy from hematoma or fat necrosis. This patient (see the figures), who had undergone breast conservation therapy 4 years ago, presented with a new left breast mass and pain and had no recollection of a traumatic event. There was no bruising in the skin at the time she presented. It is helpful if the patient recalls a traumatic event and bruising because this can steer the diagnosis toward hematoma. However, many women do not recall the event and do not perceive the focal finding until the bruising has passed and the hematoma is felt as a lump.
Standard imaging of mammography and ultrasound can be helpful in making the diagnosis. Typically, the mammogram and ultrasound show features of fat and water density. The mass on mammography has fat lucency and may have high-density material (blood) appearing as a dense halo (see the figures). On ultrasound, there may be an echogenic mass (blood) with varying degrees of liquefaction, appearing as cystic areas (see the figures). The ultrasound appearance can be quite variable because acute hematoma is seen as a markedly hypoechoic mass, and then as clotting begins, varying degrees of echogenic material and clot can develop. The mass should not have increased blood flow on color Doppler because the echogenic material seen within is clot, not tumor.
The management of the finding can be close follow-up if there are features of a hematoma. If the ultrasound examination is repeated in 1 month, there should be a decrease in the size of the mass (see the figures). The time to full resolution varies, but it is helpful to follow the mass to complete resolution, if possible. Malignant masses are prone to bleeding if subjected to trauma, and this possibility must always be considered when following a hematoma, particularly in a high-risk patient. If the mass increases in size or does not change in 1 month, biopsy should be considered.
CASE 76
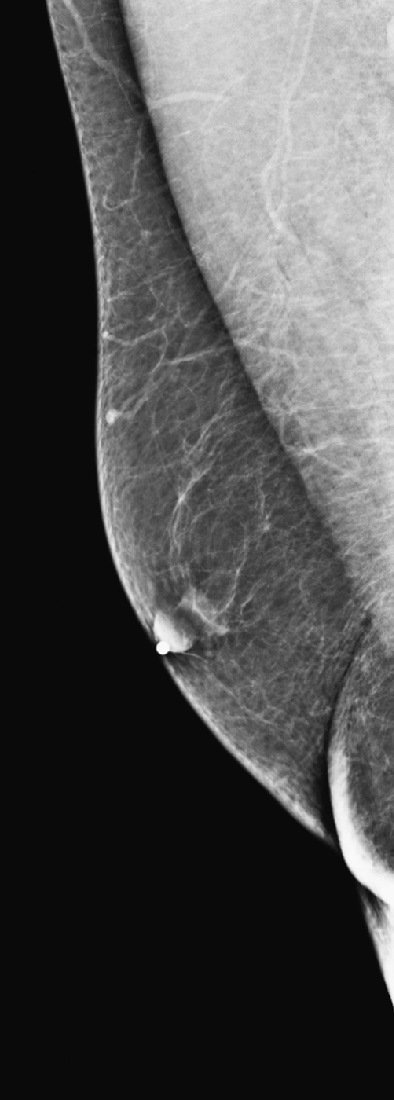
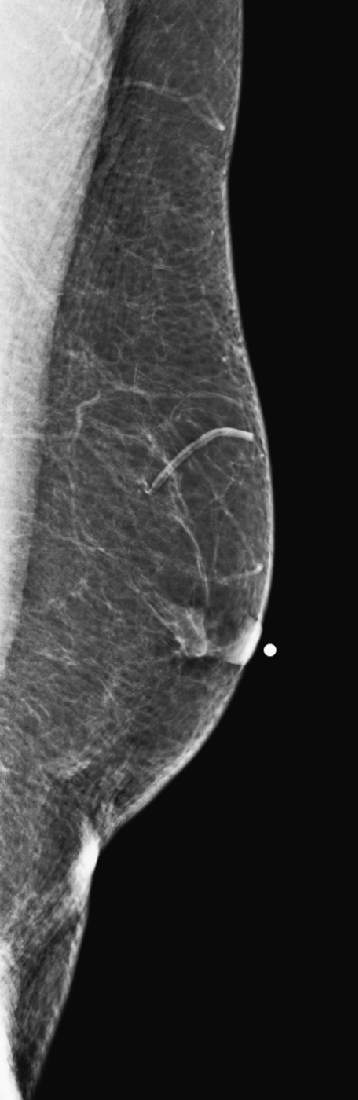
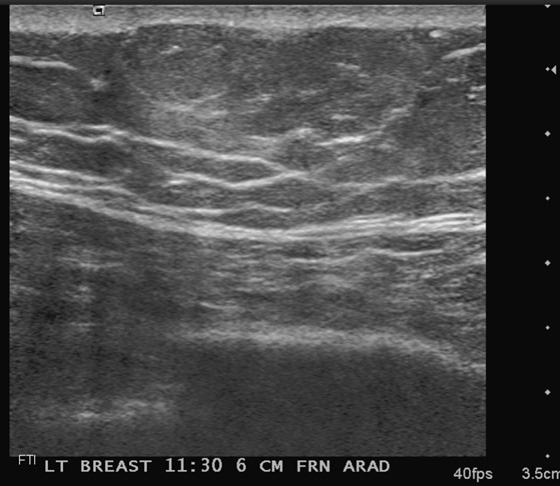
History: A 55-year-old man presents with a palpable mass in his left upper breast.
1. What is your differential diagnosis for palpable masses in the male breast? (Choose all that apply.)
2. What is your interpretation of the findings presented on mammography and ultrasound?
A. Normal mammogram, mass isoechoic to fat on ultrasound, likely lipoma
B. Benign-appearing mass on mammography or ultrasound
C. Left mammogram and ultrasound consistent with gynecomastia
D. Malignant mass behind left nipple, not demonstrated on ultrasound
3. What is your recommendation and Breast Imaging Reporting and Data System (BI-RADS) code based on the imaging presented?
A. Biopsy is needed, BI-RADS 4
B. Incomplete exam, recommend MRI of breasts, BI-RADS 0
D. Probably benign, recommend 6-month follow-up, BI-RADS 3
4. Why is it important to perform a mammogram in a man presenting with a breast mass?
A. Men should be screened annually or semiannually for breast cancer with a mammogram.
C. Mammography is not necessary; the exam should begin with ultrasound of the area of concern.
ANSWERS
CASE 76
Palpable Mass in a Male Patient (Lipoma)
1. A, B, C, and D
2. A
3. C
4. B
References
Bancroft LW, Kransdorf MJ, Peterson JJ, O’Connor MI. Benign fatty tumors: classification, clinical course, imaging appearance, and treatment. Skeletal Radiol. 2006;35(10):719–733.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:137.
Comment
Male patients can present with palpable masses in the breast. The findings fall into three broad categories: breast cancer, gynecomastia, and masses that could arise anywhere in the body, deriving from the skin, lymph system, or blood vessels. As in female patients, the most important consideration for the radiologist is to evaluate for the possibility of cancer. Thus, male patients who present with a palpable finding may first have a bilateral mammogram, followed by a directed ultrasound exam if needed. (Ultrasound might not be necessary when the mammogram demonstrates a characteristic appearance of gynecomastia.)
Lipomas are common masses in male and female patients. They arise from the subcutaneous fat, and they may be palpable owing to the superficial location. They can become large and may be cosmetically unappealing, but they are not otherwise clinically important. The lipoma is usually not recognized on a male mammogram (see the figures) because the normal male breast is composed predominantly of subcutaneous fat. In the female breast, the lipoma may be recognized on the mammogram if it is outlined by dense glandular tissue. On ultrasound, the lipoma typically is horizontally aligned and isoechoic or hyperechoic to the surrounding fat. The margins are smooth, and a thin, echogenic capsule may be seen (see the figures). The lipoma may be difficult to distinguish from the surrounding subcutaneous fat. The lipoma is soft, and it can compress on pressure of the transducer.
CASE 77
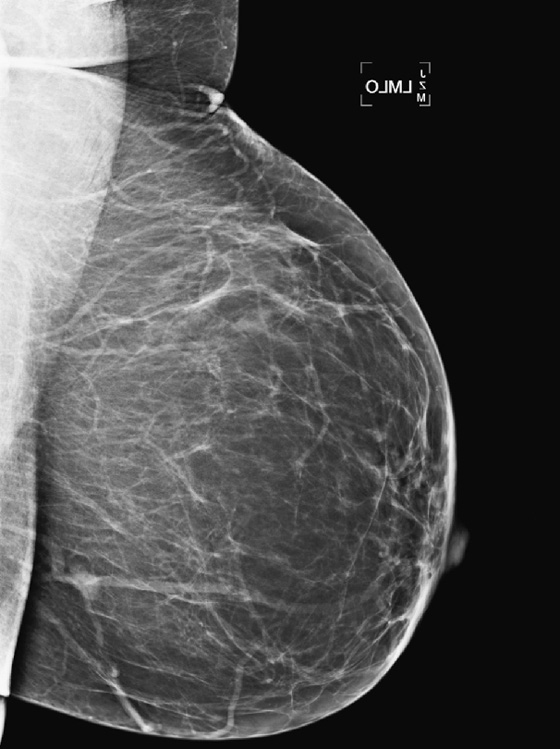
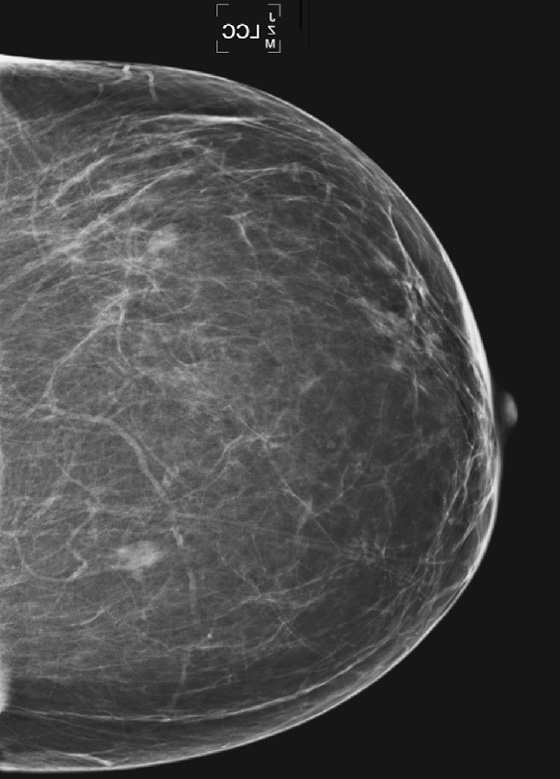
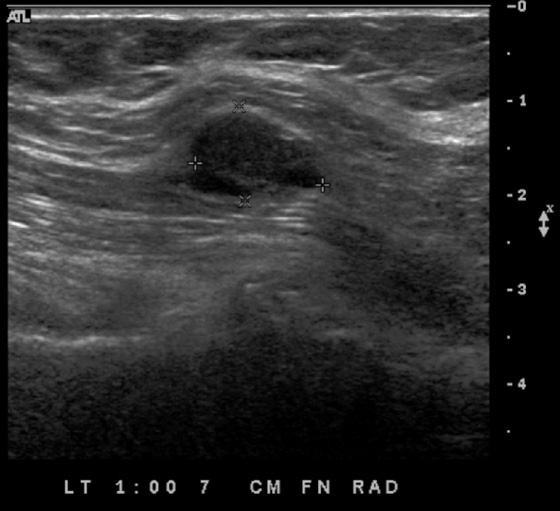
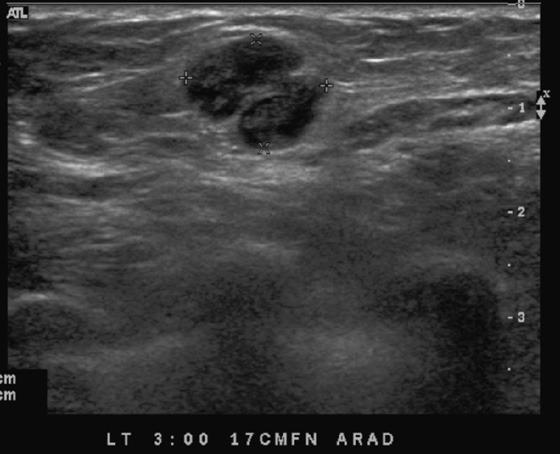
History: A 65-year-old woman who had a melanoma removed from her right leg 10 years ago undergoes a routine screening mammogram. The left breast only is shown.
1. What should be included in the differential diagnosis for the two developing masses in the left breast? (Choose all that apply.)
B. Metastatic masses in the breast
C. Primary carcinoma of the breast
2. Which of the following statements is true about hematogenous spread of tumor to the breast?
A. There are almost always multiple masses in both breasts.
B. Masses are most often spiculated.
C. Masses are usually in a ductal distribution.
D. Masses are typically hypervascular.
3. What is the most common cancer to spread to the breast?
4. Would you be more likely to recommend biopsy of a benign-appearing breast mass in a woman with known malignancy such as melanoma?
B. Yes, you should perform biopsy of a new mass in the breast regardless of appearance.
D. A patient’s cancer history bears no relationship to the decision to recommend biopsy.
ANSWERS
CASE 77
Metastatic Melanoma
1. A, B, and C
2. D
3. A
4. C
References
Akçay MN. Metastatic disease in the breast. Breast. 2002;11(6):526–528.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:117.
Comment
Nonbreast primary tumors can spread hematogenously and can involve the breast. When single or multiple new masses are seen in a nonductal pattern in the breast in a patient with a known cancer, metastases should be considered. Metastatic involvement of the breast is rare, representing approximately 1% of all breast malignancies. The most common cause of metastatic involvement is from a contralateral breast cancer; melanoma is the most frequent nonbreast cancer to spread to the breast. Lymphoma, lung cancer, and ovarian cancer are the next most frequent cancers to metastasize to the breast. Sometimes metastases are the first indication that a cancer is present. This is more common in the case of enlarged axillary nodes seen on mammogram that herald a previously unknown malignancy.
Metastatic deposits in the breast are often multiple, as in this patient (see the figures), bilateral, and well defined. Microcalcification in the masses is rare, as are spiculated margins. The mammographic and ultrasound appearance overlaps with many benign masses, such as cysts and fibroadenomas. On mammography, the masses are round or oval, with circumscribed margins. On ultrasound, the masses usually are well defined, without shadowing, and without a thick echogenic halo because there is typically no desmoplastic reaction in the surrounding tissue. They are often hypoechoic or even appear anechoic. Color flow Doppler should show increased vascularity because the masses are spread hematogenously. It is important to recognize the possibility of metastases and to perform a biopsy of lesions with imaging guidance. Biopsy may avoid unnecessary breast surgery and lead to appropriate oncologic management.
This patient was diagnosed with melanoma 10 years previously and had no metastasis until the mammogram showed masses. The patient was initially recalled for additional views of the left breast and ultrasound evaluation. When she returned for the biopsy appointment 2 months after the mammogram, ultrasound revealed a new mass deep in the breast at the 1 o’clock position, near the pectoral muscle (see the figures), and another at 3 o’clock (see the figures), which had not been seen on the mammogram. Biopsy specimens of these two masses were obtained with core needle technique and ultrasound guidance, and a diagnosis of melanoma metastases was made.
CASE 78
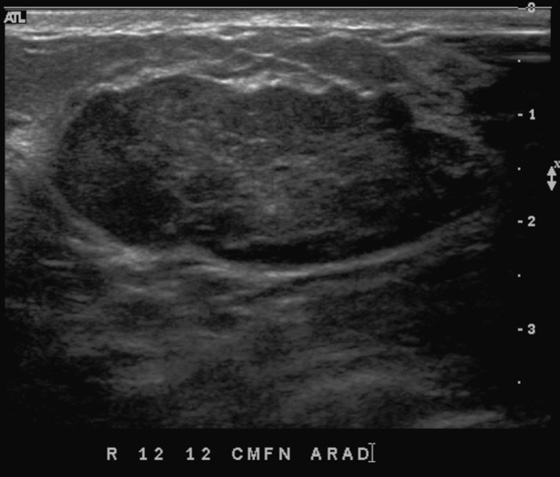
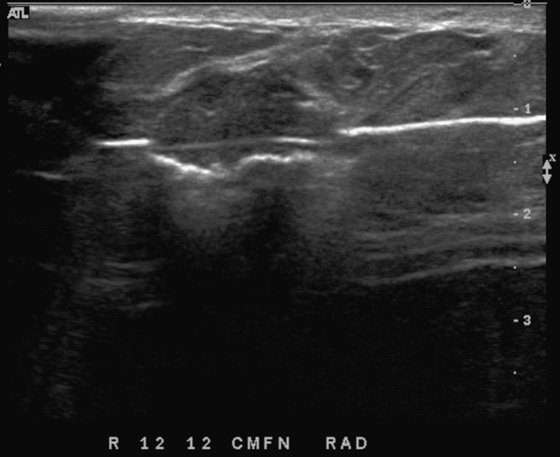
History: A 19-year-old woman who is 2 months postpartum presents for evaluation of a mass in the right upper breast.
1. What should be included in the differential diagnosis for this breast mass? (Choose all that apply.)
2. How do you evaluate a 19-year-old woman with a mass?
A. Mammogram must always be performed in the setting of a palpable mass.
B. A patient of this age should always have surgical evaluation first.
C. Ultrasound of the palpable mass should be performed.
D. MRI of both breasts should be performed.
3. Is the mass related to the patient’s recent pregnancy?
A. Yes, the mass likely developed during pregnancy.
B. No, tubular adenomas are related to radial scars, not pregnancy.
C. No, pregnancy and breast masses are not related.
D. Yes, all breast masses in young women develop during pregnancy.
4. Are fibroadenomas, tubular adenomas, and lactating adenomas the same pathology?
A. No, they are not related; the names are similar, but the pathology is very different.
C. No, they are not the same pathology, but they are related.
D. Yes, they are the same pathology, differing only in size.
ANSWERS
CASE 78
Tubular Adenoma
1. A, B, and D
2. C
3. A
4. C
References
Soo MS, Dash N, Bentley R, et al. Tubular adenomas of the breast: imaging findings with histologic correlation. AJR Am J Roentgenol. 2000;174(3):757–761.
Stavros AT. Breast Ultrasound. Philadelphia: Lippincott Williams & Wilkins,; 2004. pp 554–557
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:127. 379
Comment
Tubular adenoma is a relatively rare, benign mass that develops during the reproductive years. This mass is related to lactating adenoma, which is more common and typically develops during pregnancy and lactation. These two adenomas have similar pathologic features, consisting mainly of epithelial cells, with very little stromal component. Fibroadenoma, much more common, has a greater degree of stromal cells.
The ultrasound and mammographic appearance of tubular adenoma is similar to fibroadenoma: oval shape, wider than tall, and possibly enhanced through-transmission. The mass surface may be smooth or microlobulated (see the figures). The microlobulated surface is a feature that can be seen in malignancy, making the lesion a BI-RADS (Breast Imaging Reporting and Data System) 4 when this feature is present. Calcifications can occur within the mass, although in one series, calcifications were seen only in tubular adenomas of older women.
Histologic diagnosis is indicated in this patient owing to the ultrasound features. Patients may choose a surgical resection, especially when the mass is large and palpable. However, they may choose follow-up, rather than excision, if the histology is known. Ultrasound-guided core needle biopsy can provide the histologic diagnosis, and a patient can choose follow-up or excision at her leisure.
This patient chose a needle biopsy, which was performed with a vacuum-assisted device (see the figures). Histology was tubular adenoma.
CASE 79
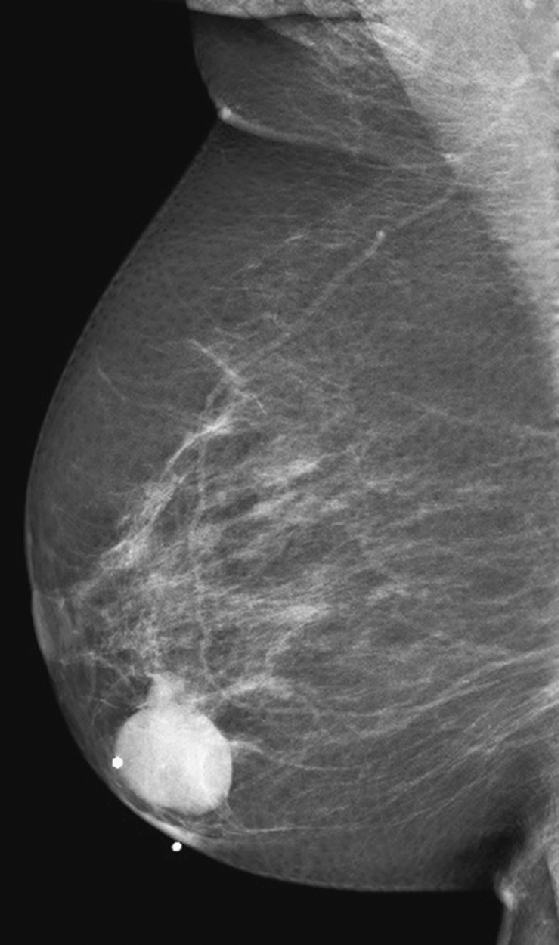
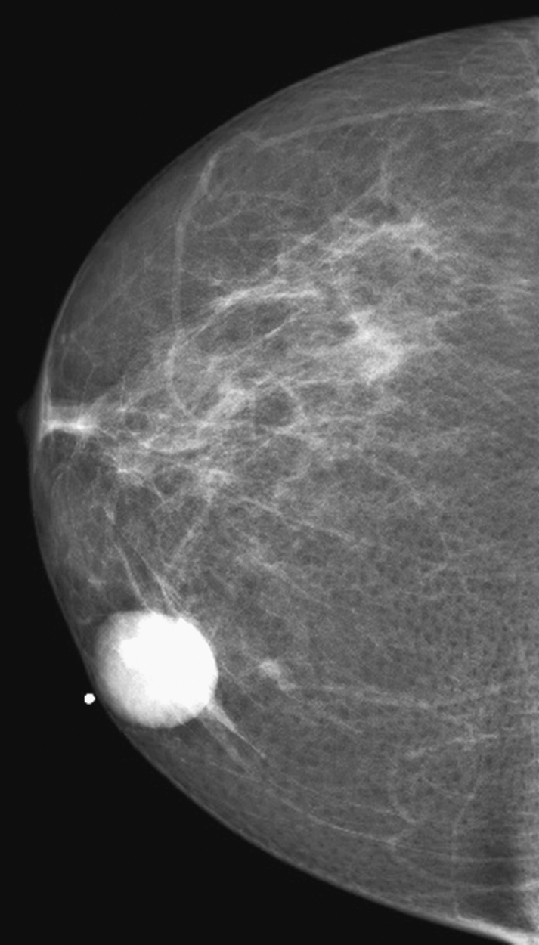
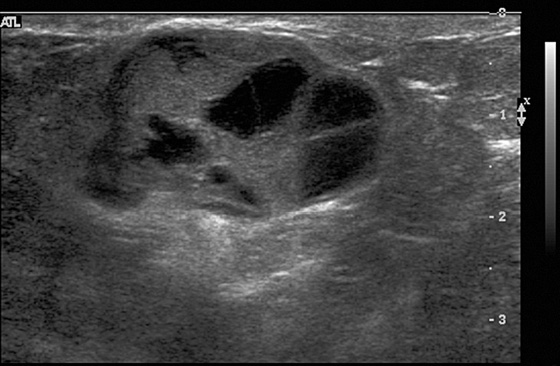
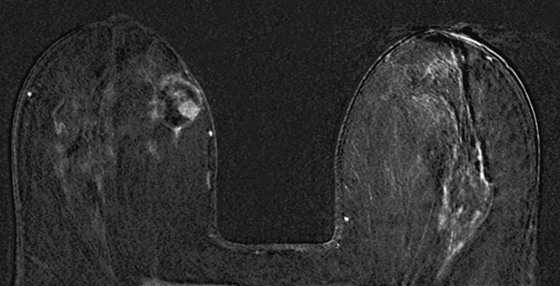
Subtraction view after contrast enhancement.
History: A 73-year-old woman presents with a palpable lump in the inner right breast. She has no family history of breast cancer.
1. What is the differential diagnosis? (Choose all that apply.)
2. Which of the following statements regarding papillary carcinomas is true?
A. Papillary carcinomas are common.
B. Papillary carcinomas are more common in premenopausal women.
C. Treatment consists of radiation and hormonal treatment.
D. The overall prognosis of papillary carcinomas is excellent.
3. Which of the following statements regarding imaging of papillary carcinomas is true?
A. The presence of multiple masses excludes the diagnosis of papillary carcinoma.
B. Small lesions manifest as architectural distortion or nipple retraction or both.
C. Papillary carcinomas may manifest as complex cystic lesions or solid masses.
D. On MRI, the fluid component is dark on T1-weighted images and bright on T2-weighted images.
4. Which of the following statements is false?
A. Diagnosis of papillary carcinoma is made by biopsy.
B. Histologically, papillary carcinomas are a heterogeneous group of lesions.
C. Papillary carcinomas arise from benign papillomas.
D. Distinction between benign papillary lesions and papillary carcinoma is important.
ANSWERS
CASE 79
Papillary Carcinoma
1. C and D
2. D
3. C
4. C
References
Bhosale SJ, Kshirsagar AY, Sulhyan SR, et al. Invasive papillary breast carcinoma. Case Rep Oncol. 2010;3(3):410–415.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:116.
Comment
Papillary carcinomas are rare, accounting for approximately 1% of all newly diagnosed breast cancers. They are more common in older patients (average age of onset is approximately 70 years) and frequently manifest as a solitary palpable mass in the subareolar location or multiple peripheral masses; they may also manifest as bloody nipple discharge or be found incidentally on routine imaging. Retraction of the nipple or skin may be an associated clinical finding.
Mammographic findings are usually nonspecific. Lesions commonly appear as a dense, round or lobulated mass, with architectural distortion or nipple retraction as they become larger (see the figures). Less commonly, multiple or bilateral masses may be seen.
On ultrasound, lesions appear as complex cystic masses, with solid mural nodules with or without septa (see the figures). The solid component is often highly vascular. They may also appear as entirely solid masses.
MRI usually shows a well-circumscribed, heterogeneous round or oval mass, with cystic component and mural solid nodules (see the figures). The solid component has intermediate signal intensity, whereas the cystic areas may have a different appearance depending on the fluid composition (serous content appears hypointense on T1-weighted images and hyperintense on T2-weighted images; hemorrhagic content appears hyperintense on both T1-weighted and T2-weighted images). With addition of contrast agent, there is marked enhancement of the walls, septa, and solid nodules.
Biopsy should be performed to obtain a definitive diagnosis. Ultrasound-guided core needle biopsy establishes the initial diagnosis, as it can accurately target the solid components. Histologically, papillary carcinomas are a morphologically heterogeneous group of lesions, having in common a growth pattern characterized by arborescent fibrovascular stalks lined by epithelial cells. The mass is surrounded by thick stromal fibrosis. Papillary carcinomas can be distinguished from benign papillary lesions by the absence of myoepithelial cells in the malignant form. Distinction between benign papillary lesions and papillary carcinoma is important because it has an impact on treatment.
Treatment of papillary carcinoma, as in any type of breast cancer, includes surgery (lumpectomy or mastectomy) in association with radiation and hormonal treatment for hormone-sensitive tumors, chemotherapy, or both chemotherapy and radiation. The overall prognosis of papillary carcinoma is excellent because it tends to be low grade and slow growing. The survival rate approaches 100% at 10 years. Lymph node metastases occur less commonly than in invasive ductal carcinomas not otherwise specified.
CASE 80
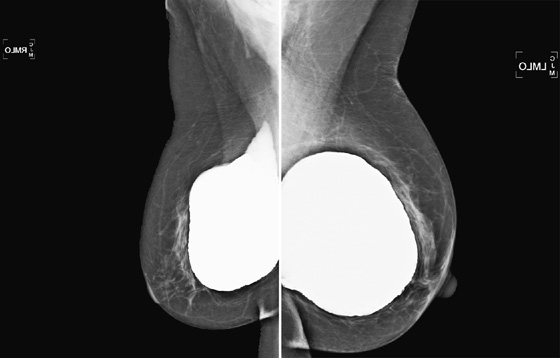
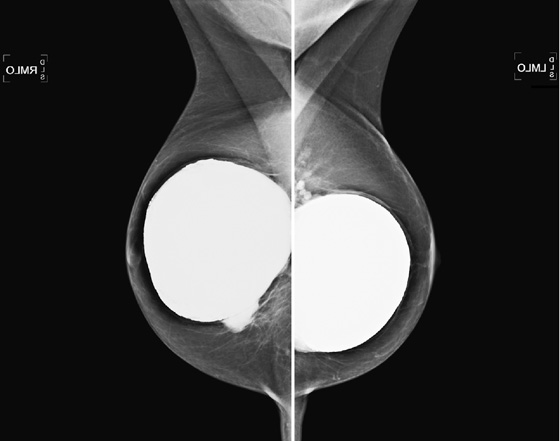
History: A 60-year-old asymptomatic woman with silicone implants placed 20 years ago undergoes mammography.
1. What should be included in the differential diagnosis for the images shown in the first figure? (Choose all that apply.)
A. Saline implants with right saline implant rupture
B. Mass suspicious for cancer, developing at the chest wall on the right
C. Right silicone implant rupture
D. Bilateral silicone implant rupture
2. What is the location of the implants, and what type are they?
3. What is the next step in imaging for this patient?
A. MRI is needed to evaluate integrity of the right implant.
B. Ultrasound is needed to evaluate possible right implant rupture.
C. No further work-up is needed.
D. Additional mammographic views are needed.
4. What is the management of this problem?
B. Recommend that the patient discuss implant rupture with her clinician.
C. The patient must be referred to a surgeon for implant removal.
D. Silicone granulomas must be surgically removed because of autoimmune sequelae.
ANSWERS
CASE 80
Mammography of Silicone Implant Leak
1. C
2. A
3. C
4. B
Reference
Berg WA, Caskey CI, Hamper UM, et al. Diagnosing breast implant rupture with MR imaging, US, and mammography. Radiographics. 1993;13(6):1323–1336.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:343.
Comment
Asymptomatic silicone implant rupture is seen in approximately 5% of implants on screening mammography. Several factors predispose to implant rupture: subglandular location (as in this patient), implant age, and closed capsulotomy (a procedure in which pressure is applied to the implant to break the fibrous capsule and keep the implant from feeling hard).
When interpreting a mammogram of a woman with implants, it is important to distinguish the type of implant. Implant type is distinguished by assessing the density of the fluid—silicone is denser than saline—and checking for the presence of a valve—saline implants have a valve, but silicone implants do not. It is important to know the type of implant because when saline implants rupture, they collapse, and the saline is reabsorbed by the body. Only the implant shell remains, flattened against the posterior breast. For this reason, MRI is not used to evaluate for saline implant rupture: It is not a diagnostic dilemma; the rupture is clinically obvious. When silicone implants rupture, silicone gel leaks out of the implant shell, but the bulk of the silicone remains in the shell. The silicone leaks into the capsule (intracapsular rupture) and then may leak into the breast if there is also a rupture of the fibrous capsule around the implant (extracapsular rupture) (see the figures).
The second figure shows a different patient, also asymptomatic, with bilateral extracapsular silicone implant rupture. Silicone that has leaked outside the capsule can form into silicone granulomas, which can be found in the breast and axilla and in the rest of the body; silicone also can be taken up by the lymph nodes. A patient may be asymptomatic, similar to both of the patients discussed in this case; symptoms may be minimal; or the patient may experience pain and lumps. The American College of Rheumatology issued a report in 1995 stating that silicone implants do not expose patients to additional risk for connective tissue disease.