CASE 60 Chronic Occlusion of a Superficial Femoral Artery
Case presentation
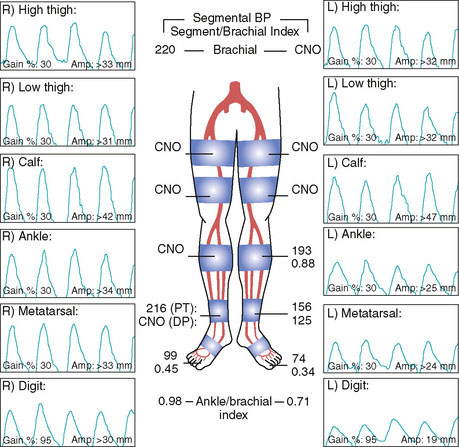
A 76-year-old woman developed worsening leg claudication, progressing nearly to rest pain. She had numerous atherosclerotic risk factors including diabetes, hypertension, hyperlipidemia, and prior tobacco abuse, and had an extensive atherosclerosis history including coronary artery disease, renal artery stenosis, cerebrovascular disease, and peripheral arterial disease. Six months prior to her current presentation, she underwent lower extremity angiography and was found to have bilateral iliac artery lesions along with disease in the superficial femoral arteries. At that point, her physician decided to first treat the inflow disease with stents and then reevaluate the patient’s symptoms. Following the bilateral iliac stenting procedure, she continued to have lifestyle-limiting claudication and her right leg symptoms progressed to occasional nocturnal rest pain. The noninvasive studies obtained after iliac stenting showed severe arterial insufficiency in both legs at the level of the thigh (Figure 60-1) consistent with the disease in the superficial femoral artery (SFA). Her physician recommended repeat angiography and possible SFA intervention.
Angiography
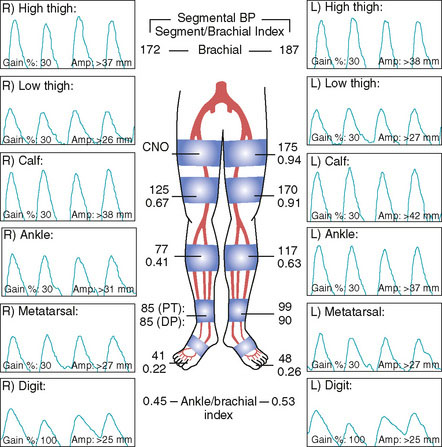
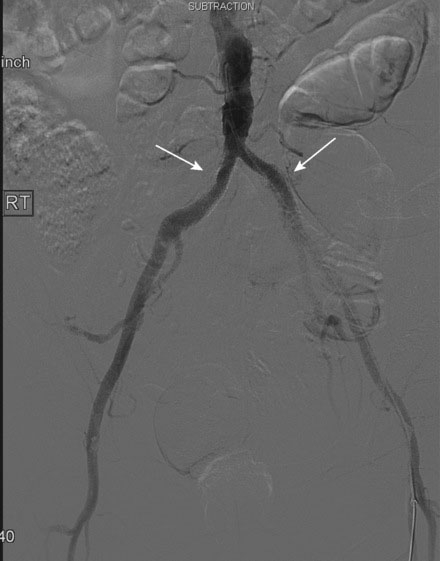
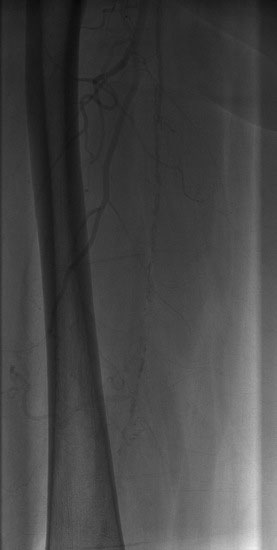
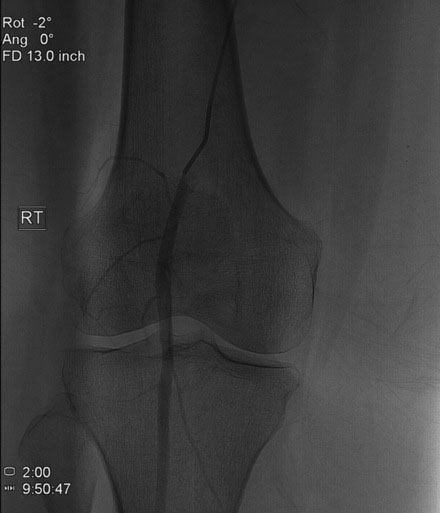
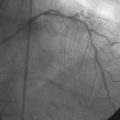
Arterial access was obtained in the left common femoral artery and a 4 French Omniflush catheter was used to perform angiography. Aortography confirmed wide patency of the common iliac stents (Figure 60-2). Selective right femoral angiography and runoff was performed by passing a hydrophilic glide wire over the bifurcation using the Omniflush catheter and then exchanging for a 4 French RIM catheter. Angiography found a mild stenosis in the common femoral artery, followed by a diffusely diseased segment of the proximal SFA of moderate stenosis and then a long segment of total occlusion (Figure 60-3). There was reconstitution of the distal SFA above the adductor canal via collaterals from the profunda femoris (Figures 60-4 through 60-6). Below the knee, the anterior tibial was occluded; however,two-vessel runoff was present to the foot via the posterior tibial and peroneal arteries. Based on the patient’s profound symptoms, her anatomy, and her comorbid conditions, it was decided to proceed with an intervention to the right SFA.
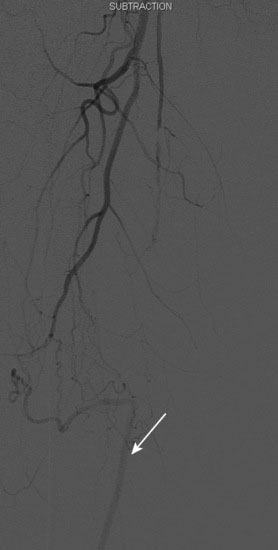
FIGURE 60-4 A long occlusion of the mid-SFA is present and reconstitutes at the popliteal artery (arrow).
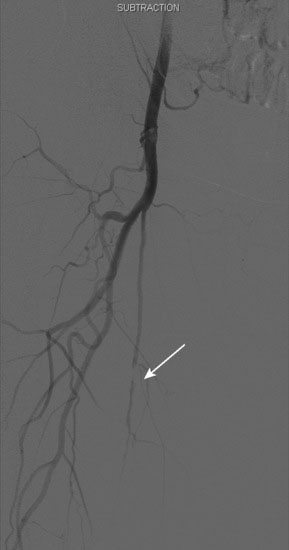
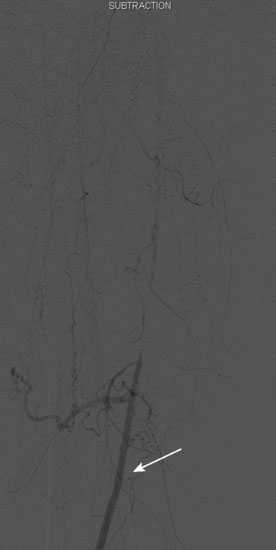
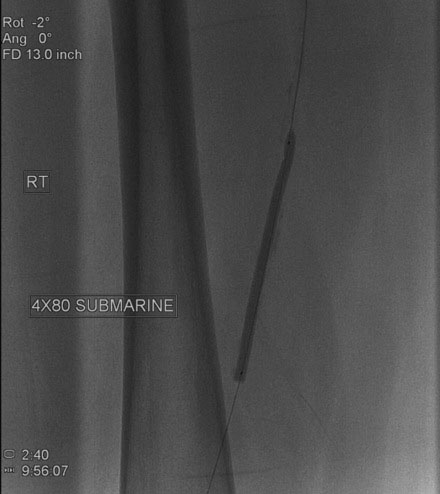
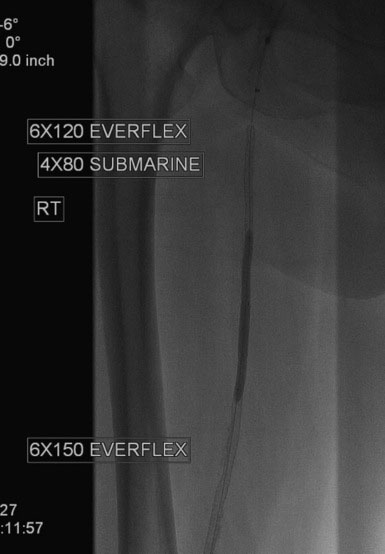
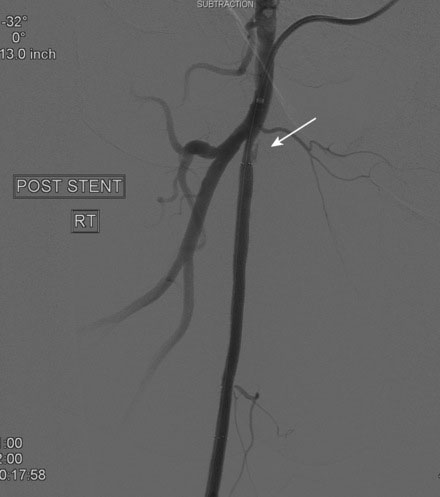
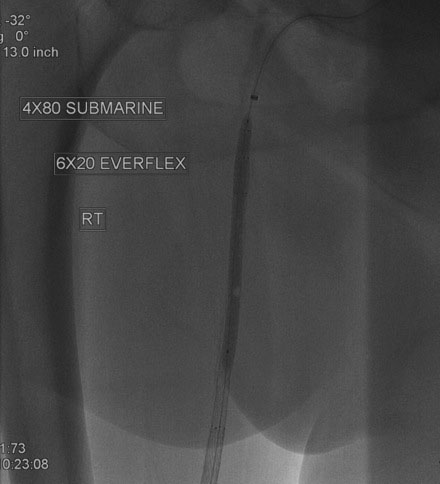
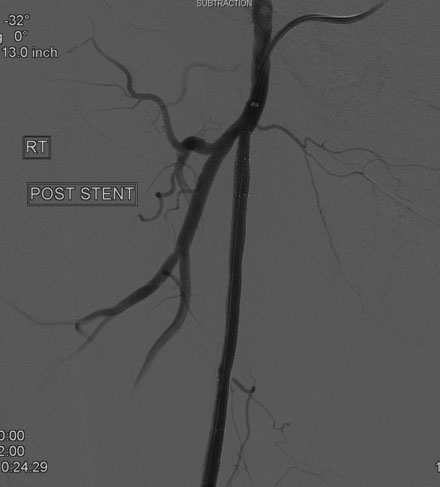
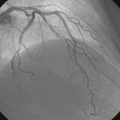
An Amplatz wire was placed through the RIM catheter in the right external iliac. Attempts were made to get a 6 French long Ansel sheath to pass over the bifurcation from the left femoral artery over the Amplatz wire; however, the sheath would not advance past the previously placed iliac stents. A longer dilator and a 0.018 inch system were tried without success. In order to avoid damage to the prior iliac stents, the operator decided to access the right femoral artery in an antegrade fashion. Access to the right common femoral artery was obtained with a micropuncture kit and a 6 French Ansel sheath was inserted. Following the administration of unfractionated heparin, the occluded segment of the right SFA was probed with a glide wire backloaded on a hydrophilic catheter (VERT catheter). With gentle manipulations, the catheter and wire successfully crossed the SFA occlusion and the distal tip of the guidewire appeared to move freely within the popliteal artery. The glide wire was removed; contrast injected through the catheter confirmed intraluminal placement (Figure 60-7). Systemic heparin was administered to achieve a therapeutic activated clotting time of more than 250 seconds. A 0.018 inch interventional guidewire was inserted and the hydrophilic catheter exchanged for a balloon catheter. The entire SFA was then dilated with a 4 mm diameter by 80 mm long balloon (Figure 60-8). Substantial residual stenosis and recoil remained after balloon dilatation, and thus the operator decided to place multiple stents. Beginning in the distal SFA, a 6 mm diameter by 150 mm long, self-expanding nitinol stent was deployed, followed by a 6 mm diameter by 120 mm long self-expanding nitinol stent. A 1 to 2 cm gap was initially left at the ostium of the SFA. The stented segment and ostial SFA were further dilated with the 4 mm by 80 mm balloon to high atmospheres (Figure 60-9). Subsequent angiography confirmed an excellent result in the stented segment (Figure 60-10); however, the ostium of the SFA had greater than 30% residual stenosis and an ulcerated dissection (Figure 60-11). The operator placed another stent (6 mm diameter by 20 mm long self-expanding nitinol), taking great care to avoid impingement on the profunda femoris and postdilated with a 4 mm diameter by 80 mm long balloon (Figure 60-12). The final angiogram demonstrated sealing of the dissection with no residual stenosis, no impingement on the profunda femoris, and no signs of distal embolization (Figure 60-13).
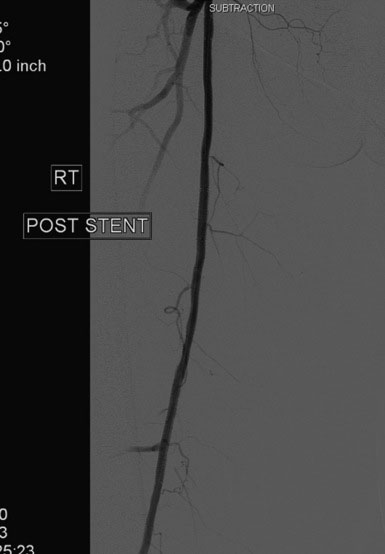
FIGURE 60-10 Angiogram of the mid-SFA obtained after stent deployment and postdilatation showing an excellent result.
Postprocedural course
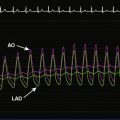
Both sheaths were left in place and removed by manual compression when the activated clotting time fell below 180 seconds. The patient remained on bed rest for 4 hours following sheath removal and was hydrated with normal saline. After she was able to ambulate without difficulty, she was discharged the same day. At follow-up 1 month later, she had experienced resolution of her right leg symptoms. Noninvasive testing found near-normalization of her right lower extremity ankle-brachial index (ABI) (Figure 60-14). Her left leg symptoms were only moderate and she decided to continue with medical therapy and exercise. At 6 months, she noted stable symptoms. Noninvasive testing found a slight drop in her ABIs, likely due to moderate restenosis of her right SFA stents.
Discussion
Disease of the SFA is found in 80% to 90% of patients with claudication.1 Guidelines were created to help manage patients with peripheral arterial disease involving the femoral-popliteal arteries.2 The TASC criteria classifies femoropopliteal lesions from A to D, with TASC A and B lesions typically being treated percutaneously and TASC D lesions being treated surgically, due to the poor long-term patency of endovascular therapy for this subset. Surgery is preferred for TASC C lesions unless the patient has substantial comorbid conditions resulting in excessive surgical risk. The lesions in the case presented here would be classified TASC C or D and the long term patency would likely have been greater with surgical rather than endovascular revascularization. However, the decision regarding the method of revascularization is complex and must consider other medical conditions, patient preference, and technical feasibility. For example, in this case, the presence of multiple comorbid conditions including advanced age, diabetes, and both prior myocardial infarction and stroke increased her risk of surgery. Endovascular therapy was thought to be lower-risk and appeared technically feasible. Importantly, this mode of revascularization would be unlikely to interfere with surgical options if needed at a later date, leading to the decision to pursue endovascular therapy.
Once a wire has been positioned distally, a variety of techniques have been proposed to treat the occluded segment. These include balloon angioplasty, directional or orbital atherectomy, self-expanding stents, and covered self-expanding stents. There are little data available comparing these techniques and most information is generated from registries and self-reported case series. With this in mind, at the present time, there does not appear to be a clearly superior mode of therapy for the treatment of a chronically occluded SFA. The relatively high rate of restenosis, on the order of 20% to 50%, remains a major limitation for all of these devices.3 There are also limited data available addressing the issue of restenosis. Nevertheless, with current endovascular techniques, aggressive medical therapy, and close follow-up, the majority of these patients have an improvement in their quality of life.
1 Creager M.A., Loscalzo J. Vascular Diseases of the Extremities. In: Fauci A.S., Braunwald E., Kasper D.L., Hauser S.L., Longo D.L., Jameson J.L., Loscalzo J., editors. Harrison’s Principles of Internal Medicine. ed 17. New York, NY: McGraw-Hill; 2008:1568-1570.
2 Norgren L., Hiatt W.R., Dormandy J.A., Nehler M.R., Harris K.A., Fowkes F.G.R., TASC II Working Group. Inter-Society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33:S1-S70. on behalf of the
3 Laird J.R. Limitations of percutaneous transluminal angioplasty and stenting for the treatment of disease of the superficial femoral and popliteal arteries. J Endovasc Ther. 2006;13(Suppl II):II 30-II 40.