CHAPTER 6 Transfusion Therapy
4 Historically, a hemoglobin level of 10 g/dl (hematocrit of 30) was used as a transfusion trigger. Why is this is no longer an accepted practice?
It is also interesting to note that men and women tend to be treated equally when the decision to transfuse is made, despite the fact that normal women are anemic relative to men. Using the same transfusion triggers hardly seems rational. Finally there is a concern that a transfusion might not substantially increase oxygen delivery (see discussion of blood storage lesions in question 8). These are strong arguments for closely scrutinizing the consideration to transfuse.
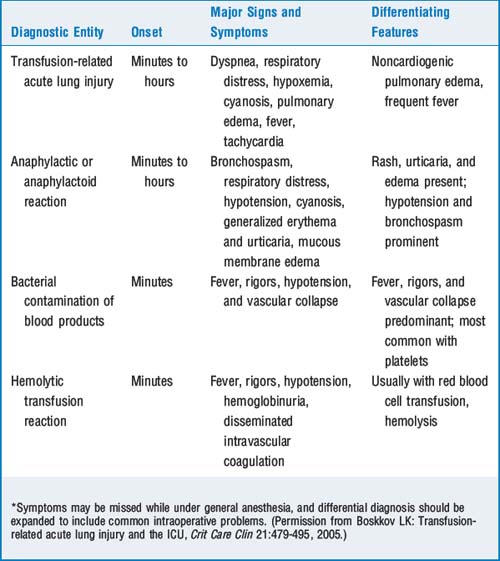
7 Review the major transfusion-related reactions
 Hemolytic transfusion reactions caused by ABO incompatibility are most commonly caused by clerical errors and transfusion of the wrong unit. Mistransfusion is thought to occur with a frequency between 1:14,000 and 1:18,000. Most reactions occur during or shortly after a transfusion. Clinical manifestations include fever; chills; chest, flank, and back pain; hypotension; nausea; flushing; diffuse bleeding; oliguria or anuria; and hemoglobinuria. General anesthesia may mask some of the clinical manifestations, and hypotension, hemoglobinuria, and diffuse bleeding may be the only signs. It should be noted that the signs of a severe hemolytic reaction might be missed while the patient is under general anesthesia or attributed to another cause.
Hemolytic transfusion reactions caused by ABO incompatibility are most commonly caused by clerical errors and transfusion of the wrong unit. Mistransfusion is thought to occur with a frequency between 1:14,000 and 1:18,000. Most reactions occur during or shortly after a transfusion. Clinical manifestations include fever; chills; chest, flank, and back pain; hypotension; nausea; flushing; diffuse bleeding; oliguria or anuria; and hemoglobinuria. General anesthesia may mask some of the clinical manifestations, and hypotension, hemoglobinuria, and diffuse bleeding may be the only signs. It should be noted that the signs of a severe hemolytic reaction might be missed while the patient is under general anesthesia or attributed to another cause. Anaphylactic reactions are caused by binding of IgE; present with bronchospasm, edema, redness, and hypotension; and require urgent treatment with epinephrine, fluid infusions, corticosteroids and antihistamines, and other therapies as indicated by severity and progression of symptoms.
Anaphylactic reactions are caused by binding of IgE; present with bronchospasm, edema, redness, and hypotension; and require urgent treatment with epinephrine, fluid infusions, corticosteroids and antihistamines, and other therapies as indicated by severity and progression of symptoms. Febrile reactions may be an early sign of hemolytic transfusion reaction (but other symptoms should be present) or bacterial contamination of the blood product. Febrile nonhemolytic transfusion reactions usually occur in patients who have had prior transfusions; headache, nausea, and malaise are associated symptoms. The reaction is caused by leukocyte antibodies, and leukocyte-depleted red blood cells may be indicated for these patients. Antipyretics may decrease the symptoms if given before the transfusion; meperidine may decrease the severity of chills.
Febrile reactions may be an early sign of hemolytic transfusion reaction (but other symptoms should be present) or bacterial contamination of the blood product. Febrile nonhemolytic transfusion reactions usually occur in patients who have had prior transfusions; headache, nausea, and malaise are associated symptoms. The reaction is caused by leukocyte antibodies, and leukocyte-depleted red blood cells may be indicated for these patients. Antipyretics may decrease the symptoms if given before the transfusion; meperidine may decrease the severity of chills. Transfusion-related acute lung injury (TRALI) is in the top three of transfusion-related deaths, having a mortality of 50%. A form of noncardiogenic pulmonary edema, TRALI is also immune related and is usually noted within 6 to 12 hours after transfusion. Symptoms include hypoxia, dyspnea, fever, and pulmonary edema; treatment is supportive.
Transfusion-related acute lung injury (TRALI) is in the top three of transfusion-related deaths, having a mortality of 50%. A form of noncardiogenic pulmonary edema, TRALI is also immune related and is usually noted within 6 to 12 hours after transfusion. Symptoms include hypoxia, dyspnea, fever, and pulmonary edema; treatment is supportive.8 What are the current standards for the length of storage of blood? What is a blood storage lesion?
12 Discuss the criteria for diagnosis of transfusion-related acute lung injury
 Acute onset: often occurring in less than 2 hours after a transfusion, but usually less than 6 hours
Acute onset: often occurring in less than 2 hours after a transfusion, but usually less than 6 hours Pulmonary arterial occlusion pressure ≤18 mm Hg or lack of clinical evidence of left atrial overload (i.e., the problem is noncardiogenic pulmonary edema)
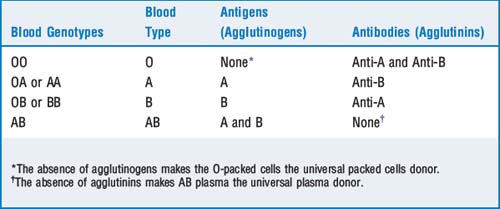
Pulmonary arterial occlusion pressure ≤18 mm Hg or lack of clinical evidence of left atrial overload (i.e., the problem is noncardiogenic pulmonary edema)14 Review the ABO and Rh blood genotypes and the associated antibody patterns
Blood type is determined by two alleles of three types: O, A, and B. A and B refer to antigens on the red blood cell surface. An individual can have either A or B, both A and B, or neither (blood type O). If an individual does not have the type A antigen, over time anti-A antibodies (also known as agglutinins) form. A patient with type AB blood has both antigens and will form no agglutinins. Individuals with type O blood have no antigen and develop both A and B antibodies (Table 6-2). The antibodies are primarily immunoglobulin (Ig)M or IgG. Acute hemolytic reactions are caused by complement activation and release of proteolytic enzymes that digest the red cell membrane.
15 What is the difference between a type and screen and a crossmatch?
The patient’s blood is typed for ABO and Rh group by placing his or her red cells with commercially available anti-A and anti-B reagents and reverse typing the patient’s serum against A and B reagent cells. A screen for antibodies involves placing the patient’s serum with specially selected red cells containing all relevant blood group antigens. In a crossmatch the patient’s serum is also incubated with a small quantity of red cells from the proposed donor unit to verify in vitro compatibility. A crossmatch also detects more unique antibodies (Table 6-3).
| Degree of Crossmatch | Chance of Compatible Transfusion |
|---|---|
| ABO-Rh type only | 99.8% |
| ABO-Rh type + antibody screen | 99.94% |
| ABO-Rh type + antibody screen crossmatch | 99.95% |
17 What are some of the complications of massive blood transfusion?
 Coagulopathy secondary to dilutional thrombocytopenia, lack of labile coagulation factors V and VIII, and disseminated intravascular coagulation
Coagulopathy secondary to dilutional thrombocytopenia, lack of labile coagulation factors V and VIII, and disseminated intravascular coagulation18 If suspected, how should a major transfusion reaction be managed?
 Massive hemolysis can result in hyperkalemia. Follow serum potassium levels and continuously monitor the electrocardiogram for electrocardiographic signs of hyperkalemia.
Massive hemolysis can result in hyperkalemia. Follow serum potassium levels and continuously monitor the electrocardiogram for electrocardiographic signs of hyperkalemia. Disseminated intravascular coagulation may occur. The best treatment is identifying and treating the underlying cause. Follow prothrombin, partial thromboplastin, fibrinogen, and d-dimer levels.
Disseminated intravascular coagulation may occur. The best treatment is identifying and treating the underlying cause. Follow prothrombin, partial thromboplastin, fibrinogen, and d-dimer levels.19 What alternatives are there to transfusion of donor blood?
 Autologous transfusion (the collection and reinfusion of the patient’s own blood). It should be noted that only about 55% of predonated units are returned to the patient. The patient scheduled to autologous transfusion still runs the risk of clerical errors and bacterial infection. There is also a report of a patient who received an autologous transfusion developing TRALI.
Autologous transfusion (the collection and reinfusion of the patient’s own blood). It should be noted that only about 55% of predonated units are returned to the patient. The patient scheduled to autologous transfusion still runs the risk of clerical errors and bacterial infection. There is also a report of a patient who received an autologous transfusion developing TRALI. Preoperative use of erythropoietin to stimulate erythrocyte production. Erythropoietin stimulates erythrocyte production in 5 to 7 days and has been shown to reduce use of allogeneic blood in patients with renal insufficiency and anemia of chronic disease and when transfusion is refused.
Preoperative use of erythropoietin to stimulate erythrocyte production. Erythropoietin stimulates erythrocyte production in 5 to 7 days and has been shown to reduce use of allogeneic blood in patients with renal insufficiency and anemia of chronic disease and when transfusion is refused.20 What are the limitations, advantages, and disadvantages of alternative hemoglobin solutions?
1. American Society of Anesthesiologists Task Force on Perioperative Blood Transfusions. Practice guidelines for perioperative blood transfusion and adjuvant therapies. Anesthesiology. 2006;105:198-208.
2. Hebert P.C., Tinmouth A., Corwin H. Anemia and red cell transfusion in critically ill patients. Crit Care Med. 2003;31(Suppl):S672-S677.
3. Madjdpour C., Spahn D.R. Allogenic red blood cell transfusions: efficiency, risks, alternatives and indications. Br J Anaesth. 2005;98:33-42.
4. Rajagopalan S., Mascha E., Na J., et al. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology. 2008;108:71-77.
5. Spahn D.R., Kocian R. Artificial oxygen carriers: status in 2005. Curr Pharm Des. 2005;11:4099-4114.