Section 6 Respiratory
6.1 Upper respiratory tract
Introduction
Urgent conditions requiring immediate attention or intervention are those likely to compromise the airway. Protection and maintenance of airway, breathing and circulation (the ABCs) take precedence over history taking, detailed examination or investigations. Possible causes of airway obstruction are listed in Table 6.1.1.
Triage and initial evaluation
Symptoms and signs of airway obstruction include dyspnoea, stridor, altered voice, dysphonia and dysphagia. Evidence of increased work of breathing includes subcostal, intercostal and suprasternal retraction, flaring of the nasal alae, as well as exhaustion and altered mental state. The presence of these signs may vary with age and accompanying conditions. Cyanosis is a late sign. Further examination will be directed by the presenting complaint and initial findings and includes:
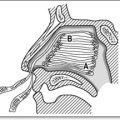
Upper airway obstruction
Obstruction may be physiological, with the patient unable to maintain and protect an adequate airway owing to a reduced conscious state. Mechanical obstruction may be due to pathology within the lumen (aspirated foreign body), in the wall (angioedema, tracheomalacia) or to extrinsic compression (Ludwig’s angina, haematoma, external burns). Obstruction may be due to a combination of physiological and mechanical causes. A summary of potential causes of upper airway obstruction is provided in Table 6.1.1. Despite the plethora of possible causes, the initial treatment to secure the airway is the same.
Investigation
Imaging
Neck X-rays
A lateral soft tissue X-ray of the neck is sometimes helpful once the patient has been stabilized. Metallic or bony foreign bodies, food boluses or soft tissue masses may be seen. A number of subtle radiological signs have been described in epiglottitis (Table 6.1.2).
| The ‘thumb’ sign | Oedema of the normally leaf-like epiglottis resulting in a round shadow resembling an adult thumb. The width of the epiglottis should be less than one-third the anteroposterior width of C4. |
| The vallecula sign | Progressive epiglottic oedema resulting in narrowing of the vallecula. This normally well-defined air pocket between the base of the tongue and the epiglottis may be partially or completely obliterated. |
| Swelling of the aryepiglottic folds | |
| Swelling of the arytenoids | |
| Prevertebral soft tissue swelling | The width of the prevertebral soft tissue should be less than half the anteroposterior width of C4. |
| Hypopharyngeal airway widening | The ratio of the width of the hypopharyngeal airway to the anteroposterior width of C4 should be less than 1.5. |
Computed tomography
In the patient with a mechanical obstruction a computed tomography (CT) scan of the neck and upper thorax may be helpful in diagnosing the cause of the obstruction as well as the extent of any local involvement. It may aid in planning further management, especially if surgical intervention is indicated, for example for a retrothyroid goitre or a head and neck neoplasm.
Trauma
Penetrating trauma
Clinical
Penetration of the airway should be suspected if there is difficulty breathing, a change in voice, pain on speaking, subcutaneous emphysema or bubbling from the wound. This is often associated with great vessel or pulmonary injuries, and the patient may require an emergency airway procedure. Uncontrolled haemorrhage is a potentially life-threatening condition. Other causes include head and neck malignancies eroding vascular structures, or following radiotherapy. Uncontrolled haemorrhage may lead to exsanguination as well as compromising the airway, and requires prompt surgical intervention.
Investigations
Endoscopy
Endoscopy includes both laryngoscopy and bronchoscopy, performed in the operating theatre, as urgent surgical intervention may be required. Fuhrman et al. (see Further reading) suggest a classification system for the severity of blunt upper airway injury based on endoscopic and radiological findings (Table 6.1.3).
| Grade | Endoscopic and radiological findings |
|---|---|
| I | Minor laryngeal haematoma without detectable fracture |
| II | Oedema, haematoma or minor mucosal disruption without exposed cartilage, or non-displaced fractures on CT |
| III | Massive oedema, tears, exposed cartilage, immobile cords |
Infections
Non-specific upper airway infections
Pharyngitis/tonsillitis
Sore throat is one of the top 10 presenting complaints to EDs in the USA. The differential diagnosis is large and includes a number of important conditions (Table 6.1.4). Pharyngitis has a wide range of causative bacterial and viral agents, most of which produce a self-limited infection with no significant sequelae. The major role for antibiotics in treating pharyngitis is for suspected group A β-haemolytic streptococcal infection (GABHS) or Streptococcus pyogenes. Timely use of appropriate antibiotics reduces the duration of symptoms by an average of 8 hours but increases the rate of adverse effects. Antibiotic use also reduces the incidence of suppurative complications such as otitis media, quinsy and retropharyngeal abscess. If given in the first 9 days they prevent the development of acute rheumatic fever. Antibiotics have not been shown to reduce the incidence of post-streptococcal glomerulonephritis, which is related to the subtype of streptococcus. Antibiotic therapy is also recommended for patients from the following groups: patients with scarlet fever, with known rheumatic heart disease, or from populations with high incidence of acute rheumatic fever, including some Aboriginal populations in Central and Northern Australia.
Table 6.1.4 Differential diagnosis of sore throat in the adult
| Infective pharyngitis |
Other abscesses
Parapharyngeal abscesses involve the lateral or pharyngomaxillary space. Presentation and treatment are similar to those of Ludwig’s angina, from which they may develop. As well as the complications of Ludwig’s angina, including airway obstruction and spread to contiguous areas, there is the added risk of internal jugular vein thrombosis and erosion of the carotid artery, which has a mortality of 20–40%.
Epiglottitis
Sore throat and odynophagia are the most common presenting symptoms. Drooling and stridor are infrequent. Factors shown to be associated with an increased risk of airway obstruction include stridor, dyspnoea, sitting upright, and short duration of symptoms. A number of X-ray changes have been described in epiglottitis, which are listed in Table 6.1.2. Management requires admission and i.v. ceftriaxone or cefotaxime. The role of steroids and nebulized or parenteral epinephrine (adrenaline) is controversial. Chloramphenicol may be used in patients with cephalosporin sensitivity. Most adults can be treated conservatively without the need for an artificial airway.
Alcaide ML, Bisno AL. Pharyngitis and epiglottitis. Infectious Disease Clinics of North America. 2007;21:449-469.
American Heart Association. Guidelines for cardiopulmonary resuscitation and emergency cardiac care. Circulation. 112, 2005. Supplement
Ames WA, Ward WMM, Tranter RMD, et al. Adult epiglottitis: an under-recognized, life-threatening condition. British Journal of Anaesthesia. 2000;85:795-797.
Atkins BZ, Abbate S, Fischer S, et al. Current management of laryngotracheal trauma: case report and literature review. Journal of Trauma. 2004;56:185-190.
Atkins BZ, Abbate S, Fischer S, et al. Current management of laryngotracheal trauma: case report and literature review. Journal of Trauma. 2004;56:185-190.
Bisno AL, Gerber MA, Gwaltney JM, et al. Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clinical Infectious Disease. 2002;35:113.
Centor RM, Witherspoon JM, Dalton HP, et al. The diagnosis of strep throat in adults in the emergency room. Medical Decision Making. 1981;1:239-246.
Cicala RS. The traumatized airway. In: Benumof JE, editor. Airway management: principles and practice. Mosby-Year Book: St Louis; 1996:736.
Cooper RJ, Hoffman JR, Bartlett JG, et al. Principles of appropriate antibiotic use for acute pharyngitis in adults: Background. Annals of Internal Medicine. 2001;134:509-517.
Del Mar CB, Glasziou PP, Spinks AB. Antibiotics for sore throat. Cochrane Database of Systematic Reviews. (4):2006. CD000023. DOI: 10.1002/14651858.CD000023.pub3. Accessed December 2007
Fuhrman GM, Stieg FH, Buerk CA. Blunt laryngeal trauma: Classification and management protocol. Journal of Trauma. 1990;30:87-92.
Frantz TD, Rasgon BM, Quesenberry CP. Acute epiglottitis in adults. Journal of the American Medical Association. 1994;272:1358-1360.
Gonzales R, Bartlett JG, Besseer RE, et al. Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults: Background, specific aims, and methods. Annals of Emergency Medicine. 2001;37:690-697.
Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of nonspecific upper respiratory tract infections in adults: Background. Annals of Emergency Medicine. 2001;37:698-702.
Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections and bronchitis by ambulatory care physicians. Journal of the American Medical Association. 1997;278:901-904.
Howes DS, Dowling PJ. Triage and initial evaluation of the oral facial emergency. Emergency Medicine Clinics of North America: Oral-Facial Emergencies. 2000;8:371-378.
Hurley MC, Heran MKS. Imaging studies for head and neck infections. Infectious Disease Clinics of North America. 2007;21:305-353.
Linder JA, Chan JC, Bates DW. Evaluation and treatment of pharyngitis in primary care practice: the difference between guidelines is largely academic. Archives of Internal Medicine. 2006;166:1374-1379.
McCollough M. Update on emerging infections from the centers for disease control and prevention: commentary. Annals of Emergency Medicine. 1999;34:110-111.
Minard G, Kodak KA, Croce MA, et al. Laryngotracheal trauma. American Surgeon. 1992;58:181-187.
Nemzek WR, Katzberg RW, Van Slyke MA, et al. A reappraisal of the radiologic findings of acute inflammation of the epiglottis and supraglottic structures in adults. American Journal of Neuro Radiology. 1995;16:495-502.
Richardson MA. Sore throat, tonsillitis, and adenoiditis. Medical Clinics of North America: Otolaryngology for the Internist. 1999;83:75-84.
Schamp S, Pokieser P, Danzer M, et al. Radiological findings in acute adult epiglottitis. European Radiology. 1999;9:1629-1631.
Scott PMJ, Loftus WK, Kew J, et al. Diagnosis of peritonsillar infections: a prospective study of ultrasound, computerized tomography and clinical diagnosis. Journal of Laryngology and Otology. 1999;113:229-232.
Stewart MH, Siff JE, Cydulka RK. Evaluation of the patient with sore throat, earache, and sinusitis: an evidence based approach. Emergency Medicine Clinics of North America: Evidence Based Emergency Medicine. 1999;17:153-188.
Steyer TE. Peritonsillar abscess: diagnosis and treatment. American Family Physician. 2002;65:93-96.
Thierbach AR, Lipp MDW. Airway management in trauma patients. Anesthesiology Clinics of North America. 1999;17:63-82.
Victorian Medical Postgraduate Foundation. Therapeutic Guidelines: Antibiotic Version 12, 2006.
6.2 Asthma
Pathophysiology
Pathophysiologically the effects of acute asthma are:
There is increasing evidence that there are different phenotypes of both acute and chronic asthma. For acute asthma, a rapid onset may be closer to anaphylaxis in pathology, with minimal inflammation, and may respond more quickly to treatment.
Clinical assessment
Examination
Physical findings vary with the severity of the attack and may range from mild wheeze and dyspnoea to respiratory failure. Findings indicative of more severe disease include an inability to speak normally, use of the accessory muscles of respiration, a quiet or silent chest on auscultation, restlessness or altered level of consciousness, and cyanosis. Clinical features are a good guide to the severity of attacks. Features of the major severity categories are summarized in Table 6.2.1. Pulsus paradoxus has been abandoned as an indicator of severity.
Table 6.2.1 Categorization of asthma severity based on clinical features. (Modified from Guidelines for Emergency Management of Adult Asthma, Canadian Association of Emergency Physicians, British Guideline on the Management of Asthma (SIGN) and Asthma Management handbook (NAC).)
| Severity category | Features | Respiratory function |
|---|---|---|
| Near death |
Management
Moderate asthma
Patients with moderate attacks may require oxygen therapy titrated to achieve oxygen saturation in excess of 92%. The mainstays of therapy are inhaled β-adrenergic agents (by MDI with a spacer or nebulizer) and systemic corticosteroids. The dosage of salbutamol is 5–10 mg by nebulizer or eight puffs by MDI and spacer, every 15 minutes for three doses. Corticosteroids are equally effective given by the oral or intravenous routes. The usual dose is 50 mg prednisolone orally or 250 mg hydrocortisone intravenously. Reassessment, including repeat pulmonary function tests, should occur at least 1 hour after the last dose of β-agonist, with a view to the need for further therapy and decision on hospital admission. For patients who are discharged, oral corticosteroids at a dose of 0.5–1 mg/kg/day, in addition to inhaled steroids at standard doses, should be continued for at least 5 days or until recovery.
Magnesium
The postulated mechanisms of action of magnesium in acute asthma are a bronchodilator effect by impeding the uptake of calcium ions into smooth muscle cells, and an anti-inflammatory effect by attenuation of the neutrophil respiratory burst associated with asthma. Magnesium can be administered i.v. or by nebulizer.
Beveridge RC, Grunfeld AF, Hodder RV. Guidelines for the emergency management of asthma in adults. CAEP/CTS Asthma Advisory Committee. Canadian Medical Association Journal. 1996;155:25-37.
Blitz M, Blitz S, Beasley R, et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database System Review. 19(4), Oct 2005. CD003898
Fernandez MM, Villagra A, Blanch L. Non-invasive mechanical ventilation in status asthmaticus. Intensive Care Medicine. 2001;27:486-492.
Haney S, Hancox RJ. Overcoming beta-agonist tolerance: high dose salbutamol and ipratropium bromide. Two randomized controlled trials. Respiratory Research. 2007;8:19.
Kelly AM, Kerr D, Powell CVE, et al. Is severity assessment after one hour of treatment better for predicting the need for admission in acute asthma? Respiratory Medicine. 2004;98:777-781.
Kelly HW. Levalbuterol for asthma: a better treatment? Current Allergy and Asthma Reports. 2007;7:310-314.
National Asthma Council Australia. Asthma Management Handbook. National Asthma Council Australia: Melbourne, 2006.
Parameswaran K, Belda J, Rowe BH. Addition of intravenous aminophylline to beta2-agonists in adults with acute asthma. Cochrane Database Systematic Reviews. (4):2000. CD 002742
Putland M, Kerr D, Kelly AM. Adverse events associated with the use of intravenous adrenaline in emergency department patients presenting with severe asthma. Annals of Emergency Medicine. 2006;47:559-563.
Rodrigo G, Pollack C, Rodrigo C, et al. Heliox for nonintubated acute asthma patients. Cochrane Database System Review. (4):October 2006. CD002884
Rodrigo G, Rodrigo C, Pollack C, et al. Helium–oxygen mixture for nonintubated acute asthma patients. Cochrane Database Systematic Reviews. (1):2001. CD002884
Scottish Intercollegiate Guidelines Network. British guidelines on the management of asthma, 4 July 2007. http://www.sign.ac.uk/guidelines/published/support/guideline63/download.html. Accessed
6.3 Community-acquired pneumonia
Pathogenesis and aetiology
Estimates of the rates of occurrence of various organisms implicated in CAP are difficult for several reasons. Isolation of a causative organism occurs in only around 70% of cases in hospital-based studies, less so in community-based ones, and much less commonly in actual clinical practice (particularly in CAP treated in the community). The most common organism isolated in all settings and in all classes of CAP, Streptococcus pneumoniae, is one of the easiest to isolate, whereas Chlamydophila pneumoniae and psitacii (formerly Chlamydia pneumoniae and psitaccii) and the Legionella species present much greater difficulty, potentially skewing the data in favour of pneumococcus. There is a great deal of heterogeneity in the pneumonia studies with regard to underlying patient characteristics, setting, case definition, degree of diagnostic investigation and timing with relation to epidemics, which further complicates interpretation of the data.
Streptococcus pneumoniae
Outbreaks occur in crowded institutions, but these make up a small percentage of cases.
Other important organisms
Non-typable Haemophilus influenzae is a rare cause of mild CAP and is uncommon in young patients. Although it is associated with exacerbations of chronic obstructive pulmonary disease (COPD), it is no more common as a cause of CAP in these patients than in the general population. It does, however, become more common with increasing severity of pneumonia and increasing age. Less than 25% of isolates are β-lactamase producing; others are susceptible to aminopenicillins (and somewhat less so to benzylpenicillin). Moraxella catarrhalis has similar antibiotic susceptibilities and is less common than Haemophilus. Second-generation cephalosporins, tetracyclines or the combination of amoxicillin and clavulanate is adequate if amoxicillin alone fails.
Clinical features
Pneumonia should be suspected in patients with:
A normal chest examination makes pneumonia less likely but does not rule it out. The classically described progression of chest examination findings is from crackles and reduced air entry in the first days, to a dull percussion note and bronchial breathing which persists until resolution begins at around day 7–10, when crackles return. Fever is said to be persistent until a ‘crisis’, followed by resolution. Of course the actual clinical reality may bear little resemblance to this. The presence of classic findings in the chest may precede radiological abnormality by several hours, particularly in pneumococcal pneumonia.
Much has been made of the role of the clinical syndrome as a predictor of aetiology, but the evidence shows it to be unreliable. Previously ‘typical’ and ‘atypical’ pneumonia were differentiated clinically, but there is now a general consensus that these terms should be abandoned as they are misleading. The term ‘atypical organism’, however, has persisted as an umbrella term for the Chlamydophila spp., the Legionella spp. and Mycoplasma. With these caveats in mind, there are certain associations that should be considered (Table 6.3.1).
Table 6.3.1 Clinical features associated with specific organisms
| Streptococcus pneumoniae |
Investigation
Imaging
Sputum
Microscopy (generally with Gram stain, although Zeil–Neilsen stain for acid-fast bacilli should be requested if tuberculosis is suspected) can potentially provide useful guidance for empiric prescribing as well as an indication of whether the specimen is of sufficient quality for culture to be useful.
Severity assessment
A key clinical problem in patients with suspected CAP is assessment of severity. The great majority of CAP cases are mild and self-limiting, although antibiotic treatment shortens the illness. However, a significant minority of cases cause an acutely debilitating illness, and a small minority are life-threatening. Attempts at formalizing the process of severity assessment have focused on two aims: identifying those who can safely be managed at home, thereby reducing unnecessary admissions and unplanned readmissions; and identifying those who are likely to need ICU care, with the aim of reducing mortality and complications from delayed recognition of severe disease while avoiding overuse of ICU. Those seeking the former goal have met with a great deal of success, but the latter has remained elusive. A number of scoring systems have been developed and validated, including the Pneumonia Severity Index (PSI), the British Thoracic Society’s BTS, modified BTS, CRB, CURB and CURB-65 scores, the American Thoracic Society’s ATS, modified ATS (m-ATS) and revised ATS (r-ATS) scores, and the Spanish SEPAR score.
Proven markers of severity
The following have all been defined as markers of severity in various studies:
The Pulmonary Severity Index (PSI)
The PSI was derived by retrospective chart review and validated both retrospectively and prospectively in separate groups of patients in the late 1980s and early 1990s. In all, over 54 000 patients and 275 hospitals from across the USA and Canada were involved in the study. The rule is a two-step process, with low-risk patients being identified on clinical grounds alone in the first step and then all other patients being further differentiated on the basis of age and 19 dichotomized and weighted clinical and investigation features into four further groups (Tables 6.3.2 and 6.3.3).
| Step 1 | |
|---|---|
| The patient is Class I and needs no further investigation if they are ≤50 years old and have none of the following: | |
| History | |
| Factor | Score |
|---|---|
| Demographic | |
| Coexisting illness | |
| Signs on examination | |
| Investigations | |
(After Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. New England Journal of Medicine 1997; 336: 243–250.)
Table 6.3.3 Mortality and PSI class
| Score | Class | 30-Day mortality (%) |
|---|---|---|
| N/A | I | 0.1 |
| 1–70 | II | 0.6 |
| 71–90 | III | 0.9 |
| 91–130 | IV | 9.3 |
| >130 | V | 27 |
The CURB-65 score
Scores are correlated with risk of death and site of care is suggested (Table 6.3.4).
| Score | Risk of death or ICU admission (%) | Comments |
|---|---|---|
| 0 | 0.7 | Low risk. Non-severe pneumonia. May be suitable for treatment at home |
| 1 | 3.2 | |
| 2 | 13 | Increased risk of death. Consider for short inpatient or hospital-supervised outpatient treatment |
| 3 | 17 | High risk of death. Treat as inpatients with severe pneumonia. Consider use of ICU |
| 4 | 41.5 | |
| 5 | 57 |
The CURB-65 score has the significant advantage of simplicity, and has been shown to perform as well as a previous two-stage BTS score. It is easy to remember and quick to calculate. It is worth noting, for community practitioners, that the CRB-65 score (CURB-65 without the urea measurement) performs similarly well, and that patients with a CRB-65 score of 0 are generally safely managed in the community whereas those with a score of 1 or more should be assessed at hospital. The CURB-65 score has been validated in thousands of patients from the UK and other countries, and is the result of a process of refinement of a series of other validated scoring systems.
Treatment
Site of care
More severe cases should be treated in hospital, with early referral to ICU if appropriate.
General supportive care
For the admitted patient similar measures will be required. Hydration may need to be supplemented with intravenous fluids, and in severe or prolonged illness nutritional support will be required. Oxygen should be provided to maintain SpO2>95% (PaO2>60 mmHg). A lower SpO2/PaO2 may be desirable in patients with severe COAD.
Mild pneumonia
Oral therapy is preferred in patients well enough to be treated at home who are able to tolerate oral medications and are likely to be compliant with a treatment regimen. Given the known spectrum of pathogens as described above, whether to use a β-lactam alone or in combination with dedicated ‘atypical cover’ is debated. A Cochrane Review has found no benefit in the addition of ‘atypical cover’, although most of the studies examined compared fluoroquinolone monotherapy to β-lactam monotherapy. In the UK, where the 4- yearly cycle of Mycoplasma epidemics is well described, amoxicillin as a single agent is recommended, with erythromycin as an alternative, if tolerable, unless a Mycoplasma outbreak is known to be occurring. In the USA the practice of using macrolide monotherapy is well established but it can fail against DRSP. Australian guidelines recommend monotherapy with amoxicillin unless there is specific concern about atypical organisms.
Likely developments over the next 5–10 years
Acknowledgements
The author thanks Professor RM Robins-Browne, Department of Immunology and Microbiology, University of Melbourne, for advice on microbiological detail.
Antibiotic Expert Group. Community-acquired pneumonia. Therapeutic Guidelines: antibiotic, 13th edn. Melbourne, Therapeutic Guidelines Ltd, 2006.
Dremsizov T, Clermont G, Kellum JA, et al. Severe sepsis in community-acquired pneumonia: when does it happen, and do systemic inflammatory response syndrome criteria help predict course? Chest. 2006;129:968-978.
Elliott JH, Anstey NM, Jacups SP, et al. Community-acquired pneumonia in northern Australia: low mortality in a tropical region using locally-developed treatment guidelines. International Journal of Infectious Diseases. 2005;9:15-20.
Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. New England Journal of Medicine. 1997;336:243-250.
Gottlieb T, Collignon P, Robson J. on behalf of the Australian Group for Antimicrobial Resitance (AGAR). Streptococcus pneumoniae, 2007.Survey 2005 Antibicrobial Susceptibility Report. Australian Group for Antibicrobial Resistance (AGAR), 2006. http://www.agargroup.org/surveys/spneumo%2005%20cdi%20report.pdf. Accessed July
Johnson PDR, Irving LB, Turnidge JD. Community-acquired pneumonia. Medical Journal of Australia. 2002;176:341-347.
Kennedy M, Bates DW, Wright SB, et al. Do emergency department blood cultures change practice in patients with pneumonia? Annals of Emergency Medicine. 2005;46:393-400.
Macfarlane JT, Boswell T, Douglas G, et al. BTS Guidelines for the Management of Community Acquired Pneumonia in Adults. Thorax. 56(Supplement 4), 2001.
Macfarlane JT, Boswell T, Douglas G, et al. BTS Guidelines for the Management of Community Acquired Pneumonia in Adults – 2004 Update. British Thoracic Society, 2007. http://www.britthoracic.org.uk/c2/uploads/macaprevisedapr04.pdf. Accessed July
Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clinical Infectious Diseases. 2007;44:S27-72.
Metersky ML, Ma A, Bratzler DW, et al. Predicting bacteremia in patients with community-acquired pneumonia. American Journal of Respiratory Critical Care Medicine. 2004;169:342-347.
Mylotte JM. Nursing home-acquired pneumonia. Clinical Infectious Disease. 2002;35:1205-1211.
Shefet D, Robenshtok E, Paul M. Empiric antibiotic coverage of atypical pathogens for community acquired pneumonia in hospitalized adults. Cochrane Database Systematic Review. 18(2), 2005. CD004418