CASE 6 High-Risk, Hemodynamically Supported PCI
Case presentation
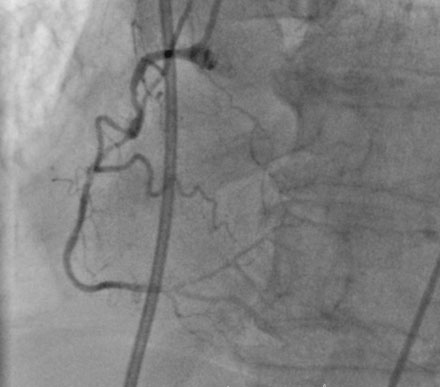
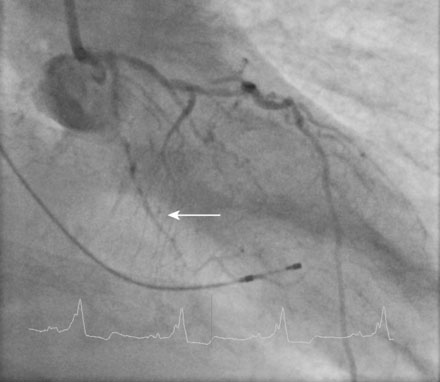
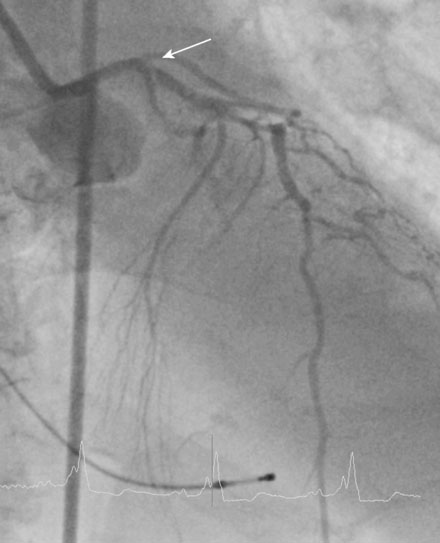
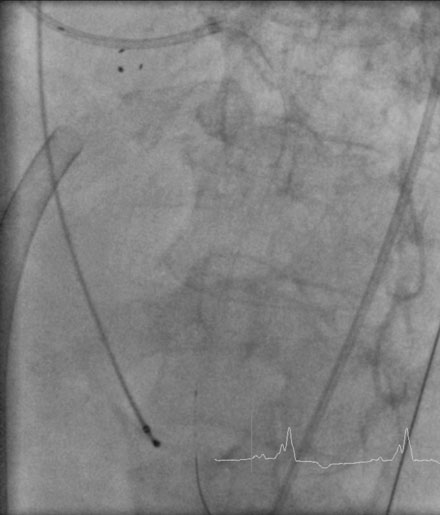
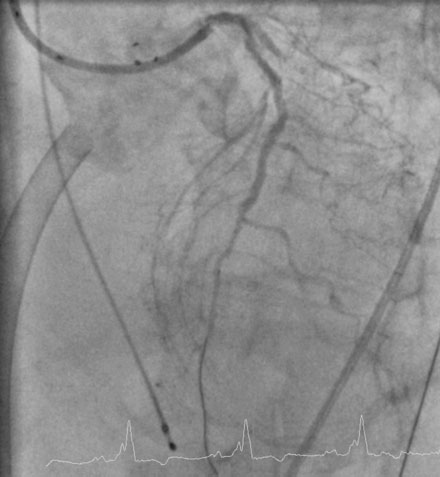
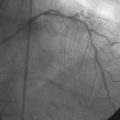
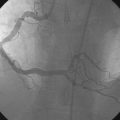
A fairly active 83-year-old man, with a past medical history notable for previous inferior infarction and ischemic cardiomyopathy, presented with a non-ST elevation myocardial infarction and congestive heart failure. His presenting electrocardiogram revealed an old left bundle branch block. Pertinent laboratory values revealed a brain natriuretic peptide (BNP) of 1473 pg/mL and a peak troponin of 2.28 ng/mL. Cardiac catheterization was performed during his initial hospitalization and revealed a severe mid-right coronary artery stenosis in a diffusely diseased, heavily calcified artery (Figure 6-1 and Video 6-1). The left coronary system was also heavily calcified and demonstrated a moderate-to-severe left main stenosis; a completely occluded circumflex with left to left collateralization (Figure 6-2 and Video 6-2); and a nearly occluded, severely diseased, ostial left anterior descending artery (LAD) (Figure 6-3 and Video 6-3). The LAD also provided collateral flow to the distal RCA territory. A cardiac MRI was subsequently performed to evaluate left ventricular function and myocardial viability. This demonstrated severely reduced left ventricular function (ejection fraction of 12.7%) and viability in the LAD territory. Transmural infarct was present in the inferolateral wall, representing the myocardium supplied by the right coronary and circumflex arteries. He was also noted to have a left ventricular thrombus and a 6 by 9 mm filling defect in the descending thoracic aorta that likely represented atheroma. Based on these findings, he was denied surgical revascularization. Thus, he was managed medically and was discharged on optimal medical therapy including a beta blocker, an ACE inhibitor, a nitrate, a diuretic, aspirin, clopidogrel, and a statin. However, within a 2-week period following discharge, he had two separate admissions for rest chest pain, heart failure, and recurrent non-ST elevation myocardial infarctions. Following the second admission, the decision was made to attempt high-risk percutaneous revascularization of the LAD.
Cardiac catheterization
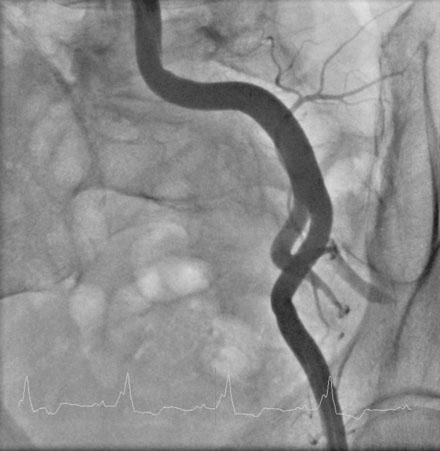
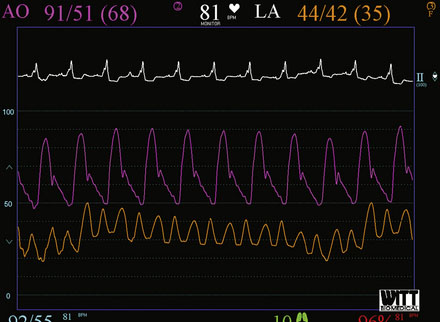
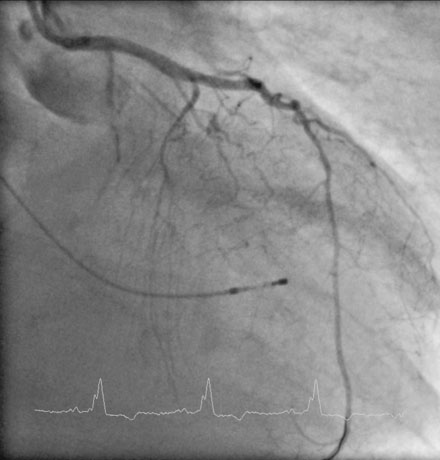
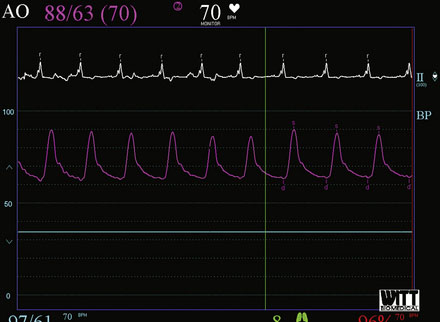
Repeat angiography at the time of intervention was unchanged. Severe three-vessel disease with left main involvement, heavily calcified coronary arteries, significant collateralization of the RCA from the LAD, and a severely reduced ejection fraction, together placed the patient at significantly high risk for percutaneous intervention. Thus, the operator chose to employ a TandemHeart for hemodynamic support during the procedure. Prior to insertion, the left internal iliac was imaged to assure that the artery was of proper size and was without significant disease burden, to allow passage of the large arterial cannula required for the procedure (Figure 6-4 and Video 6-4). The artery measured 7.7 mm in minimum diameter and thus the operator inserted a 17 French cannula in the left external iliac percutaneously through the left common femoral artery. A transseptal puncture was performed; baseline hemodynamics showed a marked elevation of the left atrial pressure and preserved arterial pressure (Figure 6-5). The transseptal sheath was exchanged for a 21 French cannula placed via the right femoral vein into the left atrium (Figure 6-6). Unfractionated heparin was used for anticoagulation following the transseptal puncture and ACT was maintained greater than 350 seconds. The arterial and venous cannulae were attached to the extracorporeal centrifugal pump and the system was purged of air. The pump was initiated and maintained at 6500 rpm with resultant flows of 4.5 L/minute. An 8 French sheath was then placed in the right femoral artery, through which the coronary intervention was performed. A temporary pacemaker was first placed prophylactically via the left femoral vein. Because of the heavy calcification of the proximal LAD, the lesion was debulked using rotational atherectomy with a 1.25 mm burr followed by a 1.5 mm burr. Sequential balloon dilation was performed following successful atherectomy with a 1.5 mm diameter by 15 mm long, a 2.0 mm diameter by 20 mm long, and a 2.5 mm diameter by 20 mm long series of compliant balloons. There was complete inflation of the 2.5 mm balloon along the entire course of the LAD that would require stenting, and the postballoon result appeared suitable for stenting (Figure 6-7 and Video 6-5). Subsequently, a 2.5 mm diameter by 28 mm long everolimus-eluting stent was deployed in the LAD and a 2.75 mm diameter by 23 mm long everolimus-eluting stent deployed in the left main. The operator postdilated the left main and LAD stents with a 3.0 mm diameter by 15 mm long semicompliant balloon. The final angiogram demonstrated excellent stent apposition and a widely patent lumen (Figure 6-8, Videos 6-6 and 6-7). Importantly, the patient maintained a normal mean arterial blood pressure of 70 mmHg during the entire procedure while on hemodynamic support (Figure 6-9). The TandemHeart was weaned and removed immediately following the procedure, with hemostasis achieved by manual compression.
Discussion
The intraaortic balloon pump (IABP) is inserted percutaneously via the femoral artery and advanced in the aorta to the level of the tracheal bifurcation. Most devices require an 8 or 9 French sheath, but can be placed sheathless if a smaller arteriotomy is desired. Balloon volumes range from 30 to 50 cc (average is 40 cc) and are chosen depending on the size of the patient. The balloon inflates during diastole and deflates during systole, augmenting diastolic pressures and reducing afterload. Hemodynamic effects of the IABP include mild increases in cardiac output and stroke volume, decreases in aortic systolic pressure, increases in aortic diastolic and mean pressures, and increases in coronary flow. While the IABP is a useful adjunct to PCI, it is dependent on a fairly stable intrinsic rhythm and cardiac output. It provides the least hemodynamic support of the devices available. Use is contraindicated in the presence of significant aortic regurgitation, aortic dissection, right to left shunts, and severe peripheral vascular disease or tortuosity.1
The Impella assist device is inserted percutaneously via the femoral artery into the left ventricle, much in the same way that a left ventricular pigtail catheter is placed. A 13 French sheath requires at least a 5 mm diameter iliofemoral arterial system. Iliac imaging is strongly recommended prior to placement of the device to assure proper vessel size and absence of significant tortuosity. The device functions by removing blood directly from the left ventricle and delivering it to the aorta at the level of the coronary arteries. The Impella provides 2.5 L/min of flow, thus augmenting cardiac output but not providing full support. Hemodynamic effects include direct ventricular unloading, decreased myocardial work and wall tension, increased cardiac output and mean arterial pressure, and increased coronary flow. A 5.0 L/min device, which can provide full left ventricular support, is available, but must be inserted surgically directly into the aorta. The Impella device is contraindicated in patients with severe peripheral vascular disease or tortuosity, mechanical prosthetic aortic valves, significant aortic stenosis, or in the presence of left ventricular thrombus.2
The TandemHeart requires percutaneous placement of a 15 to 17 Fr arterial sheath and a transseptally placed 21 Fr venous sheath. The arterial sheath is placed via the femoral artery and advanced into the iliac artery where retrograde arterial flow is delivered. The iliofemoral system should measure at least 6 mm for safe insertion. Due to the large arterial cannula size, iliofemoral imaging is mandatory prior to placement of the device to assure proper vessel size and absence of significant tortuosity or disease. Oxygenated blood is withdrawn from the left atrium via the 21 French cannula placed transseptally via the right femoral vein, and delivered in a retrograde fashion to the artery cannula via a rotary pump. The TandemHeart functions as a percutaneous left ventricular assist device and can provide full cardiac support, up to 4.5 L/min. It is useful for support of high-risk PCI when hemodynamic collapse is likely, such as left main PCI or PCI of a main artery in the setting of severe left ventricular dysfunction. The TandemHeart requires full systemic anticoagulation similar to the extent needed for cardiopulmonary bypass. Hemodynamic effects include increases in cardiac output and mean arterial pressure and decreases in pulmonary capillary wedge pressure. It is contraindicated in the presence of severe peripheral vascular disease, an inferior vena cava filter, left atrial thrombus, and in cases where anticoagulation is contraindicated or transseptal puncture cannot be performed safely.3
In the setting of high-risk PCI:
1 Brodie B.R., Stuckey T.D., Hansen C., Muncy D. Intra-aortic balloon counterpulsation before primary percutaneous transluminal coronary angioplasty reduces catheterization laboratory events in high-risk patients with acute myocardial infarction. Am J Cardiol. 1999;84:18-23.
2 Dixon S.R., Henriques J.P.S., Mauri L., Sjauw K., Civitello A., Kar B., Loyalka P., Resnic F.S., Teirstein P., Makkar R., Palacios I.F., Collins M., Moses J., Benali K., O’Neill W.W. A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (PROTECT I). JACC Cardiovasc Interv. 2009;2(2):91-96.
3 Aragon J., Lee M.S., Kar S., Makkar R.R. Percutaneous left ventricular assist device: “TandemHeart” for high risk coronary intervention. Catheter Cardiovasc Interv. 2005;65(3):346-352.