CASE 59 Stenosis in a Superficial Femoral Artery
Case presentation
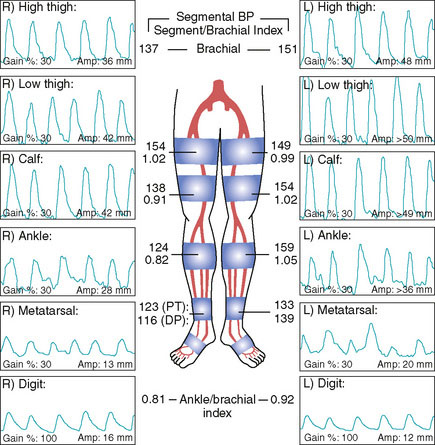
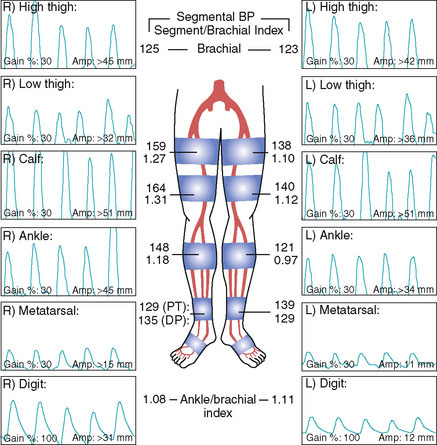
A 74-year-old diabetic man with hypertension and prior tobacco use, whose medications included aspirin, a statin, and an ACE inhibitor, developed bilateral calf claudication. Eventually, his leg symptoms progressed and he was unable to exercise, greatly limiting his lifestyle. His physician detected diminished pulses distally and assessed these symptoms with noninvasive studies (Figure 59-1). These demonstrated moderate bilateral arterial insufficiency at the level of the superficial femoral artery (SFA). He was referred for angiography.
Angiography
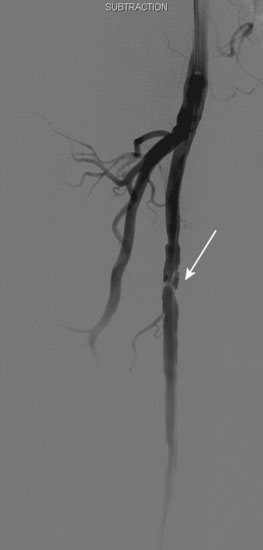
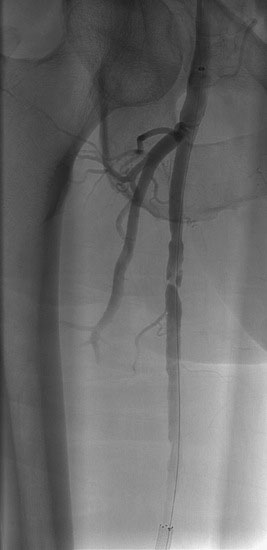
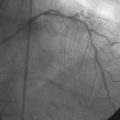
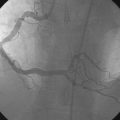
The patient’s symptoms were worse in the left leg; thus angiography of the left leg was performed first from an arterial sheath placed in the right femoral artery. It showed high-grade, sequential lesions in the mid-SFA (not shown). These were successfully treated with a self-expanding stent. Following that procedure, diagnostic angiography of the right leg was performed and demonstrated two high-grade lesions in the SFA with an ulcerated stenosis of the proximal segment of the right SFA (Figures 59-2, 59-3) and a longer, severe stenosis in the mid- to distal segment (Figure 59-4). The patient was discharged after this procedure on the left leg and experienced resolution of his left leg claudication with normalization of his left leg noninvasive studies. However, he continued to be limited by right leg claudication. Therefore, approximately 3 months after his left leg intervention, he presented for endovascular treatment of the right leg.
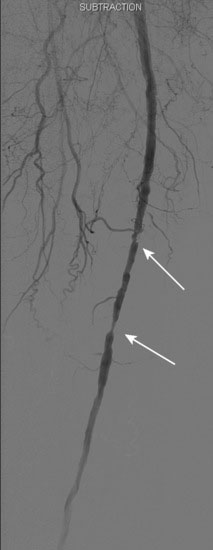
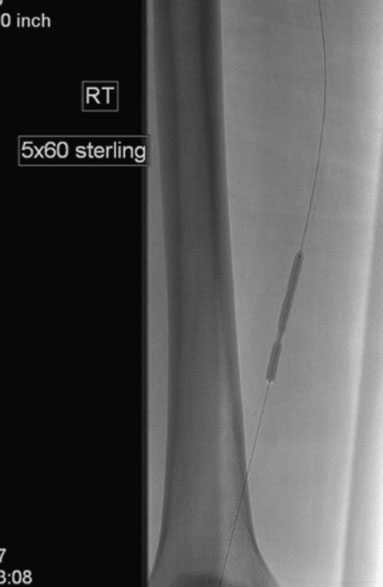
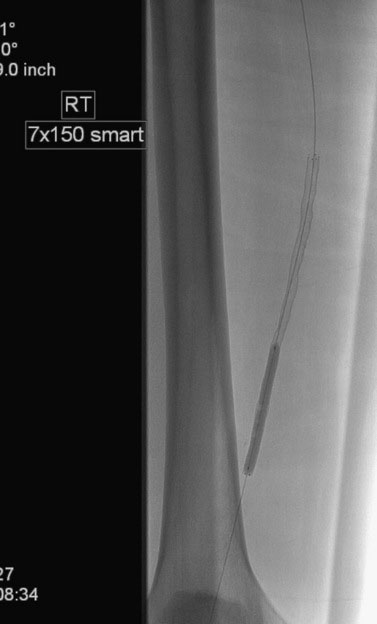
Access was obtained from the left common femoral artery. An Omniflush catheter was used to lay out the iliac vessels, and a 6 French long sheath was placed “up and over” from the left femoral to the right external iliac artery. An intravenous bolus of unfractionated heparin was administered to achieve a therapeutic activated clotting time of more than 250 seconds. The proximal and distal lesions were crossed with a hydrophilic glide wire. A catheter was then used to exchange the glide wire for a 0.018 inch guidewire. The distal SFA was treated first using a 5 mm diameter by 60 mm long balloon in two overlapping locations. The distal lesion was found to be difficult to expand (Figure 59-5); ultimately, the balloon appeared fully expanded at 12 atmospheres of pressure. A post balloon angiogram showed residual stenosis and elastic recoil; thus the operator deployed a 7 mm diameter by 150 mm long self-expanding nitinol stent across the extent of the distal lesions. The stent was postdilated with the 5 mm diameter by 60 mm long balloon (Figure 59-6) and the post-stent angiogram demonstrated an excellent result (Figure 59-7).
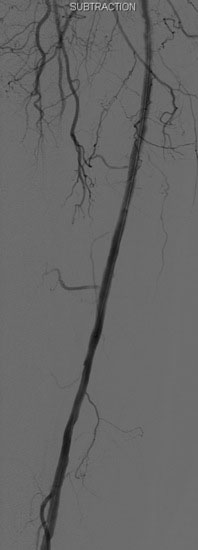
FIGURE 59-7 Angiographic result of the mid- to distal right SFA lesions after angioplasty and stenting.
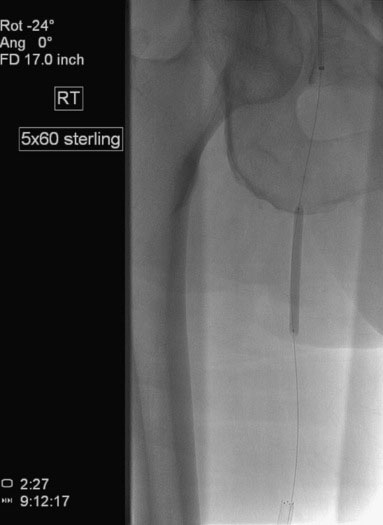
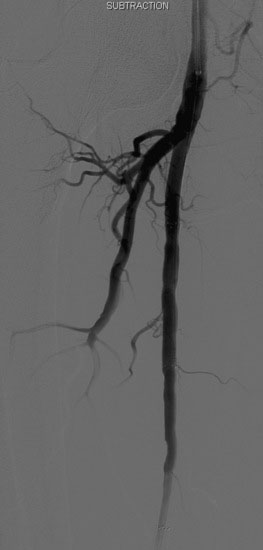
The proximal stenosis was then dilated with the 5 mm diameter by 60 mm long balloon (Figure 59-8). This disrupted the plaque, but a significant stenosis remained; therefore the operator placed a 7 mm diameter by 60 mm long self-expanding nitinol stent, postdilating it with the 5 mm diameter balloon used earlier. The final angiogram found no residual stenosis or angiographic complication (Figure 59-9).
Postprocedural course
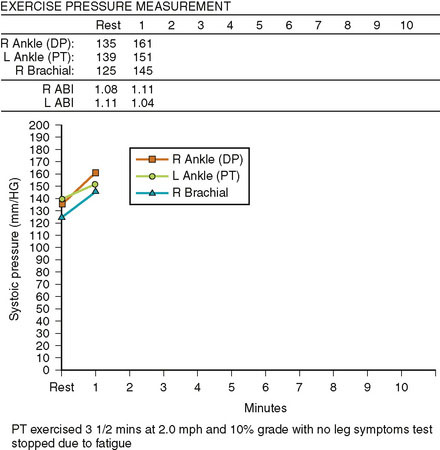
Following this procedure, the 6 French sheath was left in place until the activated clotting time fell below 180 seconds. Hemostasis was achieved with manual pressure followed by 4 hours of bed rest. He was discharged later that same day on aspirin and clopidogrel. At follow-up 1 month later, he had resolution of his symptoms and normalization of his noninvasive tests both at rest and after exercise (Figures 59-10, 59-11).
Discussion
Disease of the superficial femoral artery is present in 80% to 90% of patients with claudication.1 At the time of angiography, this vessel is often found to be completely occluded, with collateralization and reconstitution of the distal vessel from the profunda femoris. In fact, the profunda femoris is considered to be the “lifeline” to the lower leg, as the collaterals it provides are usually adequate to avoid limb-threatening ischemia. They are usually not adequate, however, to prevent claudication.
As with aortoiliac disease, the TASC working group has created a classification system for femoral/popliteal disease (Table 59-1).2 Again, similar to aortoiliac disease, endovascular treatment is preferred for TASC A and B lesions and surgery is preferred for TASC D lesions. TASC C lesions are usually treated surgically unless the patient is a poor surgical candidate, although endovascular approaches are reasonable in some patients as long as the intervention does not “burn any bridges” regarding future surgical options. The lesions presented in this case would be considered TASC B as there were multiple SFA lesions which individually were less than 5 cm each (the distal SFA lesion was actually two lesions that were treated as one).
| Classification | Characteristics |
|---|---|
| Type A |
1 Creager M.A., Loscalzo J. Vascular Diseases of the Extremities. In: Fauci A.S., Braunwald E., Kasper D.L., Hauser S.L., Longo D.L., Jameson J.L., Loscalzo J., editors. Harrison’s Principles of Internal Medicine. ed 17. New York, NY: McGraw-Hill; 2008:1568-1570.
2 Norgren L., Hiatt W.R., Dormandy J.A., Nehler M.R., Harris K.A., Fowkes F.G.R., TASC II Working Group. Inter-Society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33:S1-S70. on behalf of the