CASE 57 Percutaneous Mitral Valve Repair
Cardiac catheterization
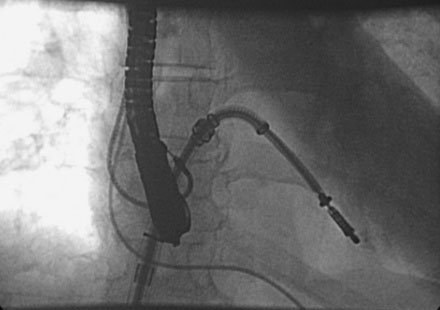
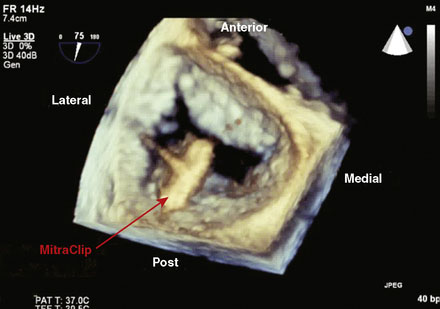
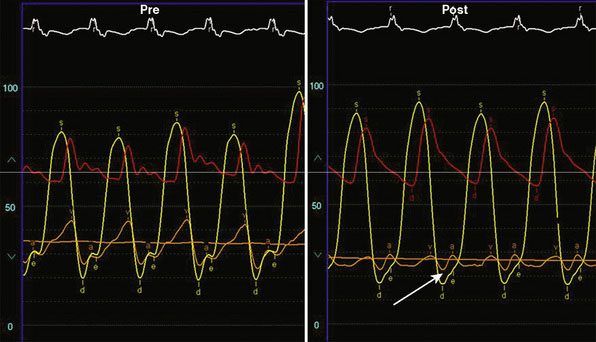
Following transseptal puncture, she received 4000 U of unfractionated heparin and maintained an activated clotting time (ACT) over 250 seconds. The transseptal sheath was exchanged for a 24 French MitraClip steerable guide. Through this guide, the MitraClip was advanced through the left atrium and oriented to the mitral valve using transesophageal echo guidance. Once the device was in the left ventricle, the clip was opened (Figure 57-1 and Video 57-1). Careful adjustments were made using echo guidance in order to position the clip at the site of maximum regurgitation. The device was gently pulled back and the leaflets were grabbed (Videos 57-2, 57-3). After the leaflets were grabbed and the clip closed (Figures 57-2, 57-3), the degree of mitral regurgitation was assessed and found to be satisfactorily reduced (Video 57-4). At this point, the clip was released. There was only minimal, 1+ residual regurgitation. Hemodynamics were reassessed. There was no diastolic pressure gradient between the left atrium and left ventricle (Figure 57-4). The guide was withdrawn to the right atrium and exchanged for a 16 French short sheath. Right heart pressures were repeated and showed an increase in cardiac output to 8.0 L/minute with a significant decrease in left atrial pressure. The sheaths were removed with manual pressure. No contrast was used, and fluoroscopy time was 18.6 minutes.
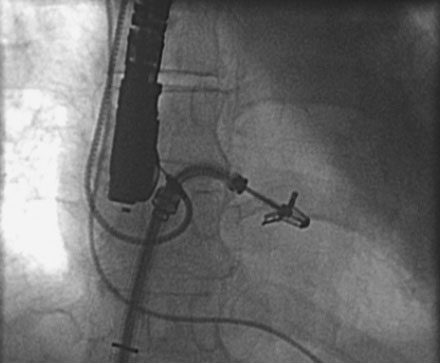
FIGURE 57-1 Via a transseptal approach, the MitraClip is positioned across the mitral valve and the clip opened.
Discussion
Mitral valve surgery is effective but carries significant morbidity. Many patients are not operative candidates or are at high risk from surgery. Quite recently, a novel percutaneous approach was has been developed for treatment of patients with nonrheumatic mitral regurgitation that involves the placement of a metal clip (MitraClip, Evalve, Menlo Park, CA) on the regurgitant portions of the mitral valve.1,2 This approach was derived from a known surgical approach of edge-to-edge leaflet repair as described by Alfieri.3,4 The MitraClip is introduced from a percutaneous, transvenous, transseptal approach to the mitral valve. The traditional imaging modality in the catheterization laboratory of fluoroscopy is of limited utility in this procedure, as it cannot visualize the mitral leaflets. Therefore, the procedure is guided by simultaneous transesophageal imaging, using both two- and three-dimensional echocardiography (see Figure 57-3). Therefore, it is important that the interventionalist be skilled in transesophageal imaging to complement his or her catheterization skills.
By placing the MitraClip on the central portions of the anterior and posterior leaflets, it acts to anchor prolapsing or flail segments, as well as to coapt tethered leaflets, so that it reduces the time and force required to close the valve. By decreasing mitral regurgitation, the left ventricular volumes are reduced, leading to beneficial effects on left ventricular remodeling.5 Anatomically, the MitraClip creates a tissue bridge between the two leaflets, which limits dilation of the mitral annulus in the septal-lateral dimension, and supports the durability of this repair.2
This novel approach to percutaneous mitral valve repair was evaluated in the EVEREST trials. EVEREST I was the safety and feasibility registry, which enrolled 55 patients in a nonrandomized clinical trial.6 EVEREST II was the pivotal clinical trial in which 279 patients with 3+ or 4+ mitral regurgitation were randomized to either the MitraClip therapy or standard surgical therapy (either mitral valve repair or replacement). It is important to note that this involved low- and moderate-risk patients, in comparison to the EVEREST High-Risk Registry, which enrolled 79 patients who were not operative candidates to the MitraClip therapy. In the initial nonrandomized experience with the MitraClip, procedural success (reduction of mitral regurgitation to 2+ or less) was achieved in 74%, and 66% were alive and without having mitral valve surgery or MR >2+ at 12 months. It is also important to note that this represents the initial experience with this novel technology, which has a steep learning curve. Ongoing follow-up from the randomized trial continues, but the initial data appear promising.
A separate registry was created for patients with severe mitral regurgitation from either degenerative (prolapse or flail) or functional etiologies (mitral regurgitation secondary to ischemic or nonischemic cardiomyopathy) who were not operative candidates – the EVEREST High-Risk Registry. This registry rapidly enrolled 79 patients, as there was significant support for it from surgical colleagues. Data presented showed that while at 30 days the predicted mortality for the group was 18.2%, the actual mortality was 7.7%, with 76% survival at 1 year and 79% of the survivors being in the New York Heart Association symptom class I or II.7 These data give support to the concept that particularly for these nonoperative patients, novel percutaneous options are attractive.
1 Fann J.I., St Goar F.G., Komtebedde J., Oz M.C., Block P.C., Foster E., Butany J., Feldman T., Burdon T.A. Beating heart catheter-based edge-to-edge mitral valve procedure in a porcine model: efficacy and healing response. Circulation. 2004;110(8):988-993.
2 St Goar F.G., Fann J.I., Komtebedde J., Foster E., Oz M.C., Fogarty T.J., Feldman T., Block P.C. Endovascular edge-to-edge mitral valve repair: short-term results in a porcine model. Circulation. 2003;108(16):1990-1993.
3 Alfieri O., Maisano F., De Bonis M., Stefano P.L., Torracca L., Oppizzi M., La Canna G. The double-orifice technique in mitral valve repair: a simple solution for complex problems. J Thorac Cardiovasc Surg. 2001;122(4):674-681.
4 Maisano F., Torracca L., Oppizzi M., Stefano P.L., D’Addario G., La Canna G., Zogno M., Alfieri O. The edge-to-edge technique: a simplified method to correct mitral insufficiency. Eur J Cardiothorac Surg. 1998;13(3):240-245. discussion 245–246
5 Herrmann H.C., Kar S., Siegel R., Fail P., Loghin C., Lim S., Hahn R., Rogers J.H., Bommer W.J., Wang A., et al. Effect of percutaneous mitral repair with the MitraClip device on mitral valve area and gradient. EuroIntervention. 2009;4(4):437-442.
6 Feldman T., Kar S., Rinaldi M., Fail P., Hermiller J., Smalling R., Whitlow P.L., Gray W., Low R., Herrmann H.C., et al. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge Repair Study) cohort. J Am Coll Cardiol. 2009;54(8):686-694.
7 Whitlow P.L., Kar S., Pederson W., Lim S., Foster E., Glower D., Feldman T. MitraClip therapy in the EVEREST High Risk Registry: One Year Results. EuroIntervention. 2008;4:437-442.