CASE 56 Mitral Balloon Valvuloplasty
Case presentation
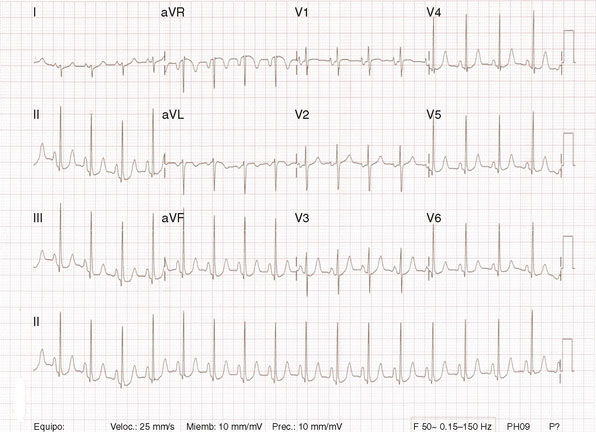
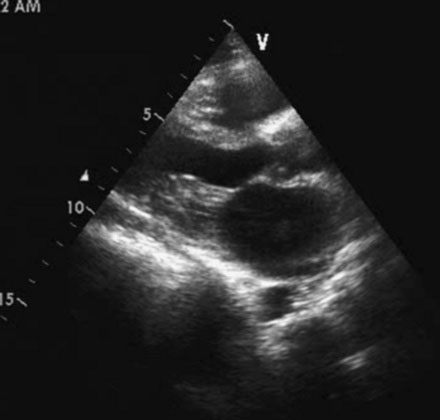
Several members of the Cardiovascular Division of the University of Virginia visit a medical clinic in Santo Domingo, Dominican Republic each year to provide tertiary care services for indigent patients with rheumatic heart disease. During one of these excursions, a 33-year-old Dominican woman presented with a 1-year history of worsening dyspnea on exertion. She reported fatigue and severe dyspnea with mild exertion but denied orthopnea or paroxysmal nocturnal dyspnea. She had been treated with a diuretic and aspirin with minimal improvement. On physical examination, she appeared healthy with a blood pressure of 110/80 mmHg and a regular pulse at 72 bpm. Her jugular veins were normal and lung fields clear. Cardiac exam revealed a loud first sound, an opening snap, and a diastolic rumble consistent with mitral stenosis without evidence of regurgitation. Her chest x-ray revealed left atrial enlargement, and a 12-lead electrocardiogram showed sinus rhythm with a right axis, evidence of right ventricular hypertrophy, and atrial enlargement (Figure 56-1). Her echocardiogram (Figure 56-2) confirmed the presence of rheumatic mitral stenosis with left atrial enlargement and a mean gradient by Doppler of 5 to 8 mmHg with only trace mitral regurgitation. The Wilkins score calculated to 8, based on the following characteristics: leaflets were severely restricted at the tips with the base showing normal mobility (score of 2); leaflet thickening appeared primarily at the margins (score of 2); there was only minimal leaflet calcification (score of 2); and there appeared to be modest thickening of the subvalvular apparatus (score of 2).
Cardiac catheterization
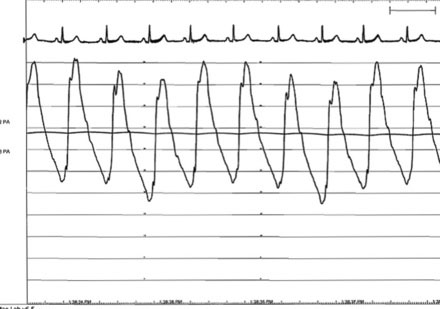
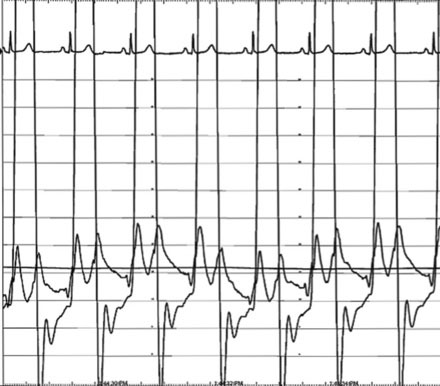
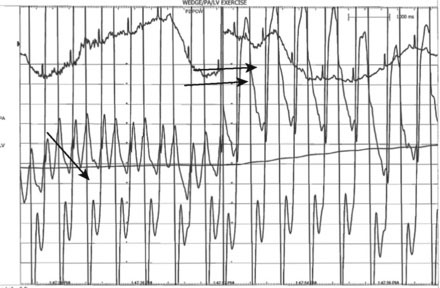
The pulmonary artery pressure measured 50/22 mmHg (Figure 56-3) and the baseline cardiac output measured 5.3 L/min. Simultaneous left ventricular and pulmonary capillary wedge pressures are shown in Figure 56-4. This confirmed a mean transmitral gradient of about 5 mmHg, and the valve area calculated to 1.4 cm2. Her symptoms appeared to be out of proportion to these findings and therefore, while on the cardiac catheterization laboratory table, exercise was performed by having the patient repetitively raise and lower a one liter bag of saline in each arm. The hemodynamic measurements were repeated after just a few minutes of exercise and showed a marked increase in pulmonary artery pressure (systolic pressure >65 mmHg) and an increase in the transmitral gradient to more than 10 mmHg (Figure 56-5). Based on the hemodynamic data, the patient’s symptoms, and the favorable echocardiographic findings, the operator proceeded with mitral balloon valvuloplasty.
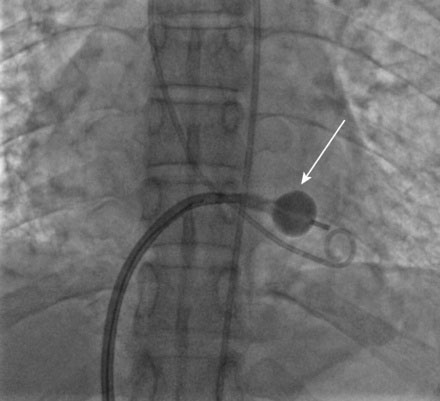
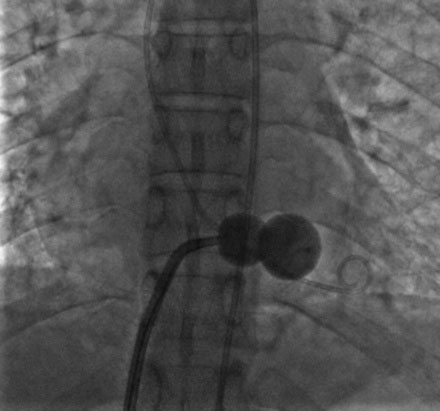
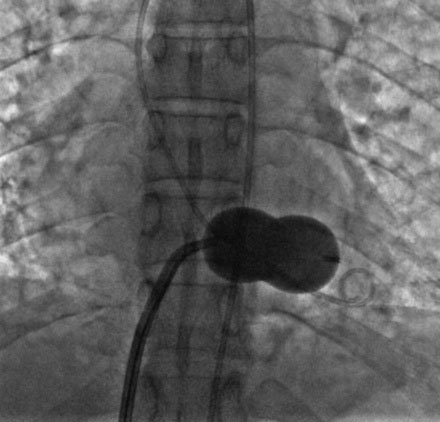
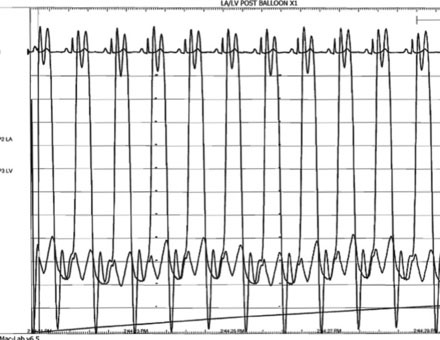
A transseptal puncture was accomplished and a transseptal sheath placed in the left atrium. Based on the patient’s height (142 cm), a 26 mm Inoue balloon was prepared and calibrated to inflate first to 24 mm. The Inoue guidewire was advanced into the left atrium and the atrial septum dilated with a 14 French dilator to accommodate the Inoue balloon. The balloon catheter was then advanced over the wire into the left atrium and the guidewire replaced with a steering wire and advanced to the tip of the balloon. The tip of the balloon was steered across the mitral valve and into the left ventricle. The operator began balloon inflation, first inflating the left ventricular side. The balloon catheter was then pulled back snugly against the valve (Figure 56-6). With continued inflation, the left atrial side inflated (Figure 56-7) and then the central portion of the balloon expanded, resulting in dilatation of the mitral annulus and valve (Figure 56-8 and Video 56-1). The steering wire was removed and simultaneous left ventricular and left atrial pressures were measured, with no significant residual transmitral gradient found (Figure 56-9). An echocardiogram found no mitral regurgitation; this was confirmed by ventriculography (Video 56-2).
Discussion
Medical therapy of rheumatic mitral stenosis includes antibiotic prophylaxis, beta-blockers to allow increased diastolic filling time, diuretics, and warfarin for thromboembolic protection in patients with atrial fibrillation.1 These agents are useful during the long asymptomatic or minimally symptomatic phase of the disease. Exertional symptoms are often present in patients with valve areas less than 1.6 cm2 and in patients that develop pulmonary hypertension. Patients with severe mitral stenosis (valve areas less than 1.2 cm2) usually are significantly impaired by the condition and typically require relief of valve obstruction either surgically or by balloon valvuloplasty. Both open commissurotomy and mitral valve replacement are effective surgical options and percutaneous mitral balloon valvuloplasty appears as effective as open commissurotomy in patients with appropriate valvular anatomy.2
The early generation techniques for mitral balloon valvuloplasty relying on a single or double balloon were less effective and associated with a higher complication rate, including acute mitral regurgitation or cardiac perforation from balloon slippage. The Inoue balloon, used in this case, is characterized by differential expansion and allows the balloon to inflate in a more stable and predictable manner.2 This device has improved the efficacy and safety of this procedure and is currently the most commonly used device for percutaneous mitral valvuloplasty.
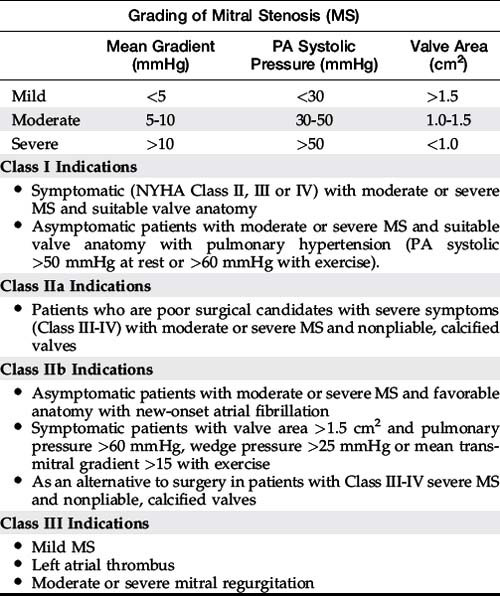
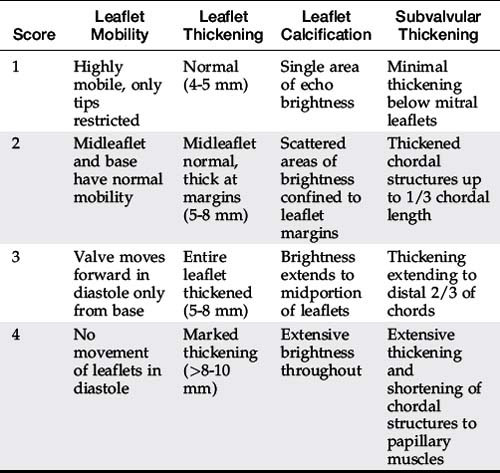
Indications for mitral balloon valvuloplasty are listed in Table 56-1.3 The pathology of the mitral valve as seen by echocardiography is an important predictor of success.4,5 The Wilkins score takes into consideration the important pathologic features predicting success (Table 56-2). A score of 1 to 4, ranging from mild to severe abnormalities, is used to describe four variables: leaflet mobility, leaflet thickening, leaflet calcification, and subvalvular thickening. A Wilkins score less than 8 is favorable for mitral balloon valvuloplasty.4,5 Although a score over 8 is associated with inadequate hemodynamic results and a higher complication rate, patients with significant symptoms who are poor operative candidates may still derive benefit from mitral valvuloplasty.
The long-term efficacy of mitral balloon valvuloplasty is excellent. Young patients with favorable anatomy do better than older patients with more calcified valves. Overall, between 60% and 90% of patients are alive and free of symptoms with no further intervention on the mitral valve 5 to 10 years after the procedure.2 In a large cohort of patients treated in the U.S. between 1986 and 2000, 82% of patients with a Wilkins score of less than 8 were alive at 12-year follow-up.5 Balloon valvuloplasty can be repeated in patients who have had prior balloon valvuloplasty (i.e., mitral valve restenosis) and in patients who have had prior open commisurotomy, although the results are less favorable as compared to an initial procedure.2
1 Carabello B.A. Modern management of mitral stenosis. Circulation. 2005;112:432-437.
2 Nobuyoshi M., Arita T., Shirai S., Hamasaki N., Yokoi H., Iwabuchi M., Yasumoto H., Nosaka H. Percutaneous balloon mitral valvuloplasty. A review. Circulation. 2009;119:e211-e219.
3 Bonow R.O., Carabello B.A., Chatterjee K., et al. ACC/AHA 2006 practice guidelines for the management of patients with valvular heart disease: Executive summary. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease). J Am Coll Cardiol. 2006;48:598-675.
4 Wilkins G.T., Weyman A.E., Abascal V.M., Block P.C., Palacios I.F. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J. 1988;60:299-308.
5 Palacios I.F., Sanchez P.L., Harrell L.C., Weyman A.E., Block P.C. Which patients benefit from percutaneous mitral balloon valvuloplasty? Prevalvuloplasty and postvalvuloplasty variables that predict long-term outcome. Circulation. 2002;105:1465-1471.