CASE 53 Renal Artery Stenosis
Catheterization
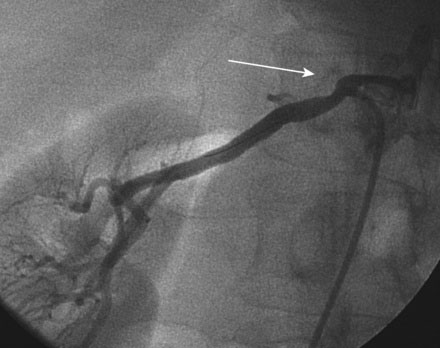
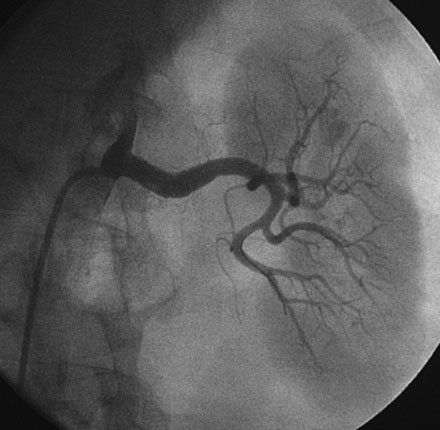
Selective right and left renal angiography was performed using a 6 French, 55 cm long internal mammary guide catheter. Selective right renal angiography uncovered a nonsignificant stenosis of the ostium (Figure 53-1 and Video 53-1). Left renal angiography confirmed the findings on MRA with a very severe eccentric stenosis of the proximal segment of the left renal artery apparent (Figure 53-2 and Video 53-2).
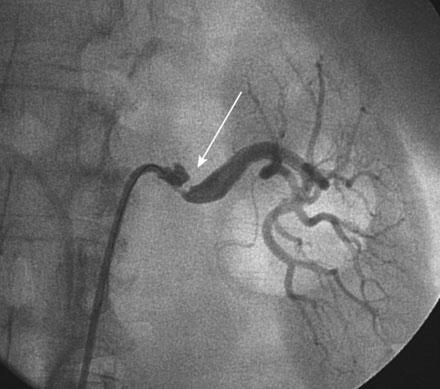
FIGURE 53-2 Selective left renal angiogram revealing a severe stenosis in the left renal artery (arrow).
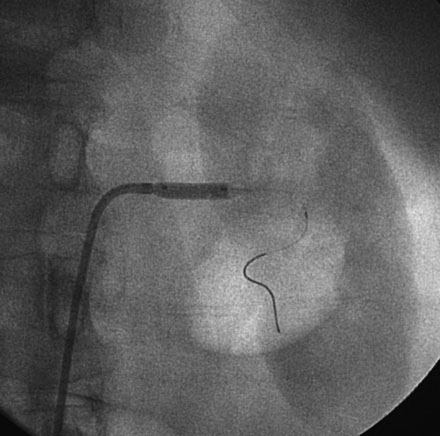
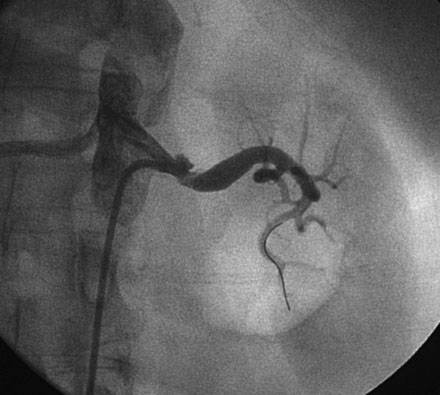
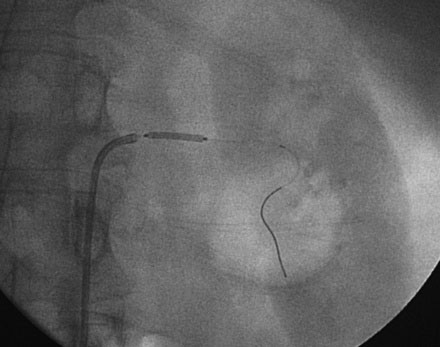
The decision was made to perform left renal artery stenting and, after administration of a bolus followed by an infusion of bivalirudin, the operator positioned a 0.014 inch floppy-tipped guidewire in the left renal artery and performed predilatation with a 4.5 mm diameter by 15 mm long rapid exchange balloon (Figures 53-3, 53-4). After balloon predilatation, a 6.0 mm diameter by 15 mm long bare-metal stent on a rapid exchange, a 0.014 inch platform was positioned in the proximal segment of the left renal artery (Figure 53-5). The stent was deployed by inflating the balloon to 10 atmospheres of pressure. An excellent angiographic result was obtained (Figure 53-6 and Video 53-3).
Discussion
Renal artery stenosis is the most common cause of secondary hypertension. The underlying pathophysiology of renal artery stenosis is atherosclerosis in 90% of cases and fibromuscular dysplasia in the remaining 10%.1,2 As demonstrated in this case, most atherosclerotic renal artery lesions affect the ostium and proximal segment of the renal artery while fibromuscular dysplasia, a condition primarily seen in young females, affects the middle third of the artery.2
Diagnosis of renal artery stenosis is based on a clinical suspicion of the entity, as symptoms and physical findings are usually absent. Guidelines suggest screening for renal artery stenosis in patients with: 1) onset of hypertension either before age 30 or after age 55; 2) accelerated, malignant, or resistant hypertension; 3) new or worsening renal insufficiency after initiation of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy; 4) unexplained renal atrophy; or 5) unexpected pulmonary edema.2 Several imaging methods are useful for diagnostic screening including duplex ultrasound, CTA, or MRA, with angiography reserved for patients with abnormal screening tests.
Treatment of renal artery stenosis includes medical therapy, stenting, and surgical revascularization. At present, the data supporting renal artery stenting over optimal medical therapy are limited.3 Trials comparing medical therapy to renal artery revascularization are difficult to design and implement. First, the hemodynamic significance of renal artery lesions is often unclear, particularly in the presence of moderate stenoses,4,5 and this may create a heterogenous group of patients in any given study depending on their inclusion criteria. Second, there has not been clear consensus on the need for adjunctive pharmacology or embolic protection.6 Third, there is a large selection bias involved in these trials, as many physicians believe they “know the answer” already and are reluctant to enroll patients in a trial in which their patient may be randomized to medical therapy instead of receiving a stent; thus the studies may consist mostly of moderate lesions, as the most severe lesions may not be enrolled. Finally, a variety of endpoints may be used, including blood pressure control, renal function, quality of life and cardiovascular mortality.
Initially, the only available randomized controlled studies consisted of a small number of patients treated with balloon angioplasty rather than stenting, and relied on study endpoints of unclear relevance.3 These studies showed no difference in blood pressure control between patients treated with balloon angioplasty and those treated with medical therapy; however, these studies did find that the balloon angioplasty group required less antihypertensive drugs to achieve that control. Given the fact that renal artery stenting, with high procedural success rates and low rates of restenosis, is clearly superior to balloon angioplasty, it was believed that the results of these trials would be different with stenting.
A recently published randomized controlled trial of medical therapy versus stenting for renal artery stenosis in over 800 patients found no clinical benefit from revascularization in terms of renal function, blood pressure, and cardiovascular events but several procedural complications in the revascularization group.7 Additional, large-scale randomized controlled trials designed to compare renal artery stenting to optimal medical therapy are underway and their results are highly anticipated. The study design, primary endpoints, and results of these trials will likely be exhaustively debated over the years.
Until we have the evidence from these trials, current guidelines recommend renal artery stenting for significant renal artery stenosis in the following clinical settings2:
1 Safian R.D., Textor S.C. Renal artery stenosis. N Engl J Med. 2001;344:431-442.
2 Shetty R., Amin M.S., Jovin I.S. Atherosclerotic renal artery stenosis: Current therapy and future developments. Am Heart J. 2009;158:154-162.
3 Schwarzwalder U., Zeller T. Critical review of indications for renal artery stenting: Do randomized trials give the answer? Catheter Cardiovasc Interv. 2009;74:251-256.
4 DeBruyne B., Manoharan G., Pijls N.H.J., Verhamme K., Madaric J., Bartunek J., Vanderheyden M., Heyndrickx G.R. Assessment of renal artery stenosis severity by pressure gradient measurements. J Am Coll Cardiol. 2006;48:1851-1855.
5 Leesar M.A., Varma J., Shapira A., Fahsah I., Raza S.T., Elghoul Z., Leonard A.C., Meganathan K., Ikram S. Prediction of hypertension improvement after stenting of renal artery stenosis. Comparative accuracy of translesional pressure gradients, intravascular ultrasound and angiography. J Am Coll Cardiol. 2009;53:2363-2371.
6 Cooper C.J., Haller S.T., Coyler W., et al. Embolic protection and platelet inhibition during renal artery stenting. Circulation. 2008;117:2752-2760.
7 The ASTRAL Investigators. Revascularization versus medical therapy for renal artery stenosis. N Engl J Med. 2009;361:1953-1962.