CASE 51 Percutaneous Aortic Valve Replacement
Case presentation
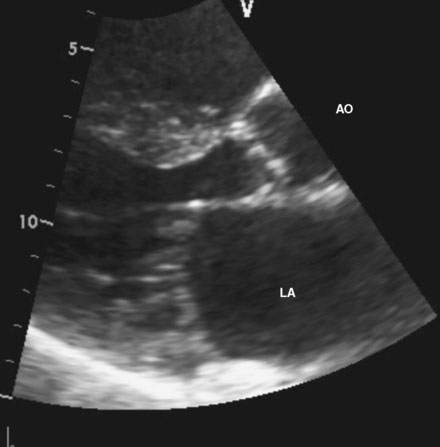
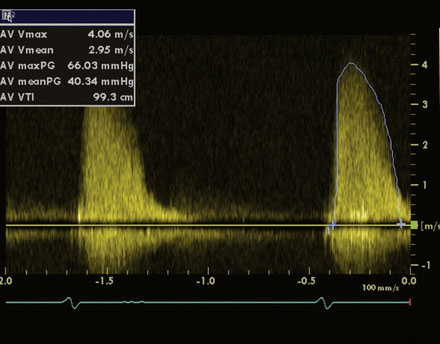
An 86-year-old man with previously known, asymptomatic aortic stenosis presented for an outpatient follow-up after a recent hospital admission for congestive heart failure and progressive angina. Before his admission with heart failure, he had noted several months of increasing shortness of breath with exertion and associated with chest tightness. During the office visit, his cardiologist obtained an echocardiogram that revealed severe, calcific aortic stenosis with a peak velocity of 4 m/sec, a peak instantaneous gradient of 66 mmHg, and a calculated valve area of 0.7 cm2, with preserved left ventricular function and an estimated pulmonary artery pressure of 70 mmHg (Figures 51-1, 51-2 and Video 51-1). In addition to the known aortic stenosis, his past medical history is extensive and includes chronic obstructive pulmonary disease, persistent atrial fibrillation, non–insulin-dependent diabetes mellitus, hypertension, dyslipidemia, obstructive sleep apnea, and coronary artery disease, with coronary bypass surgery performed 13 years earlier consisting of a left internal mammary artery to the left anterior descending artery, and saphenous vein grafts to the first and second obtuse marginals, to a large first diagonal, and to the right coronary arteries. He also has mild dementia, manifested primarily by short-term memory loss, but he remains functional and interactive with his family. Medications included metformin, ramipril, aspirin, furosemide, atorvastatin, and warfarin. He was referred for cardiac catheterization for further evaluation.
Cardiac catheterization
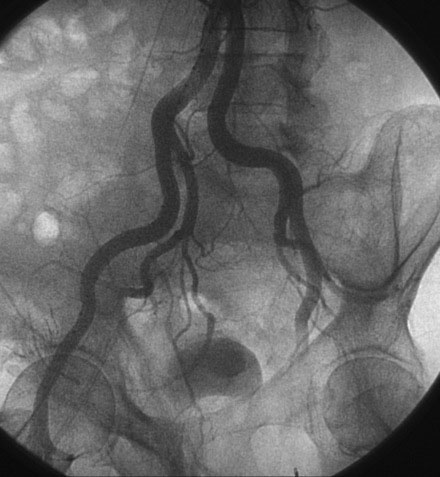
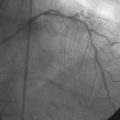
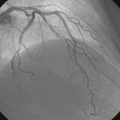
A hemodynamic evaluation found elevated right-sided heart pressures with a mean right atrial pressure of 19 mmHg and prominent V waves consistent with severe tricuspid regurgitation, pulmonary artery pressure of 63/18 mmHg (mean of 37 mmHg), and mean pulmonary capillary wedge pressure of 26 mmHg, with a cardiac output of 4.8 L/min and a valve area of 0.8 cm2. The native coronary arteries were all occluded but the left internal mammary and saphenous vein grafts remained patent and free of disease. Abdominal aortography found normal, large-caliber iliac arteries with moderate left-sided tortuosity (Figure 51-3).
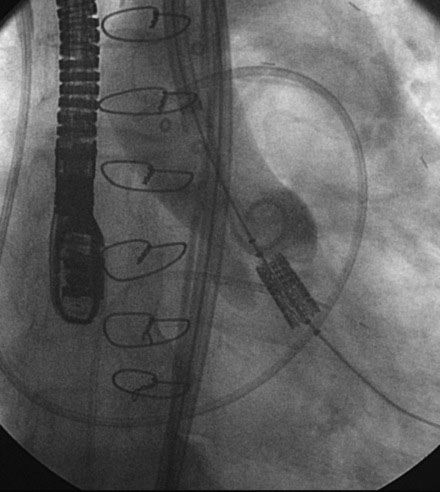
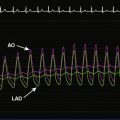
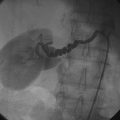
He returned to the cardiac catheterization laboratory for percutaneous aortic valve replacement. With an annulus size of 20 mm, the operator planned to insert a 26 mm valve. After the administration of general anesthesia, a 24 French sheath was inserted into the left common femoral artery under direct surgical exposure. A temporary transvenous pacemaker was positioned in the right ventricular apex via a 6 French venous sheath in the right femoral vein and a Swan-Ganz catheter was positioned in the pulmonary artery from the right internal jugular vein, allowing measurement of baseline pressures and cardiac output. Heparin was administered intravenously. The operator crossed the aortic valve with a straight wire and advanced a dual-lumen pigtail catheter to measure simultaneous left ventricular and aortic pressures. A 0.035 inch extra-stiff Amplatz wire was inserted and the dual-lumen pigtail catheter was exchanged for a valvuloplasty balloon. Using first a 20 mm diameter by 6 cm long, followed by a 22 mm diameter by 3 cm long balloon, each balloon was centered on the aortic valve and, during balloon inflation, rapid ventricular pacing at 170 beats/min was performed to decrease the cardiac output and prevent the balloon from sliding into the aorta or ventricle. The balloon catheter was removed over an extra-stiff Amplatz wire and exchanged for a 26 mm Edwards SAPIEN valve using the Retroflex I delivery catheter. The valve prosthesis was advanced over the wire and centered on the calcified valve using aortography to guide positioning. Optimal position was present when two thirds of the prosthesis lay in the ventricular side and one third on the aortic side (Figure 51-4 and Video 51-2). Rapid ventricular pacing was performed and the balloon was inflated, deploying the valve (Video 51-3). After valve insertion, hemodynamic assessment found no pressure gradient and aortography confirmed excellent position without aortic regurgitation (Video 51-4).
Discussion
Surgical replacement of the aortic valve is an effective method of relieving symptoms and reducing mortality in patients with symptomatic, severe calcific aortic stenosis.1 Unfortunately, many patients with severe, calcific aortic stenosis are usually very elderly and have accrued other comorbid conditions during their long lives that may result in an unacceptable surgical risk. In addition to advanced age, these include pulmonary disease, renal insufficiency, prior stroke, prior cardiac surgery, and a general frail status from coexisting arthritis, degenerative neurological conditions, and diminished functional status. These patients are often deemed poor surgical candidates and have few other options. Medical therapy is ineffective and balloon aortic valvuloplasty offers no more than a temporary palliative effect.2
Percutaneous aortic valve replacement offers the potential for a lower-risk alternative to conventional surgery. First described by Cribier in 2000 using an anterograde transseptal approach,3 current devices are inserted either by a transfemoral retrograde approach as shown in this case, or, alternatively, by a minimally-invasive transapical approach if the iliofemoral vessels are diseased, too small, or too tortuous to allow passage of the large devices.
To date, there are two devices in advanced stages of development, the Edwards SAPIEN valve and the CoreValve. The Edwards device consists of a valve constructed of equine pericardium mounted within a balloon expandable stent and is available in a 23 mm (for annulus sizes of 18 to 20 mm) and 26 mm size (for annulus sizes of 20 to 24 mm). The first generation devices are mounted on 22 French and 24 French catheters for 23 mm and 26 mm valves, respectively. The CoreValve consists of a bovine pericardial valve within a self-expanding nitinol frame and is available in 24 and 28 mm sizes; both valves are mounted on an 18 French catheter. Both valves have been shown to be safe and effective in registry reports of patients with severe, symptomatic aortic stenosis at high risk for aortic valve replacement.4,5 The results of randomized controlled trials comparing these devices to conventional surgery in high-risk patients are currently in progress and their role in low-risk patients is unknown at present.
1. Schwarz F., Baumann P., Manthey J., Hoffmann M., Schuler G., Mehmel H.C., Schmitz W., Kubler W. The effect of aortic valve replacement on survival. Circulation. 1982;66:1105-1110.
2. Lieberman E.B., Bashore T.M., Hermiller J.B., Wilson J.S., Pieper K.S., Keeler G.P., Pierce C.H., Kisslo K.B., Harrison J.K., Davidson C.J. Balloon aortic valvuloplasty in adults: Failure of procedure to improve long-term survival. J Am Coll Cardiol. 1995;26:1522-1528.
3. Cribier A., Eltchaninoff H., Tron C., Bauer F., Agatiello C., Sebagh L., Bash A., Nusimovici D., Litzler P.Y., Bessou J.P., Leon M.B. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol. 2004;43:698-703.
4. Webb J.G., Pasupati S., Humphries K., Thompson C., Altwegg L., Moss R., Sinhal A., Carere R.G., Munt B., Ricci D., Ye J., Cheung A., Lichtenstein S.V. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation. 2007;116:755-763.
5. Piazza N., Grube E., Gerckens U., den Heijer P., Linke A., Luha O., Ramondo A., Ussia G., Wenaweser P., Windecker S., Laborde J.-C., de Jaegere P., Serruys P.W. Procedural and 30-day outcomes following transcatheter aortic valve implantation using the third generation (18 Fr) CoreValve ReValving System: results from the multicentre, expanded evaluation registry 1-year following CE mark approval. EuroIntervention. 2008;4:242-249.