CHAPTER 50
Lumbar Spinal Stenosis
Zacharia Isaac, MD; Edrick Lopez, MD, JD
Definition
Lumbar spinal stenosis is classically defined as narrowing of the spinal canal, nerve root canals, or tunnels of the intervertebral foramina at the lumbar level [1]. A significant portion of the general population may have anatomic spinal stenosis without symptoms. Studies have indicated that 20% to 25% of asymptomatic people older than 40 years have significant narrowing of the lumbar spinal canal [2]. Stenosis causes symptoms only when there is sufficient impingement on neural structures, including the cauda equina or exiting nerve roots. Therefore spinal stenosis as a clinical entity is considered significant only if it results in symptomatic pain and compromised function.
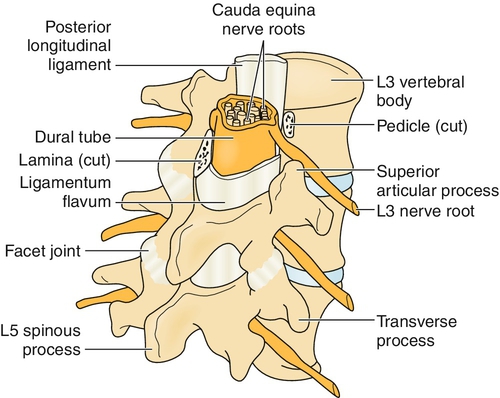
The significant anatomic elements of the lumbosacral spine include the five lumbar vertebrae L1 to L5, the sacrum, the intervertebral discs, the ligamentum flavum, the zygapophyseal joints, the lumbar spinal nerve rootlets, the spinal nerve roots, and the cauda equina (Fig. 50.1).
Various schemes for classification of spinal stenosis have been devised and are listed in Table 50.1.
Congenital lumbar spinal stenosis is less common, representing 9% of cases [3]; symptoms first appear in patients during their 30s. Achondroplastic dwarves often have stenosis secondary to hypoplasia of the pedicles. Acromegaly may cause spinal stenosis by enlargement of the synovium and cartilage, which results in decreased cross-sectional area of the canal. Symptomatic spinal stenosis secondary to purely isolated congenital causes is rare. More commonly, patients will have a combination of congenital and acquired stenosis; a developmentally smaller canal predisposes them to symptoms once acquired changes occur to the surrounding anatomy. Developmental factors that lead to a small canal include statistically significantly shorter pedicles and a trefoil-shaped canal.
The acquired types of spinal stenosis are numerous, but degenerative is the most common. The first stage in the degenerative process is generally degradation of the hydrophilic proteoglycans within the intervertebral disc, attendant disc desiccation, and loss of disc height. This causes a shift of load onto the posterior structures of the canal, in particular the facet joints, which normally provide 3% to 25% of the support during axial loading but may bear up to 47% with degeneration of the disc [4]. As the facets bear more of the burden, they undergo degeneration, one aspect of which is osteophyte formation. This further diminishes the cross-sectional area of the canal and can also result in stenosis of the neural foramina (termed foraminal stenosis). The ligamentum flavum undergoes buckling with decreased intervertebral disc height, leading to further encroachment of the canal. Epidural fat may contribute to reduced canal space in some patients [5]. Patients can have degenerative facet synovial cysts that focally can encroach on the spinal canal and nerve rootlets and cause radicular pain.
Spinal stenosis can be differentiated by anatomic location, including central canal, lateral recess, foraminal, and extraforaminal (Table 50.2). Additional subcategorization includes division of the lateral canal into three regions: entrance zone, mid-zone, and exit zone.
Canal Measurements
The normal adult central canal has a midsagittal diameter of at least 13 mm. Relative stenosis is defined as a canal diameter between 10 and 13 mm; patients may or may not be symptomatic. Absolute stenosis occurs at a diameter of less than 10 mm, and patients are usually symptomatic. Extraforaminal nerve root compression can occur with disc herniation, degenerative scoliosis, or isthmic spondylolisthesis. Far-out syndrome, described by Wiltse and colleagues [6], involves L5 root impingement between the L5 transverse process and sacral ala in patients with spondylolisthesis. Extension of the spine can reduce foraminal cross-sectional area by 20% and central canal volume by up to 67% [7].
Finally, spinal stenosis has been categorized according to the patient’s presenting pain syndrome of mechanical back pain, radicular pain, or neurogenic claudication. The pain pattern can be an indication of the anatomic and pathophysiologic mechanism of the patient’s particular case of spinal stenosis.
Symptoms
Patients with acquired lumbar spinal stenosis usually have symptoms during their 50s and 60s. Symptomatic lumbar spinal stenosis may result in both back pain (from axial components, such as facet degeneration) and leg pain (from radicular components of nerve root compression, either central or lateral). Leg pain is often greater than back pain, and depending on which nerve roots are impinged, leg pain can be unilateral or bilateral and monoradicular or polyradicular. Patients with acquired, degenerative lumbar spinal stenosis tend to have a history of chronic low back pain and develop leg pain later in their course. In a study of 100 patients with lumbar spinal stenosis, back pain had been present for an average of 14 years and leg pain for an average of 2 years [8]. The classic symptom of lumbar spinal stenosis is neurogenic claudication, also known as pseudoclaudication, which typically is manifested as buttock, thigh, and calf pain exacerbated with walking, standing, or lumbar extension and alleviated with sitting, lying, or lumbar flexion. Symptoms also commonly involve cramping, numbness, tingling, heaviness, and spasms. Because the spinal canal and neural foramina widen with flexion and become narrower with extension, pain often improves with squatting and walking tolerance increases with a flexed posture, such as while walking uphill or on an inclined treadmill or while pushing a shopping cart. From a diagnostic point of view, neurogenic claudication was found in one study to have a sensitivity of 63% and a specificity of 71% compared with a combination of clinical, radiologic, and imaging test findings [9].
Symptoms that have been reported to have high sensitivity for lumbar spinal stenosis are as follows: best posture with regard to symptoms is sitting (89%) [10], worst posture with regard to symptoms is standing or walking (89%) [10], pain below buttocks (88%) [11], pain in legs worsened by walking and relieved by sitting (81%) [10], radiating leg pain (81%) [9], and age older than 65 years (77%) [11]. Symptoms with high specificity for lumbar spinal stenosis include no pain when seated (93%) and symptoms improved when seated (83%) [11]. A systematic review of the accuracy of the clinical history (the information in this sentence is about symptoms and not about findings in clinical examination) for the diagnosis of lumbar spinal stenosis, encompassing four studies and 741 patients, found that having no pain when seated (likelihood ratio [LR], 7.4; 95% confidence interval [CI], 1.9-30), improvement of symptoms when bending forward (LR, 6.4; 95% CI, 4.1-9.9), presence of bilateral buttock or leg pain (LR, 6.3; 95% CI, 3.1-13), and neurogenic claudication (LR, 3.7; 95% CI, 2.9-4.8) were the most useful individual findings for identifying the syndrome of lumbar spinal stenosis.
Physical Examination
Unlike the history, no physical examination findings are considered classic for lumbar spinal stenosis. Abnormal findings in lumbar spinal stenosis are similar to those in other disorders that cause peripheral neurologic deficits, but they are often not present. On physical examination, having a wide-based gait (LR, 13; 95% CI, 1.9-95) and an abnormal Romberg test result (LR, 4.2; 95% CI, 1.4-13) also increased the likelihood of the clinical syndrome of lumbar spinal stenosis. On the other hand, absence of neurogenic claudication (LR, 0.23; 95% CI, 0.17-0.31) decreased the likelihood of the diagnosis [12]. In the study by Amundsen and colleagues [8], abnormal findings within the 100-subject cohort included sensory dysfunction (51%), diminished deep tendon reflexes (47%), positive Lasègue test result (24%), and leg weakness (23%), among others. Physical and functional findings with high sensitivity include longer recovery time after level versus inclined treadmill walking (82%) [10] and no pain with lumbar flexion (79%) [11]. Highly specific aspects of examination and testing include improved walking tolerance on inclined versus level treadmill (92%), earlier onset of symptoms on level versus inclined treadmill (83%) [10], absent Achilles reflex (78%), lower extremity weakness (78%), pinprick or vibration deficit (> 75%), wide-based gait (> 75%), and presence of Romberg sign (> 75%) [11]. Checking arterial pulses is important and necessary in patients thought to have lumbar spinal stenosis, but it is not sufficient to rule out arterial disease. A prospective study examining the rate of peripheral arterial disease in patients with intermittent claudication with concurrent lumbar spinal canal stenosis found that peripheral arterial disease was present in 26% of the subjects. Screening for peripheral arterial disease by ankle-brachial index and toe-brachial index tests should be considered in patients with intermittent claudication and lumbar spinal stenosis [13].
Functional Limitations
Worsening leg and back pain from walking, back extension, and prolonged standing is the primary contributor to activity limitation. As such, patients with lumbar spinal stenosis tend to have difficulties with walking long distances, going down stairs, and household or yard work (e.g., dishwashing, lawn mowing, and vacuuming) as well as with tasks that require overhead work (which may induce spinal extension). Balance deficits from sensory deficits may increase fall risk.
Diagnostic Studies
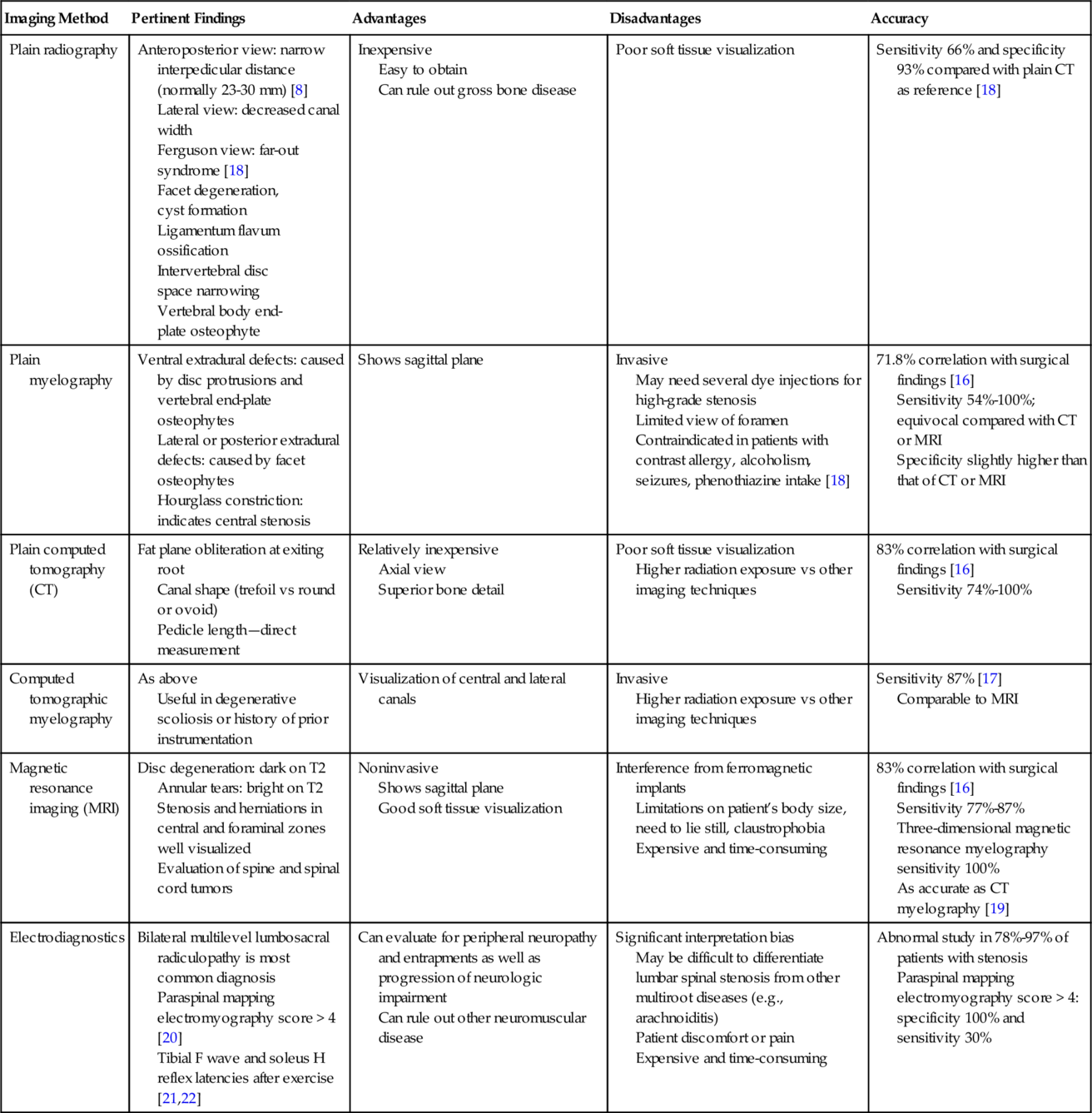
Diagnostic studies can provide useful information on structural and neurologic changes associated with spinal stenosis, but they must be interpreted in the context of the patient’s clinical presentation. Several studies have demonstrated that there is no correlation between radiologic findings and clinical or functional outcomes [14]. However, another study found that severity of lumbar spinal stenosis as measured on functional myelography predicted long-term disability independent of therapeutic intervention [15]. The various qualities of the diagnostic tests are summarized in Table 50.3.
Table 50.3
Comparison of Various Diagnostic Tests for Lumbar Spinal Stenosis
| Imaging Method | Pertinent Findings | Advantages | Disadvantages | Accuracy |
| Plain radiography | Anteroposterior view: narrow interpedicular distance (normally 23-30 mm) [8] Lateral view: decreased canal width Ferguson view: far-out syndrome [18] Facet degeneration, cyst formation Ligamentum flavum ossification Intervertebral disc space narrowing Vertebral body end-plate osteophyte |
Inexpensive Easy to obtain Can rule out gross bone disease |
Poor soft tissue visualization | Sensitivity 66% and specificity 93% compared with plain CT as reference [18] |
| Plain myelography | Ventral extradural defects: caused by disc protrusions and vertebral end-plate osteophytes Lateral or posterior extradural defects: caused by facet osteophytes Hourglass constriction: indicates central stenosis |
Shows sagittal plane | Invasive May need several dye injections for high-grade stenosis Limited view of foramen Contraindicated in patients with contrast allergy, alcoholism, seizures, phenothiazine intake [18] |
71.8% correlation with surgical findings [16] Sensitivity 54%-100%; equivocal compared with CT or MRI Specificity slightly higher than that of CT or MRI |
| Plain computed tomography (CT) | Fat plane obliteration at exiting root Canal shape (trefoil vs round or ovoid) Pedicle length—direct measurement |
Relatively inexpensive Axial view Superior bone detail |
Poor soft tissue visualization Higher radiation exposure vs other imaging techniques |
83% correlation with surgical findings [16] Sensitivity 74%-100% |
| Computed tomographic myelography | As above Useful in degenerative scoliosis or history of prior instrumentation |
Visualization of central and lateral canals | Invasive Higher radiation exposure vs other imaging techniques |
Sensitivity 87% [17] Comparable to MRI |
| Magnetic resonance imaging (MRI) | Disc degeneration: dark on T2 Annular tears: bright on T2 Stenosis and herniations in central and foraminal zones well visualized Evaluation of spine and spinal cord tumors |
Noninvasive Shows sagittal plane Good soft tissue visualization |
Interference from ferromagnetic implants Limitations on patient’s body size, need to lie still, claustrophobia Expensive and time-consuming |
83% correlation with surgical findings [16] Sensitivity 77%-87% Three-dimensional magnetic resonance myelography sensitivity 100% As accurate as CT myelography [19] |
| Electrodiagnostics | Bilateral multilevel lumbosacral radiculopathy is most common diagnosis Paraspinal mapping electromyography score > 4 [20] Tibial F wave and soleus H reflex latencies after exercise [21,22] |
Can evaluate for peripheral neuropathy and entrapments as well as progression of neurologic impairment Can rule out other neuromuscular disease |
Significant interpretation bias May be difficult to differentiate lumbar spinal stenosis from other multiroot diseases (e.g., arachnoiditis) Patient discomfort or pain Expensive and time-consuming |
Abnormal study in 78%-97% of patients with stenosis Paraspinal mapping electromyography score > 4: specificity 100% and sensitivity 30% |
Treatment
Initial
There is an overall lack of high-quality prospective controlled studies examining the efficacy of various noninvasive treatments of lumbar spinal stenosis. Moreover, many of the existing recommendations are in regard to back pain in general, without differentiation of pain associated specifically with lumbar spinal stenosis. Therefore much of clinical practice is based on extrapolation of recommendations, anecdotal experience, and expert opinion.
The oral analgesics used in lumbar spinal stenosis are acetaminophen, nonsteroidal anti-inflammatory drugs, muscle relaxants, anti–neuropathic pain medications including anticonvulsants and antidepressants, tramadol, and opioids. However, a number of systematic reviews have evaluated the use of pain medications in nonspecific low back pain. The review by Mens [23] stated that acetaminophen, nonsteroidal anti-inflammatory drugs, and mild opioids are potential first-line drugs, but there is no evidence that one is more effective than another. Nonbenzodiazepine muscle relaxants were listed as second-line drugs for acute low back pain. The efficacy of these medications for the treatment of symptomatic lumbar spinal stenosis is still unclear, and the sedating quality of these medications poses an increased risk of adverse events in elderly patients. Antidepressants with mixed-receptor or predominantly noradrenergic activity, including tricyclics, bupropion, venlafaxine, and duloxetine, are somewhat effective [24]. First-generation (e.g., carbamazepine and phenytoin) and second-generation (e.g., gabapentin and pregabalin) antiepileptic drugs are also somewhat effective.
Rehabilitation
To our knowledge, there are no randomized controlled trials in which the effectiveness of physical or manual therapies in the treatment of patients specifically with lumbar spinal stenosis is evaluated. General recommendations include relative rest (avoidance of pain-exacerbating activities while staying active to minimize deconditioning) and a flexion-biased exercise program, including inclined treadmill and exercise bicycle. Flexion biasing increases the cross-sectional area of the spinal canal compared with exercises performed in neutral or extension, thereby maximizing activity tolerance [25]. Whitman and associates [26] reported a case series of three patients who demonstrated significant improvements in pain and function at 18 months after undergoing specialized physical therapy programs that included spinal manipulation, flexion and rotation spine mobilization exercises, hip joint mobilization, hip flexor stretching, muscle retraining (lower abdominal, gluteal, and calf muscles), body weight–supported ambulation, and daily walking with properly prescribed orthotics. Body weight–supported ambulation acts to decrease the axial loading of the spine to increase the cross-sectional area of the neural foramina, and studies have provided some support of this strategy [27]. Physical modalities such as cold or hot packs, ultrasound, iontophoresis, and transcutaneous electrical nerve stimulation may be helpful, but data of clinical efficacy are lacking. Physicians and therapists must be aware of any medical comorbidities, such as cardiovascular and pulmonary disease, osteoporosis, cognitive deficits, and other musculoskeletal or neuromuscular conditions, that may have an impact on therapy tolerance. A retrospective analysis of predictors of walking performance and walking capacity showed that body mass index, pain, female sex, and age predict walking performance and capacity in people with lumbar spinal stenosis, those with low back pain, and asymptomatic control subjects. The authors of this analysis concluded that obesity and pain are modifiable predictors of walking deficits that could be targets for future intervention studies aimed at increasing walking performance and capacity in both the low back pain and lumbar spinal stenosis populations [28].
Procedures
Recommendations for the use of nonsurgical interventional procedures in the symptomatic control of lumbar spinal stenosis, particularly fluoroscopically guided epidural steroid injections, are somewhat controversial. A systematic review by Abdi and colleagues [29] concluded that for interlaminar, transforaminal, and caudal epidural steroid injections, there is strong evidence for short-term relief and limited to moderate evidence for long-term relief of lumbar radicular pain. A number of studies have cited short-term success rates of 71% to 80% [30,31] and long-term success rates of 32% to 75% [30,32]. Furthermore, symptomatic management with epidural steroid injections may delay surgery an average of 13 to 28 months [33,34]. A randomized controlled trial found that patients with lumbar central spinal stenosis received significant pain relief and improved their Oswestry disability scores after receiving lumbar interlaminar injections with or without steroids [35]. A study looking at the effects of fluoroscopic transforaminal epidural steroid injections in patients with degenerative lumbar scoliosis combined with spinal stenosis and radicular pain found them to be a more effective short-term option compared with lidocaine injections [36]. In general, epidural steroid injections can be considered a safe and reasonable therapeutic option for symptomatic management before surgical intervention is pursued.
Surgery
Patients with persistent symptoms despite conservative measures may benefit from surgical treatment. There are no universally accepted indications for and contraindications to surgery. A key feature in the selection of patients is ensuring that the symptoms indeed arise from nerve root compression. It is also important to screen for depression. A prospective clinical study of lumbar spinal stenosis patients who underwent surgery found at 2-year follow-up that patients with depressive symptoms had poorer surgical outcomes than those with normal mood [37]. Surgery generally consists of decompressive laminectomy with medial facetectomy. The decompression relieves central canal stenosis; the medial facetectomy and attendant dissection along the lateral recesses decompress areas of foraminal stenosis.
The Maine Lumbar Spine Study [38] prospectively examined the outcome of initial surgical versus nonsurgical treatment in 148 patients at 1, 4, and 8 to 10 years. Rates of improvement in predominant symptom, low back pain, and leg pain at 1 year ranged from 77% to 79% in the surgery group and 42% to 45% in the nonsurgical group. At 8- to 10-year follow-up, the rates dropped to 53% to 67% in the surgery group and remained essentially stable at 41% to 50% in the nonsurgical group. As such, the benefits from surgery diminished over time, although improvements in leg pain and back-related functional status were maintained. A time-dependent decrease in benefit among older patients was evident in another study by Yamashita and colleagues [39]. In patients requiring surgery, earlier intervention is associated with improved long-term outcomes [38,40].
The Spine Patient Outcomes Research Trial compared the outcomes of patients who underwent surgical management of lumbar spinal stenosis with the outcomes of those who underwent conservative treatment. They found that at 2-year follow-up and at 4-year follow-up, intention-to-treat analysis showed significant improvement in SF-36 bodily pain and Oswestry disability index from baseline in the surgical group compared with the nonsurgical group. In addition, comparative effectiveness evidence for defined diagnostic groups from the Spine Patient Outcomes Research Trial showed good value for surgery compared with nonoperative care during 4 years. They also found that patients with predominant leg pain improved significantly more with surgery than did patients with predominant low back pain. However, patients with predominant low back pain still improved significantly more with surgery than with nonoperative treatment [41]. Patients often will have residual back pain at some point after surgical intervention. Once the patient is cleared by the operating spine surgeon, a lumbar core stabilization exercise program that emphasizes general core strength, postural education, hip girdle flexibility and strength, and cardiovascular reconditioning is important postoperatively.
Two key controversies in the surgical management of lumbar spinal stenosis are the appropriate role for a concomitant arthrodesis (lumbar fusion) and the utility of instrumentation. A comprehensive multipart systematic review by Resnick and colleagues [42,43] concluded that the literature consistently supports the addition of arthrodesis, particularly posterior lumbar fusion, to decompression surgery only in patients with lumbar spinal stenosis secondary to spondylolisthesis.
Potential Disease Complications
The natural history of lumbar spinal stenosis is not well understood, but existing literature seems to indicate that most cases do not lead to significant deterioration. Johnsson and coworkers [44] examined the symptomatic and functional outcomes of 19 patients with moderate stenosis who did not undergo surgery. Average follow-up was 31 months, at which time 26% were worse, 32% unchanged, and 42% improved. Another study by Johnsson and colleagues [45] observed 32 patients with spinal stenosis who did not receive treatment, 75% of whom had neurogenic claudication. At a mean follow-up period of 49 months, 70% were unchanged, 15% were worse, and 15% were better. In patients with worsening symptomatic stenosis, there may be a progressive increase in back or leg pain and a decrease in walking tolerance. Severe stenosis may lead to a neurogenic bladder, especially in patients with narrowed dural sac anteroposterior diameter. Cauda equina syndrome is a rare but serious complication, in which case emergent surgical decompression is generally required.
Potential Treatment Complications
Exacerbation of symptoms is possible, particularly with functional restoration programs that may attempt to condition patients to painful activities through safe repetition of pain-inducing motions. In addition, patients with significant comorbidities, such as cardiopulmonary disease, are at risk for activity-induced adverse events. Complications from medication use include liver toxicity with acetaminophen; gastritis, gastrointestinal bleeds, renal toxicity, and platelet inhibition with nonsteroidal anti-inflammatory drugs; increased risk of cardiovascular events with some cyclooxygenase 2 inhibitors; nausea and lowering of seizure threshold with tramadol; anticholinergic effects including dry mouth and urinary retention with tricyclic antidepressants; sedation, ataxia, and other cognitive side effects with anticonvulsants (although gabapentin and pregabalin are relatively safe); and sedation, constipation, urinary retention, tolerance, and central pain sensitization with opioids. Adverse gastrointestinal events related to nonsteroidal anti-inflammatory drugs can be minimized with the use of a cyclooxygenase 2 inhibitor or concomitant use of a gastroprotective agent, such as a proton pump inhibitor or H2 blocker [46].
Quantified data on complications associated with nonsurgical interventional procedures, including epidural steroid injections, are limited. A retrospective study of 207 patients receiving transforaminal epidural steroid injections [47] reported the following adverse events: transient nonpositional headaches that resolved within 24 hours (3.1%), increased back pain (2.4%), facial flushing (1.2%), increased leg pain (0.6%), vasovagal reaction (0.3%), increased blood glucose concentration in an insulin-dependent diabetic (0.3%), and intraoperative hypertension (0.3%). Other potential complications are infection at the injection site, dural puncture potentially with associated spinal headache, chemical or infectious meningitis, epidural hematoma, intravascular penetration, anaphylaxis, and nerve root or spinal cord injury leading to paresis or paralysis [48].
Complications of decompressive surgery include infection (0.5% to 3%), epidural hematoma, vascular injury (0.02%), thromboembolism including pulmonary embolism (0.5%), dural tears (< 1% to 15%), nerve root injury, postsurgical spinal instability, nonunion or hardware failure, adjacent segment degeneration, recurrence of symptoms (10% to 15%), and death (0.35% to 2%) [49].