CASE 41
History: A 60-year-old woman presents for routine screening mammography.
1. What should be included in the differential diagnosis based on the images provided? (Choose all that apply.)
A. Pathologically unilateral retracted nipple
B. Bilateral benign nipple inversion
C. Subareolar spiculated masses
2. If a patient presents with chronically inverted nipples, what is the next diagnostic step?
A. Spot compression views of both breasts, subareolar area
3. If a patient presents with new retraction of one nipple, what is the next diagnostic step?
A. Spot compression views of the retracted nipple
B. Ductography of the retracted nipple
C. MRI should be performed before any additional mammographic views
D. Surgical consultation for duct excision
4. Why is new nipple retraction a concern?
A. The retracted nipple can interfere with lactation.
B. Cancer may be present in the retroareolar breast, tethering the nipple.
C. A retracted nipple is predominantly a cosmetic concern.
D. Paget disease of the nipple is the most common reason for retracted nipple.
ANSWERS
CASE 41
Nipple Inversion
1. B and D
2. C
3. A
4. B
References
An HY, Kim KS, Yu IK, et al. Image presentation. The nipple-areolar complex: a pictorial review of common and uncommon conditions. J Ultrasound Med. 2010;29(6):949–962.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:26. 36
Comment
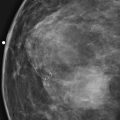
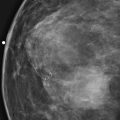
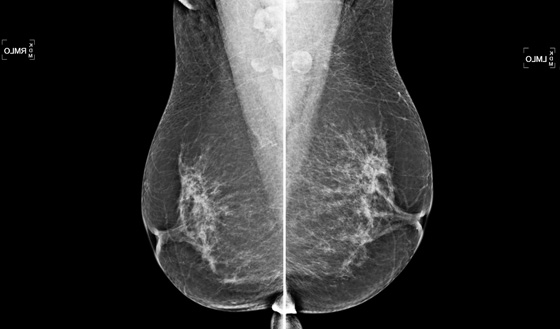
Bilateral nipple inversion is a benign condition, in which the nipples are completely beneath the level of the skin (see the figures). The nipples may evert on pressure and then resume the inverted position. This condition may interfere with lactation but is not otherwise of clinical concern and is often long standing or congenital. Benign inversion may also manifest as a slitlike area of the nipple pulled in. Nipple inversion is better seen on digital mammography than on film-screen mammography because tissue equalization algorithms allow better visualization of the skin and nipples.
A benign inverted nipple must be differentiated from a pathologically retracted nipple. The terms retraction and inversion are used interchangeably, which may cause confusion. When nipple retraction is new, it may be caused by duct ectasia, periductal mastitis, or malignancy. Cancer that develops in the retroareolar breast is typically invasive ductal carcinoma or invasive lobular carcinoma and, particularly if it develops close to the nipple, may cause the nipple to pull in, or retract. A pathologically retracted nipple does not evert on clinical examination, as a benign inverted nipple does. Paget disease of the nipple manifests as an erythematous area on the nipple-areolar complex and may cause nipple retraction. It has an association with ductal carcinoma in situ in the subareolar ducts. Inflammatory cancer of the breast can manifest with nipple retraction, but additional findings are present, with larger areas of thick, erythematous skin and peau d’orange.
The patient shown here had long-standing bilateral nipple inversion, and the mammographic appearance was stable. No further work-up is needed.
CASE 42
History: A 60-year-old woman presents for routine mammography. She has a personal history of breast cancer, which was occult on mammography and seen only on ultrasound. She was treated with breast conservation therapy. Because of her history, she requests ultrasound of both breasts annually, in addition to mammography.
1. What should be included in the differential diagnosis of the mammogram and ultrasound images shown? (Choose all that apply.)
B. Breast conservation therapy with calcified fat necrosis
C. Breast conservation therapy with calcification suspicious for recurrence
D. Calcification in the right breast related to radiation therapy
2. If you find a dense shadowing mass on ultrasound, how should you proceed?
A. All dense shadowing masses are suspicious, and biopsy should be performed.
B. Correlate the ultrasound finding with the mammogram.
C. Further work-up should be based on clinical examination.
D. Evaluate further with MRI, looking for abnormal enhancement.
3. What is the BI-RADS (Breast Imaging Reporting and Data System) for this mammogram and ultrasound?
4. If this mammogram was the patient’s first mammogram in your office, which statement regarding prior examinations is not correct?
A. It can be expensive to obtain prior mammograms.
B. No prior examinations are needed to evaluate the coarse calcification.
D. Prior mammograms are not needed for this benign mammogram.
ANSWERS
CASE 42
Calcification on Ultrasound
1. A, B, and D
2. B
3. B
4. D
Reference
Stavros AT. Breast Ultrasound. Philadelphia: Lippincott Williams & Wilkins; 2004.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:83.
Comment
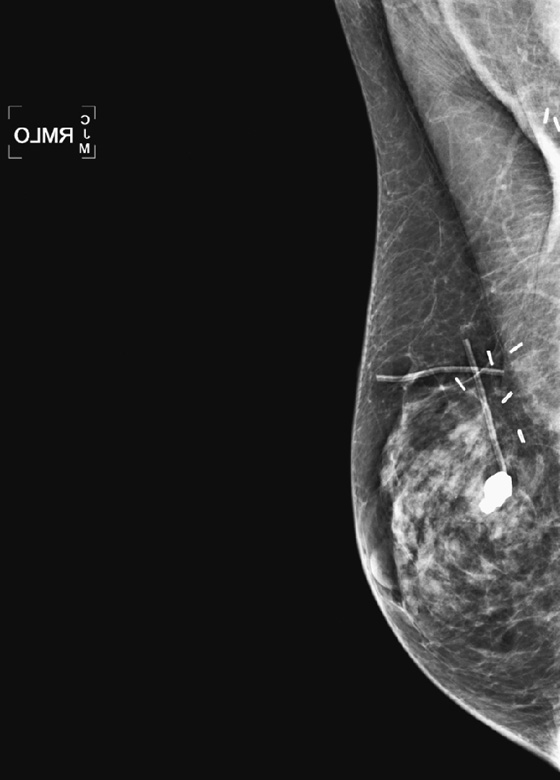
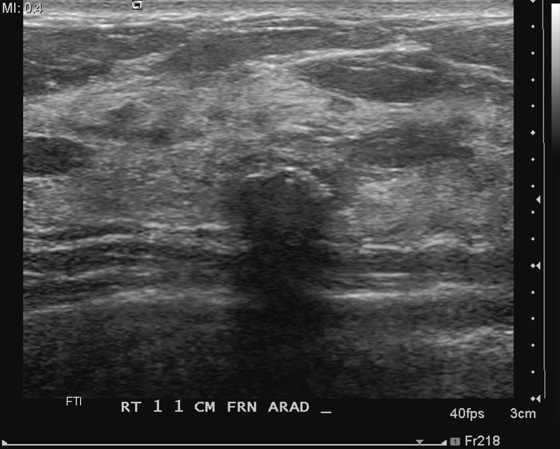
Fibroadenoma is a common breast lesion. It is often seen in all types of patients, including patients who develop malignancy. In this patient, the dense, calcified fibroadenoma existed for many years before the malignancy was detected (see the figures). Calcifications in the breast that has undergone lumpectomy need to be evaluated because recurrence may calcify. However, a large, coarse calcification such as this is benign and needs no further evaluation. This case illustrates how such a benign calcification can have a worrisome appearance on ultrasound (see the figures).
To distinguish a shadowing large calcification from a shadowing suspicious mass, look for the curvilinear dense rim along the anterior wall. This rim is the anterior margin of the calcification, which causes acoustic shadowing. The soft tissue mass of the fibroadenoma surrounding the calcification may also be seen on ultrasound. Also look at the mammogram to correlate the location of the shadowing structure with the location of the calcification on the mammogram. If the shadowing mass corresponds to the location of a coarse calcification on the mammogram, no further evaluation is needed for the shadowing mass.
CASE 43
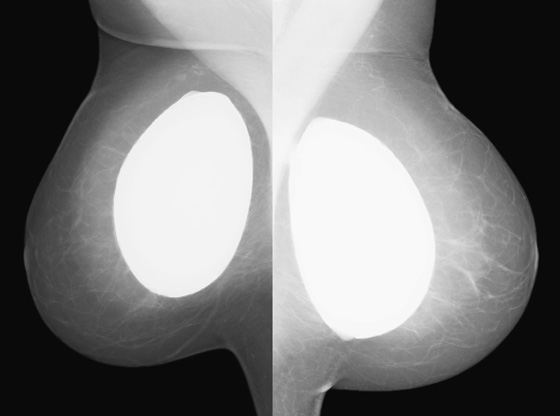
History: A 60-year-old woman had subglandular silicone implants placed 30 years ago. A mammogram obtained 6 years ago showed possible rupture, which was confirmed with MRI. She had the implants surgically removed. The routine screening mammogram following this surgery is shown.
1. What should be included in the differential diagnosis of the mammogram shown? (Choose all that apply.)
A. Fat necrosis in both breasts
B. Bilateral, locally advanced breast cancer
C. Bilateral retained implant capsule
2. What is the next step in management?
B. Referral to plastic surgeon
3. What is the “retained capsule”?
A. Free silicone that has extravasated from the implant
B. The envelope of the silicone implant that was left behind in the surgical bed
C. The fibrous capsule, formed by the patient, surrounding the implant was not removed
D. It is the same as “retained siliconoma”
4. Can saline implants also show calcification on the mammogram?
A. No, only silicone implants calcify
B. Yes, because the fibrous capsule calcifies, not the implant
C. Yes, because saline implants have a silicone envelope
D. Yes, but only in the subglandular position
ANSWERS
CASE 43
Implant Removal with Retained Capsule
1. A and C
2. B
3. C
4. B
References
Caskey CI, Berg WA, Hamper UM, et al. Imaging spectrum of extracapsular silicone: correlation of US, MR imaging, mammographic, and histopathologic findings. Radiographics. 1999;19:S39–S51. (Spec No):
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:343.
Comment
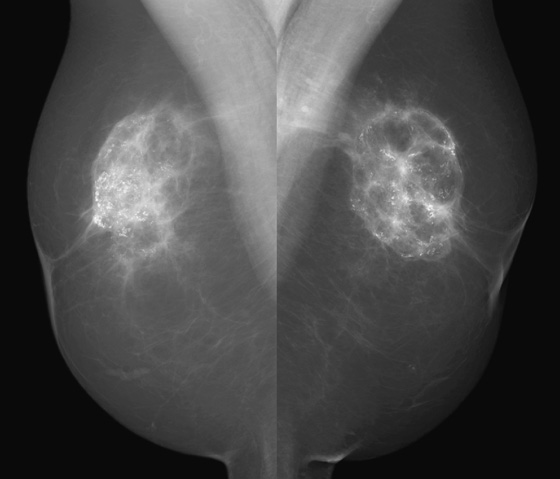
Implants are placed in the breast to augment the size of the breast. The implants are composed of a silicone elastomer shell and contain either silicone gel or saline as the filler. They are placed either in front of the pectoral muscle (see the figures), termed subglandular, or behind the pectoral muscle, termed subpectoral.
A complication of implants is the formation of the fibrous capsule. The fibrous capsule is the body’s immune response to the foreign material—an attempt to “wall off” the implant. This fibrous capsule can calcify, which often has a bizarre appearance on mammogram, as it forms plaques over the curved sheet of fibrous tissue surrounding the implant (see the figures).
When silicone implants rupture, the implant may be removed surgically. Removing the fibrous capsule was thought to be unnecessary in the past because this added morbidity and expense to the surgical procedure. There was evidence that because the capsule formed as a response to the implant, it would resorb spontaneously after the implant was removed. More recent evidence showed that spontaneous resorption did not always occur, and potential problems could arise from the retained capsule, including serous effusion, expansile hematoma, and siliconomas.
Mammography of the retained capsule most commonly shows masses at the site where the implant had been present, often with unusual calcifications (see the figures). These calcified forms have been termed “gold leaf” and “tin foil.” These calcified masses may be confused with carcinoma, so it is important to obtain the history of explantation from the patient to avoid unnecessary concern and biopsy. Free silicone and silicone granulomas are also commonly seen as well as silicone in the axillary nodes, not present in this patient. The retained capsule was excised as a separate surgical procedure in this patient.
CASE 44
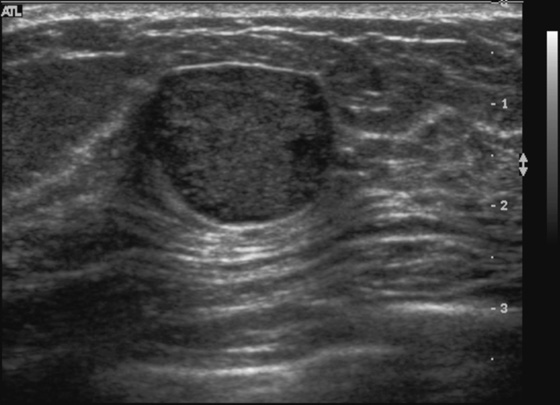
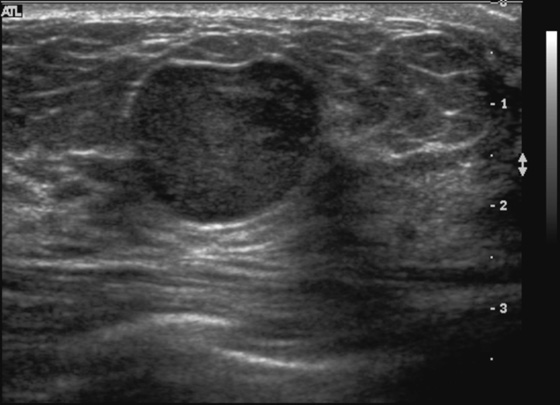
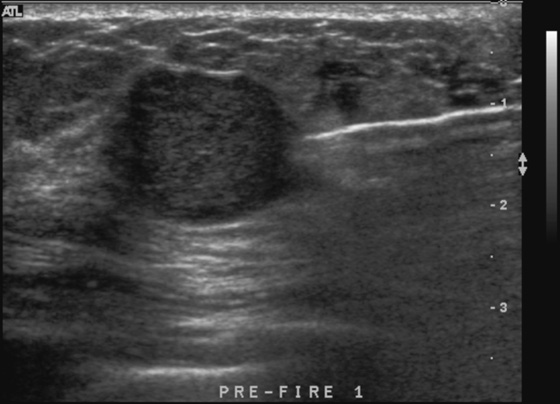
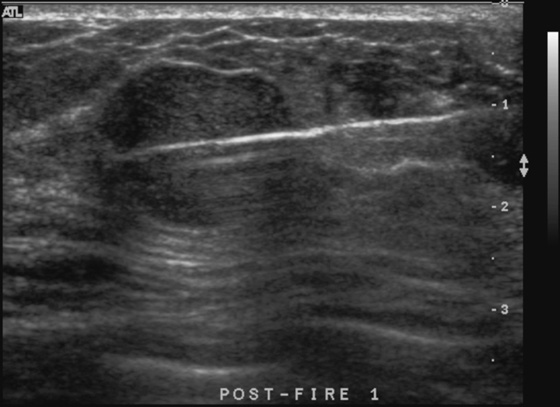
History: A 23-year-old woman feels a palpable mass in her left breast.
1. What should be included in the differential diagnosis for the images shown? (Choose all that apply.)
2. What is the BI-RADS (Breast Imaging Reporting and Data System) for this mass?
D. BI-RADS 5—highly suspicious
3. What is the next step in management of this palpable mass?
A. Biopsy must be performed because the mass is palpable.
B. Biopsy should be performed because the mass is not a BI-RADS 3.
C. The patient should be followed with ultrasound in 6 months.
D. The patient should have clinical follow-up; no further imaging follow-up is needed.
4. What are the features of a solid mass that allow follow-up rather than biopsy?
A. Elliptical shape, wider than tall, and complete thin capsule
B. Round shape with microlobulations
C. Elliptical, taller than wide, thin complete capsule
D. Elliptical, with thin capsule interrupted by one or two angular margins
ANSWERS
CASE 44
Myxoid Fibroadenoma with Biopsy
1. B, C, and D
2. C
3. B
4. A
References
Santamaría G, Velasco M, Bargalló X, et al. Radiologic and pathologic findings in breast tumors with high signal intensity on T2-weighted MR images. Radiographics. 2010;30(2):533–548.
Stavros AT. Breast Ultrasound. Philadelphia: Lippincott Williams & Wilkins; 2004.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:163. 212
Comment
Fibroadenoma is a very common mass in young women. When a patient presents with a palpable mass or a mammographic mass, and the classic features of a fibroadenoma are seen on ultrasound, the mass can be given a BI-RADS 3 designation. The patient has the following options: 6-month follow-up, core needle biopsy, or surgical excision. The BI-RADS 3 designation means that the mass has a less than 2% chance of malignancy. To have this designation, the mass must be elliptical, wider than tall, with a complete thin capsule. Two or three gentle lobulations are allowed.
Because of its round shape (see the figures), this mass is not a classic fibroadenoma and does not meet the strict BI-RADS 3 classification, and biopsy was performed (see the figures). Needle core biopsy was performed with a 14-gauge, spring-activated sampling device and ultrasound guidance. The pathology was “myxoid fibroadenoma.”
Fibroadenomas are composed of gland and stromal elements. The stromal elements can undergo myxoid change, sclerosis, hyalinization, and calcification. None of these changes affect the malignant potential; all are benign changes. However, the abundant mucin accumulated in the stroma of this lesion can be confused with a mucinous carcinoma on core needle sampling.
On ultrasound, myxoid fibroadenomas are more likely to be round and compressible. On MRI, they are bright on T2-weighted images.
There is a syndrome of myxoid lesions in the skin and heart, called Carney’s syndrome; however, most patients with a myxoid fibroadenoma do not have this syndrome. In the present case, the mass can be followed and does not need to be excised.
CASE 45
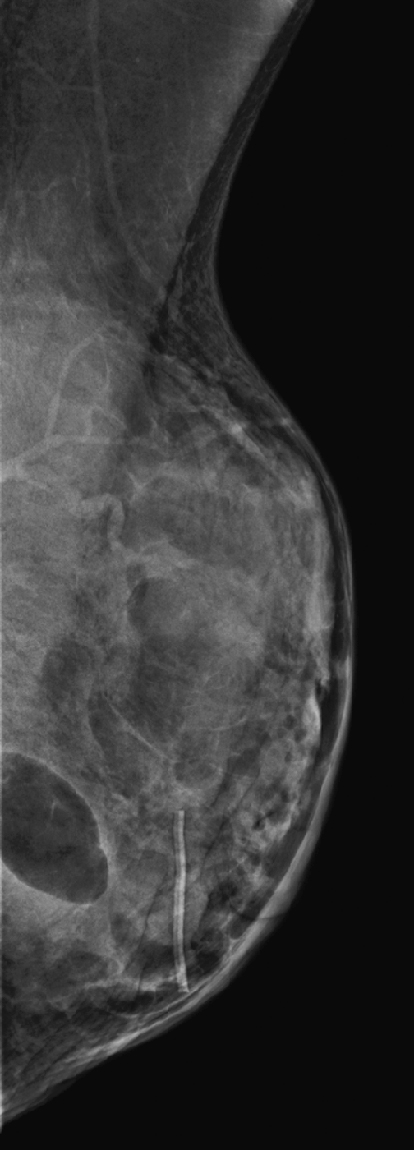
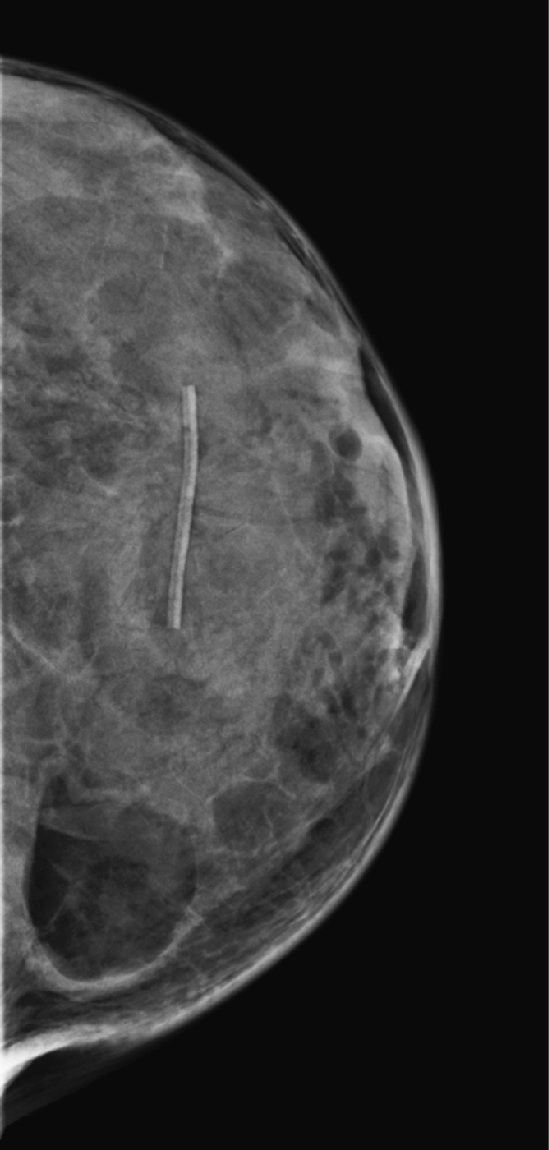
History: A 59-year-old woman presents for routine mammogram.
1. What is the differential diagnosis of the mass in the left breast? (Choose all that apply.)
A. Air in a cyst after aspiration
2. What additional studies are needed to make the diagnosis?
A. Ultrasound, to evaluate cystic versus solid
B. MRI, to evaluate for extent of disease
C. Physical exam, to check if this is palpable
3. Why is this lesion so clearly seen on this mammogram?
A. The fat density of the mass blends in with the subcutaneous fat.
B. The mass is the same density as the surrounding fatty breast tissue.
C. The mass is fat, and it contrasts with the surrounding dense breast tissue.
4. What is the Breast Imaging Reporting and Data System (BI-RADS) code and recommendation for this lesion?
A. BI-RADS 2—benign, routine follow-up
B. BI-RADS 3—probably benign, short-interval follow-up
C. BI-RADS 4—suspicious, recommend biopsy
D. BI-RADS 0—incomplete, need additional evaluation
ANSWERS
CASE 45
Lipoma
1. B, C, and D
2. D
3. C
4. A
References
Lanng C, Eriksen BO, Hoffmann J. Lipoma of the breast: a diagnostic dilemma. Breast. 2004;13(5):408–411.
Stavros AT. In: Breast Ultrasound. Philadelphia: Lippincott Williams & Wilkins; 2004:569.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:137.
Comment
Lipomas are benign tumors composed of mature fat. They are the most common soft-tissue tumor of the body. They can occur in many areas of the body, including the GI tract and spine, but they are commonly found in the subcutaneous tissues. In the breast, the lipoma is likely a variation of hamartoma, containing mostly fat, with negligible amounts of stroma and gland elements. If the mass contains a more equal distribution of fat and glandular tissue, it is an adenolipoma. The masses are slow growing and soft and have a thin fibrous capsule. The patient can present with a palpable mass, unlike the patient represented here (see the figures), who has dense, lumpy breasts and did not appreciate this mass on clinical exam.
If the mass is palpable, ultrasound can be performed to evaluate the clinical finding. This is helpful if the mass is not apparent on the mammogram, which is commonly the case. Because the lipoma may be surrounded by fat, it is often not recognized on the mammogram. Ultrasound can be done to assess the palpable finding and ensure that no suspicious mass is present. The ultrasound appearance of the lipoma is typically isoechoic with the surrounding fat or slightly hyperechoic. The mass is often in the subcutaneous fat, oval, and wider than tall. The margins can be difficult to appreciate. The mass is soft and easily deformed.
However, if the mass is not palpable and is of fat density easily seen on the mammogram, as in this patient, no further work-up is needed.
CASE 46
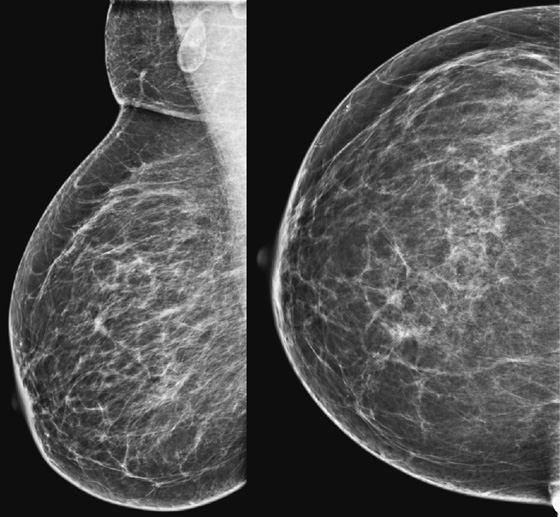
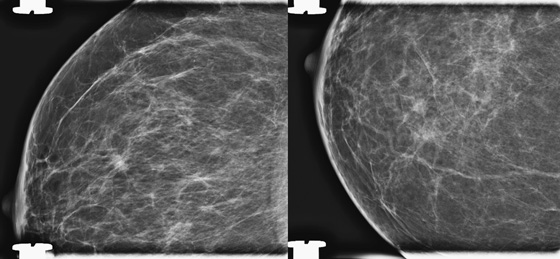
History: A 68-year-old woman undergoes a routine mammogram. She has a family history of breast cancer in a sister at age 40.
1. What should be included in the differential diagnosis of the standard views of the right breast? (Choose all that apply.)
A. Radial scar in upper inner breast
C. Infiltrating ductal carcinoma
D. Suspicious calcifications in the posterior right breast
2. What is the next step in management?
A. Perform routine mammogram in 1 year.
B. Recall patient for ultrasound.
D. Recall patient for additional views with spot compression.
3. What is the spot compression view?
A. A view of the entire breast in which more compression is applied to displace tissue better
B. A view that uses magnification to visualize microcalcifications better
C. A smaller paddle is used to image a focal area better
D. A larger paddle is used to image the entire breast better
4. What is the purpose of the spot compression view?
A. To separate true lesions from superimposition of normal structures
B. To visualize microcalcifications better
C. To determine if a mass is cystic or solid
D. To visualize better the lateral aspect of the breast on the craniocaudal (CC) view
ANSWERS
CASE 46
Spot Compression View
1. A and C
2. D
3. C
4. A
References
Ikeda DM, Andersson I, Wattsgard C, et al. Interval carcinomas in the Malmo Mammographic Screening Trial: radiologic appearance and prognostic considerations. AJR Am J Roentgenol. 1992;159(2):287–294.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:42.
Comment
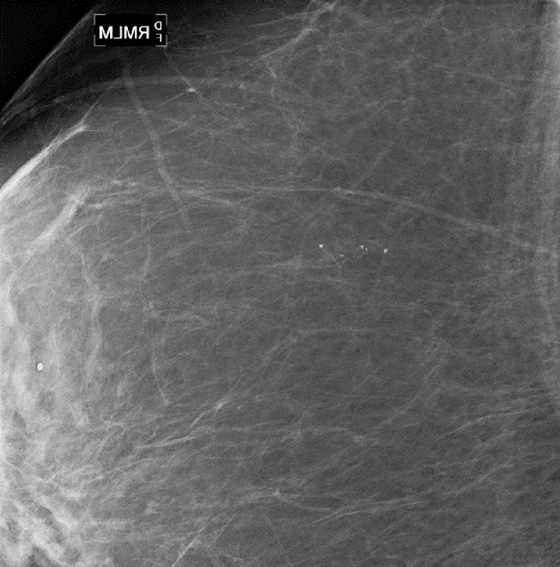
When a lesion is suspected on review of screening mammogram views, the patient is recalled for additional evaluation. The most common reason for missed cancer at screening mammography is the developing density. In a review of interval cancers, 22% were found to have subtle signs of malignancy on the previous mammogram, and most of these were developing densities. Comparison with previous mammograms is important, and if a density is new, particularly in a postmenopausal patient, the mammogram should be given a BI-RADS 0, and the patient should be recalled.
When the patient returns, it is often best to perform spot compression views first when evaluating a possible developing density. The finding is often normal, and the spot compression view can efface the lesion. When the lesion is effaced, no further work-up may be needed.
The spot compression view is performed with a small paddle applied directly over the area of concern. The greater compression applied makes a true lesion stand out from the surrounding normal tissue because the malignancy mass is denser, firmer, and less likely to compress compared with normal gland tissue.
In this patient, there was a subtle neodensity in the upper right breast on the mediolateral oblique (MLO) view (see the figures). She was recalled for additional views, and the spot compression views confirmed a true spiculated mass, seen on both views (see the figures). Ultrasound was performed for ease of biopsy. Biopsy was performed with ultrasound guidance and needle core technique and confirmed an infiltrating ductal carcinoma, grade II, 6 mm in size.
CASE 47
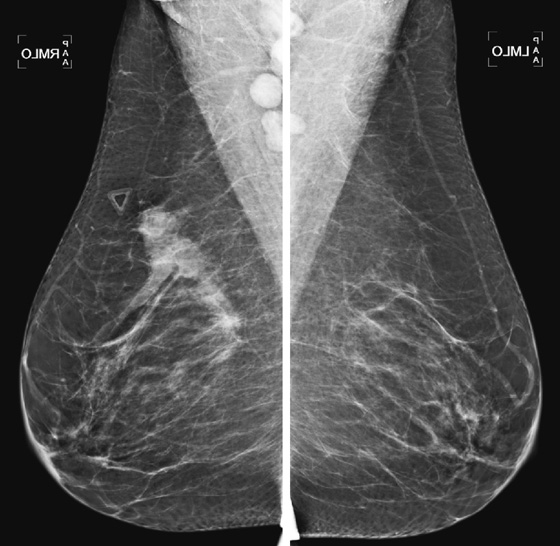
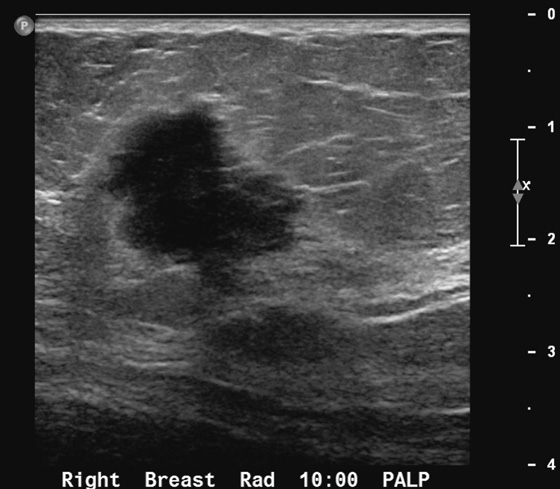
History: A 41-year-old woman presents for evaluation of a palpable mass in the right breast.
1. What should be included in the differential diagnosis? (Choose all that apply.)
A. Right breast mastitis with enlarged axillary nodes
2. What is the next step in management?
A. MRI to evaluate enhancement
3. What are the ultrasound features of suspicious lymph nodes?
A. Oval shape, with large fatty hilum
4. Can the axillary node be sampled with imaging guidance?
A. No, biopsy of the axilla is too difficult with imaging guidance.
B. No, it is difficult to position the axilla for stereotactic guidance.
D. Yes, the axillary nodes should be evaluated with ultrasound and sampled with needle biopsy.
ANSWERS
CASE 47
Mass and Axillary Node
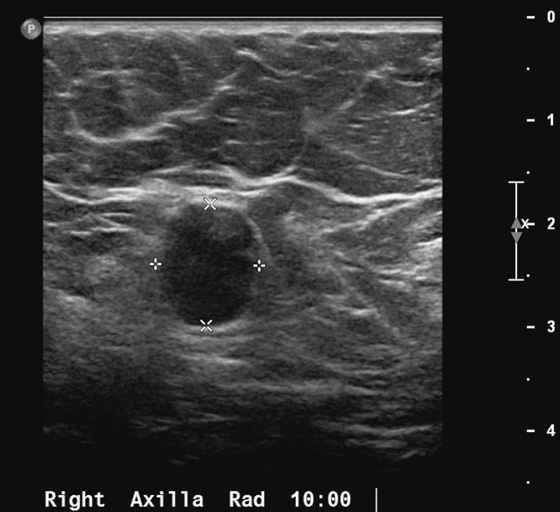
1. C and D
2. C
3. B
4. D
References
Abe H, Schmidt RA, Sennett CA, et al. US-guided core needle biopsy of axillary lymph nodes in patients with breast cancer: why and how to do it. Radiographics. 2007;27(Suppl 1):S91–S99.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:214. 395
Comment
Breast cancer may manifest as a palpable mass. Most palpable breast masses are benign tumors, such as fibroadenomas or cysts; however, malignancy should always be foremost in the differential diagnosis. The features on mammography of a suspicious mass include irregular shape, microlobulation, indistinct or spiculated margins, and high density (see the figures). Ultrasound features of a suspicious mass are similar, including round or irregular shape, microlobulated or spiculated margins, echogenic halo boundary, and hypoechoic internal echotexture (see the figures).
When a suspicious mass is seen, the surrounding breast tissue should be evaluated for the presence of satellite lesions, and all suspicious findings should be included in the biopsy planning. To evaluate extent of disease further, the axilla should be evaluated with mammography and ultrasound. The axilla should be included on a mediolateral oblique mammographic view, but if it is not well seen, a spot compression view can be obtained.
The axilla is easily evaluated with ultrasound. Malignant nodes have a characteristic appearance on ultrasound. In cases of obvious infiltration, the node is round, and the hilum is absent (see the figures). In earlier stages of infiltration, the hilum is present but may be slitlike. In even earlier infiltration, the hilum may appear normal, but there may be a focal thickening of the node cortex. Size is not an independent factor; normal nodes may be quite large, with a large fatty hilum and thin cortex. Malignant nodes may be small. Color flow Doppler should be performed of axillary nodes; increased blood supply on Doppler verifies the malignant tissue in the node. Doppler can also help map the location of axillary blood vessels before node biopsy.
If a lymph node is suspected to contain metastatic disease, needle biopsy can be performed with ultrasound guidance. Core technique or fine-needle aspiration biopsy is acceptable, although core technique is less operator dependent and has a higher success rate compared with fine-needle aspiration biopsy, and the evaluation of the material obtained does not depend on experienced cytologists. If lymph node metastasis is established before surgery, the patient need not undergo sentinel node biopsy.
CASE 48
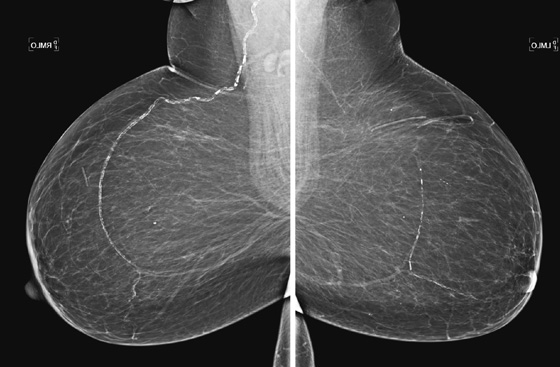
History: A 64-year-old woman presents for a routine screening mammogram. Health history includes a renal transplant 10 years ago.
1. What should be included in the differential diagnosis of the mammogram shown? (Choose all that apply.)
B. Benign mammogram with benign calcifications present
C. Suspicious linear calcifications in both breasts, possible ductal carcinoma in situ
2. What management is needed for this patient?
A. Recall for magnification views
C. Routine mammogram in 1 year
D. Doppler ultrasound to check for stenosis in the calcified arteries
3. Which patients are at risk for this condition?
B. Patients with chronic renal failure
C. Patients who are on dialysis only
D. Patients with diabetes if untreated
4. This mammographic finding may be a marker for what condition?
ANSWERS
CASE 48
Vascular Calcifications
1. A, B, and D
2. C
3. B
4. B
References
Cao MM, Hoyt AC, Bassett LW. Mammographic signs of systemic disease. Radiographics. 2011;31(4):1085–1100.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:84.
Comment
Vascular calcifications typically do not cause difficulty in diagnosis. Characteristically, they are seen as parallel lines of calcification, often with amorphous calcification within the parallel lines indicating the vessel wall en face. This condition is much more commonly seen in patients older than 60 years. These typical calcifications need no further work-up. They are found in approximately 7% to 34% of mammograms, increasing with age in one study; the prevalence exceeds 50% in women older than 65.
Vascular calcifications seen on mammography are in the media of the smaller vessel walls and the arterioles and not in the intima of the wall. The risk of the development of this calcification is increased in diabetes and chronic renal failure, and there may be an increased risk factor for coronary artery disease. The calcifications are not related to breast disease. The presence of arterial calcifications (see the figure) may be a useful marker for the presence of medial vascular calcification elsewhere in the body.
CASE 49
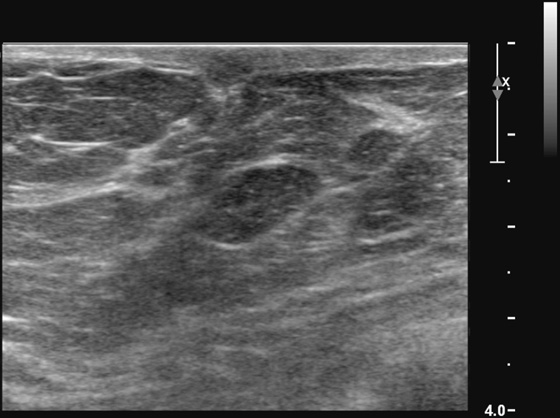
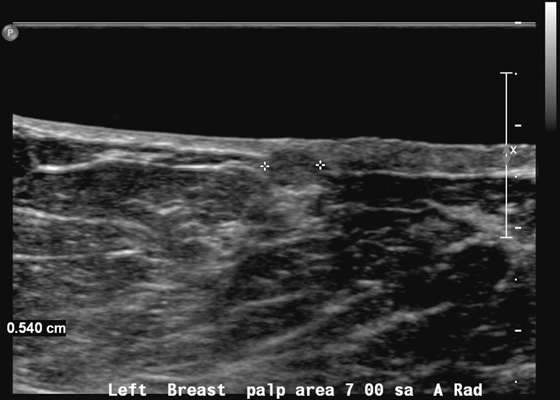
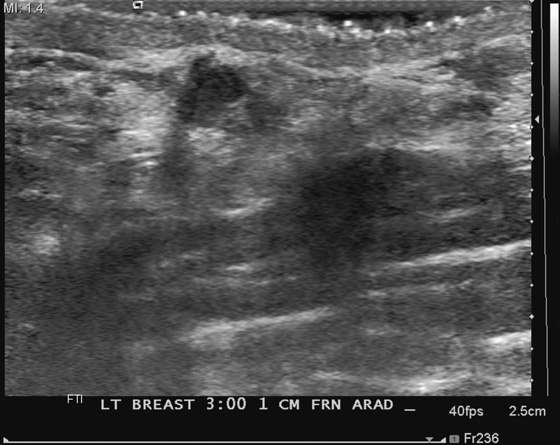
History: A 66-year-old woman presents with a palpable mass near the nipple. Mammography was negative (not shown). The third image represents a companion case.
1. What should be included in the differential diagnosis for the ultrasound images shown? (Choose all that apply.)
2. If mammogram and ultrasound are negative, but a superficial mass is clearly palpable, what examination could be performed next?
A. MRI to check for abnormal enhancement
C. Use of a standoff pad to optimize the ultrasound evaluation
D. Needle aspiration, guided by palpation
3. What is the next step in management for this lesion?
4. Why does a standoff pad help in imaging superficial structures on ultrasound?
A. The standoff pad allows visualization of the outer layer of the dermis.
C. The focal point of an ultrasound transducer is too shallow for superficial structures.
D. The standoff pad increases the volume-averaging artifact.
ANSWERS
CASE 49
Standoff Pad
1. A, B, and C
2. C
3. B
4. B
Reference
Stavros AT. Breast Ultrasound. Philadelphia: Lippincott Williams & Wilkins; 2004.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:149.
Comment
Palpable masses are of increased concern for breast cancer. Ultrasound is an important tool in the evaluation of a palpable mass. Ultrasound is typically the tool used to supplement mammography except in patients younger than 30 years and nursing or pregnant patients. In these subgroups, ultrasound is the first imaging tool.
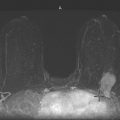
If the mass palpated by the patient is small and superficial, as in this patient (see the figures), ultrasound may not characterize the mass adequately because of constraints in the near field of the transducer. The short-axis resolution, which refers to the resolution in the superficial-to-deep plane, or elevation plane, must be optimized. For lesions in the shallowest 1 cm of the breast, a standoff pad or thicker layer of gel can yield better imaging analysis of the lesion.
The standoff pad improves resolution and decreases volume averaging of superficial structures (see the figures). There is improved margin sharpness and improved contrast resolution of the mass in the second figure compared with the same mass on the same day in the first figure. The focus zones, as adjusted by the sonographer for optimal imaging, are appropriately positioned for both images. The lesion is too small and too superficial to be adequately seen without a standoff pad. However, the standoff pad is useful only in this narrow application. It is not practical to use in evaluating the entire breast because deep lesions would be outside the focal zone and may be missed. It is also awkward to palpate the lesion while scanning with a standoff pad. The third figure demonstrates the application of a thicker layer of gel, in a different patient, to visualize a superficial palpable mass better.
CASE 50
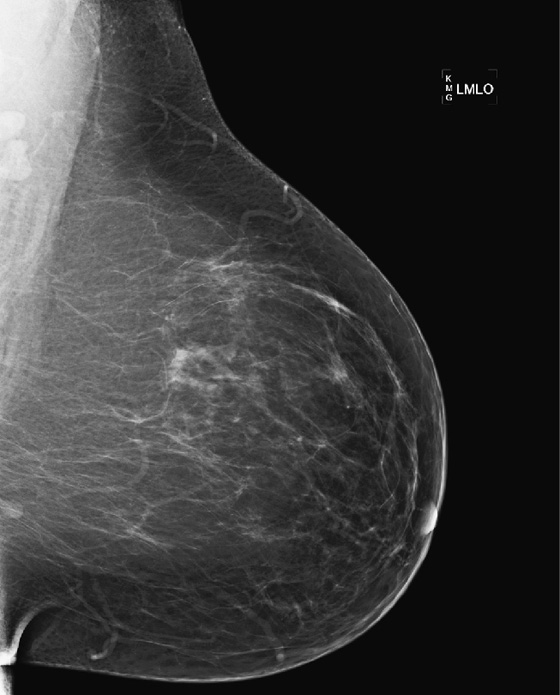
History: A 50-year-old woman presents for her baseline mammogram. A focal density is seen in the posterior left breast, but only on the mediolateral oblique (MLO) view.
1. What is the differential diagnosis for this single view of the routine mammogram? (Choose all that apply.)
A. Cyst at the edge of the image, posteriorly in the left breast
B. Highly suspicious mass at the edge of the image
C. Benign solid mass at the posterior edge of the image
D. Blood vessel at the edge of the image
2. What is the work-up?
A. No work-up is needed; the mammogram is normal.
B. Additional views with spot compression and mediolateral (ML) view are needed to help triangulate.
C. Initially, perform ultrasound.
3. How does the mediolateral (ML) view help triangulate the location of the density?
D. The ML view does not help in locating the lesion.
4. Is MRI needed in the evaluation of this patient?
A. Yes, MRI is important for complete evaluation.
B. Yes, the MRI is needed to prove that the mass is a looped blood vessel.
C. No, the MRI is unnecessary.
ANSWERS
CASE 50
Blood Vessel Presenting as a Mass at the Posterior Edge of an Image
1. A, C, and D
2. B
3. B
4. C
References
Park JM, Franken EA Jr. Triangulation of breast lesions: review and clinical applications. Curr Probl Diagn Radiol. 2008;37(1):1–14.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:42. -51
Comment
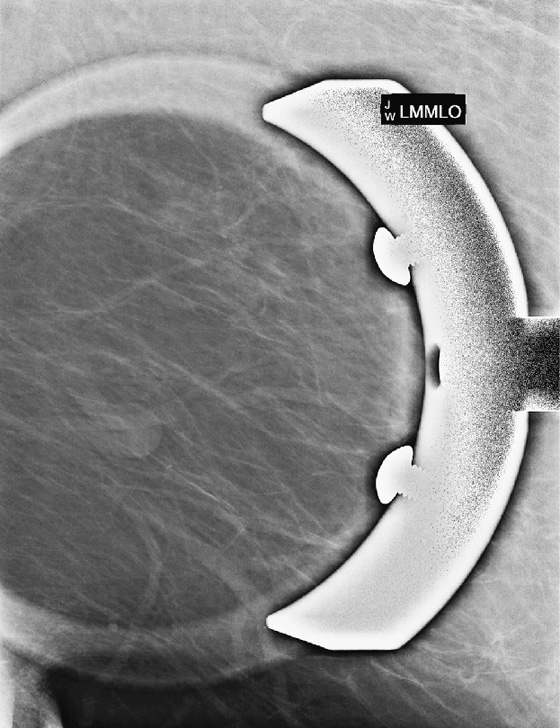
This asymptomatic patient had a routine screening mammogram, her baseline. The masslike structure was seen at the posterior edge of the image, on the left MLO view (see the figures). A spot compression view (see the figures) was performed, and the diagnosis is now apparent: The “mass” is a looped blood vessel. Blood vessels are seen on mammograms; they can loop, and the apex of the loop can be perceived as a mass. The proper diagnosis can usually be made with routine imaging, such as a spot compression view, angled views (for instance, 15 degrees from the view that showed the finding, or step-oblique images), or a rolled view if the mass is seen on the craniocaudal (CC) view.
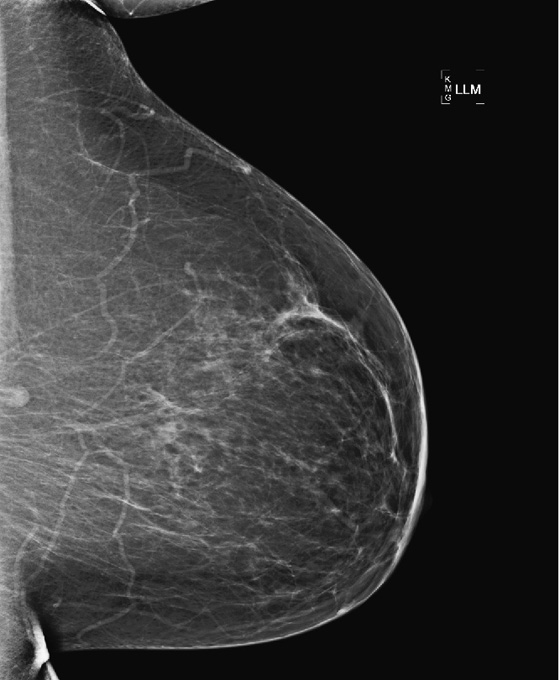
If the additional views do not help, and the lesion is seen only on the MLO view, as in this case (see the figures), then an ML view can be helpful to locate the lesion in the breast. This is called triangulation. By comparison of the MLO view to the ML view, the lesion can be determined to be in the medial or lateral breast on the CC view. Medial lesions, such as this one (see the figures) will be seen to “move up” on the ML view relative to the position on the MLO view.
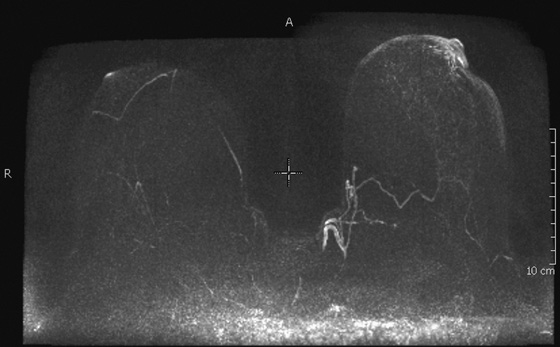
MRI is not necessary to evaluate this mammographic lesion, because it is seen to be a looped blood vessel on the spot compression view (see the figures). Further work-up with ultrasound is not likely to be helpful, and MRI, although it confirms the diagnosis in this case (see the figures), is an expensive tool for this purpose in this case. If the mass had been suspicious in appearance on mammographic images and not seen on ultrasound or felt on palpation, then stereotactic biopsy should be the next consideration. In this case, the radiologist recommended MRI for this density because stereotactic biopsy was felt to be not possible owing to the far posterior location of the density. It was not recognized as a blood vessel at the time of imaging.