CHAPTER 5. The person with type 1 diabetes
Derek Gordon and Florence Brown
Introduction 97
Epidemiology 97
The clinical presentation of type 1 diabetes 98
The aims of insulin therapy 99
Currently available insulins 102
Inhaled insulins 105
Formulations of injected insulin 105
Insulin regimens 106
Hypoglycaemia 110
Insulin adjustment 115
Nurse prescribing 117
Insulin injection technique and equipment 118
Conclusion 119
References 119
The history of the discovery of insulin in 1921 is one of intrigue, personality clashes and betrayal, and of a medical student on a summer job placement achieving the Nobel Prize. Without insulin, newly diagnosed children and young adults with diabetes faced a slow, wasting disease that could be treated only by a starvation diet and that led to an inevitable, early death. The discovery of insulin offered a chance of life to those previously living without hope.
EPIDEMIOLOGY
Type 1 diabetes accounts for approximately 10% of all people with diabetes and affects 10–20 million people worldwide. Type 1 diabetes generally affects people under the age of 40 years and 40% develop it before the age of 20 years. One of the most striking characteristics of type 1 diabetes is the large geographic variability in incidence. The Scandinavian countries and the Mediterranean island of Sardinia have the highest incidences in the world, whereas Oriental populations have the lowest incidences. The reasons for such geographical differences are not known.
THE CLINICAL PRESENTATION OF TYPE 1 DIABETES
Tom is an 18-year-old who has presented to his GP with a 3-week history of thirst. He was drinking up to 2 litres of carbonated drinks per day. He had noticed that he was passing much more urine than normal and was getting up through the night on at least three occasions. During this period of time his weight had fallen by about 4 kg. He had become increasingly tired and lethargic and had also noticed that his vision had become blurred. He also admitted to painful cracking of the foreskin. Glycosuria was confirmed as 2% and there was 3+++ of ketonuria using urine testing strips. The diagnosis of type 1 diabetes was confirmed by measurement of plasma glucose of 23.0 mmol/L. Tom was referred immediately by telephone to the local consultant diabetologist, who arranged to see him that day and insulin treatment was started.
People presenting with type 1 diabetes typically give a short and dramatic history of polydipsia, polyuria and weight loss. The lack of insulin causes a rise in blood glucose, which acts as an osmotic diuretic causing polyuria and polydipsia. In an attempt to provide energy, the body mobilises its glucose and fat reserves and, in so doing, switches into ketone production (see Chapter 1). This accounts for the acute weight loss, tiredness and lethargy. Left untreated, the person would develop diabetic ketoacidosis and coma. Nowadays, this is less frequently seen due to heightened awareness of the early diabetic symptoms by healthcare workers and the public in general.
Tom’s blurred vision was due to the presence of glucose in the lens of the eye. This causes alteration in the shape of the lens and subsequent blurring of vision due to altered refraction. This corrects itself as, with treatment, blood glucose levels return to normal, but it can take up to 6 weeks before the blurring disappears. People who are newly diagnosed with diabetes should be advised not to get their eyes tested for glasses for up to 3 months from diagnosis or 2 months from the time that their diabetes is stable.
The presence of sugar in the urine encourages the development of penile thrush, as in Tom’s case. Reducing glycosuria will eliminate the growth of organisms. In the meantime, however, Tom will also require appropriate antifungal treatment.
At the hospital clinic, Tom would be seen by the diabetes specialist team and have blood samples taken for glucose, urea and electrolytes including bicarbonate, liver function tests, full blood count and glycated haemoglobin (HbA1c). The possibility of diabetic ketoacidosis (DKA) needs to be considered. DKA is a medical emergency and requires hospital admission.
The dietician would assess Tom’s current diet and recommend dietary changes in the light of his history, lifestyle and estimated energy consumption. The DNS would start insulin therapy and teach Tom how to perform home blood glucose monitoring (HBGM) and arrange to see him frequently to continue his stabilisation and education. Tom would then enter a full education programme involving all the members of the healthcare team. This might continue over several weeks or months (see Chapter 11).
Question ‘Will my children get diabetes?’
A frequently asked question following a diagnosis of diabetes is whether other family members will be affected. In areas of the world where the risk of diabetes is moderate (e.g. the UK) the risk of developing type 1 diabetes by age 20 years is approximately 1 : 300. The risk is increased to 1 : 50 for children of women with type 1 diabetes and as high as 1 : 15 if a person’s father has type 1 diabetes. It is also estimated that by the age of 60 years approximately 10% of first-degree relatives will develop type 1 diabetes. If a child has type 1 diabetes then there is a 1 : 10 chance that another sibling will also be diagnosed with type 1 diabetes.
LESS TYPICAL PRESENTATIONS
Type 1 diabetes can affect people over the age of 40 years and can even occur in the elderly. It is now recognised that it can present with less acute symptoms. It can sometimes be difficult to decide in both young and older people whether they have type 1 or type 2 diabetes at the time of presentation. When the type of diabetes is in doubt, the diagnosis of type 1 diabetes should be considered if:
▪ ketonuria is detected, or
▪ weight loss is marked, or
▪ the person does not have features of the metabolic syndrome (see Chapter 4) or other contributing illness.
THE AIMS OF INSULIN THERAPY
▪ To preserve life.
▪ To relieve the symptoms of hyperglycaemia, i.e. polydipsia, polyuria, lethargy and weight loss.
▪ To restore ‘normal metabolism’.
▪ To prevent diabetic ketoacidosis.
The development of diabetes is associated with many subtle changes in metabolism, for example, lipids, blood clotting and connective tissue biochemistry. Many of these secondary changes in metabolism are responsible for the long-term complications of diabetes. The Diabetes Control and Complications Trial (DCCT) (DCCT Research Group 1993), as well as a number of smaller studies, have clearly demonstrated the beneficial effects of improved diabetic control in preventing the microvascular complications of diabetes (Wang et al 1993).
THE DIABETES CONTROL AND COMPLICATIONS TRIAL
Nearly 1500 people with type 1 diabetes were recruited from 29 diabetic clinics across the USA to take part in the Diabetes Control and Complications Trial (The DCCT Research Group 1993). People were randomly allocated to receive up to 10 years’ conventional or intensified treatment. Conventional treatment consisted of one or two daily injections of insulin and education about diet and exercise. People were reviewed every 3 months. Intensified treatment aimed for long-term near-normoglycaemia with at least four home blood glucose assessments a day, three or more insulin injections daily and monthly visits to a clinic with further advice freely available by telephone between clinics.
The trial was halted prematurely after people had been followed-up for periods ranging between 3 and 9 years. Those individuals in the intensively treated group maintained significantly better metabolic control throughout the study period. However, despite the intensive treatment, only 5% of this group attained the goal of near-normoglycaemia throughout the study.
Nevertheless, the study dramatically demonstrated that improving diabetic control was associated with a significant reduction in the risks of developing diabetic complications or their progression. The reduction of 2% in HbA1c in the intensively treated group of individuals was associated with:
▪ 76% reduction in the risk of newly developing retinopathy and 54% reduction in the progression of retinopathy
▪ 39% reduction in the incidence of microalbuminuria and 54% reduction in progression to proteinuria
▪ 60% reduction in development of neuropathy.
This improvement was apparent at all levels of metabolic control. In other words, a reduction in HbA1c from 16% to 14% was associated with the same improvement in outcome as a reduction from 10% to 8%. Nevertheless, it remains the case that those people with poorest control have the greatest risk of complications.
Targets for glycaemic control
The DCCT has implied that there is no threshold figure of HbA1c below which complications do not occur. However, on the basis of epidemiological studies in the DCCT and UK Prospective Diabetes Study (see Chapter 4), the microvascular risk appeared to be low once an average HbA1c was around 7.0–8.0% while macrovascular risk continued to fall with HbA1c levels down to 6.0–7.0% (DCCT standardised). This has led the National Institute for Health and Clinical Excellence (NICE) to make the following recommendations (NICE 2004):
▪ Adults with type 1 diabetes should be advised that maintaining a DCCT-harmonised HbA1c below 7.5% is likely to minimise their risk of developing diabetic eye, kidney or nerve damage in the longer term.
▪ Adults with type 1 diabetes who want to achieve an HbA1c down to, or towards 7.5% should be given all appropriate support in their efforts to do so.
▪ Where there is evidence of increased arterial risk (identified by raised albumin excretion rate, features of the metabolic syndrome, or other arterial risk factors) people with type 1 diabetes should be advised that approaching lower HbA1c level (for example 6.5%) might be of benefit.
Tight (meaning the achievement of normoglycaemia) metabolic control has its downsides. People in the DCCT intensively treated group gained weight and experienced three times as many episodes of severe hypoglycaemia as the conventionally treated group. It should be remembered that many people with type 1 diabetes fear severe hypoglycaemia more than complications in middle or late life.
Tight metabolic control might not be appropriate for the following groups of people:
▪ people with loss of warning signs of impending hypoglycaemic attack
▪ young children, particularly under the age of 7 years, when hypoglycaemia can be associated with damage to the developing brain
▪ frail, elderly people or those with limited life expectancy in whom the rigors associated with close metabolic control, would not be appropriate
▪ people who have limited abilities to treat hypoglycaemia independently.
Self-monitoring of blood glucose
Self-monitoring of blood glucose was an integral part of the intensive treatment group in the DCCT. However, self-monitoring is only likely to affect blood glucose control when used to inform self-management of diabetes. In clinical practice there is often little relationship between frequency of blood glucose self-monitoring and frequency of insulin dose self-adjustment (Gordon et al 1991).
NICE has therefore made several recommendations, including the following:
▪ Self-monitoring of blood glucose levels should be used as part of an integrated package that includes appropriate insulin regimens and education to help inform choice and achievement of optimal diabetes outcomes.
▪ Adults with type 1 diabetes should be advised that the optimal frequency of self-monitoring will depend on:
▪ the characteristics of their blood glucose control
▪ the insulin regimen
▪ personal preference in using the results to achieve the desired lifestyle.
Chapter 7 further expands on the evidence base for blood glucose monitoring.
Insulins with different chemical structures, depending on their source and manufacture, are available:
▪ Bovine (Wockhardt UK)
▪ Porcine (Wockhardt UK)
▪ ‘Human’ (NovoNordisk, Lilly, Sanofi-Aventis)
▪ Insulin analogues (NovoNordisk, Lilly, Sanofi-Aventis).
ANIMAL INSULINS
The chemical structures of these insulins differ from human insulin by a few amino acids. Bovine insulin differs from human insulin in three of its 51 amino acids, and is thus more likely to cause antibodies to be formed against it. Porcine insulin differs in only one amino acid residue (alanine in place of threonine).
‘HUMAN’ INSULINS
There are two different methods by which ‘human’ insulin is produced. Porcine insulin can be chemically altered by replacing the alanine amino acid with threonine (enzymically modified pro-insulin: emp insulin).
Alternatively, ‘human’ insulin can be produced by introducing the gene for human insulin into bacteria or yeast (prb: proinsulin recombinant bacteria, or pyr: proinsulin recombinant yeast insulins). The organisms are cultured in huge vats and the insulin is harvested and purified.
Following the introduction of ‘human’ insulins there was much concern about altered warning signs of hypoglycaemia and an increased reported incidence of severe hypoglycaemic episodes with the newer insulins. However, a subsequent literature survey of 39 studies and 12 epidemiological reports concluded that there were no significant differences in the physiological responses to hypoglycaemia or the frequency of hypoglycaemic episodes between human and porcine insulins (Jogensen et al 1994). However, a number of individuals with long-term diabetes remain unhappy about taking human insulin. Healthcare professionals should remain receptive to these views and individuals should be able to continue to use porcine or bovine insulins.
INSULIN ANALOGUES
An analogue is a chemical with a similar, but not identical, molecular structure to another chemical. Insulin analogues have been produced in order to develop insulins which have novel properties. Box 5.1 lists the insulin analogues that are now available in the UK.
Box 5.1
Insulin analogues currently available in the UK
Short-acting analogues
▪ Insulin lispro (Lilly)
▪ Insulin aspart (Novo Nordisk)
▪ Insulin glulisine (Sanofi-Aventis)
Long-acting insulin analogues
▪ Insulin glargine (Sanofi-Aventis)
▪ Insulin detemir (NovoNordisk)
The currently available short-acting insulin analogues have been created by making amino acid substitutions at one or more sites in the insulin molecule. Insulin molecules normally aggregate together forming hexamers, that is, six molecules of insulin loosely bound together. In this form the hexamer is too large to cross from the subcutaneous site into the circulation. The hexamers must first separate into dimers and then into single insulin molecules before absorption into the bloodstream can occur. This process takes some time and delays the onset of action of the conventional insulins. However, the analogue insulins have been designed to prevent this aggregation and formation of hexamers. This means that when the short-acting insulin analogues are injected into the subcutaneous space they are absorbed much more rapidly.
The short-acting insulin analogues therefore have a more rapid onset of action and a shorter duration of action than the conventional insulins (Mudaliar et al 1999, Nielsen et al 1995; Table 5.1). These insulins therefore have the advantage that they can be injected immediately before, or indeed immediately after, eating. Their peak serum concentration coincides with the postprandial rise in blood glucose and a meta-analysis of several studies has shown that rapid-acting insulin analogues are more effective than short-acting ‘human’ insulin in improving postprandial glucose control, without an increase in the rate of hypoglycaemic episodes (Davey et al 1997). The shorter duration of action should help to prevent hypoglycaemic episodes occurring before the next meal. People perceive an improvement in their quality of life with rapid-acting analogues due to the increased flexibility of injection times and less frequent hypoglycaemic episodes.
| Note: with the exception of Hypurin Bovine Lente, bovine and porcine insulins have been excluded from the table. | |||
| Insulin type | Onset of action | Peak action | Duration |
|---|---|---|---|
| Soluble (human) | |||
| Human Actrapid | 30 minutes | 2–4 hours | 5–8 hours |
| Humulin S | |||
| Insuman Rapid | |||
| Short-acting analogues | |||
| Insulin lispro (Humalog) | 5–10 minutes | 30–90 minutes | 2–4 hours |
| Insulin aspart (NovoRapid) | |||
| Insulin glulisine (Apidra) | |||
| Isophane (Human) | |||
| Humulin I | 2 hours | 6–12 hours | 18–24 hours |
| Insulatard | |||
| Insuman Basal | |||
| Insulin Zn suspension | |||
| Hypurin Bovine Lente | 2 hours | 8–12 hours | 30 hours |
| Long-acting analogues | |||
| Insulin glargine (Lantus) | 1–3 hours | Flat with no peak | 24 hours |
| Insulin detemir (Levemir) | 1–3 hours | 6–7 hours | 20–24 hours |
Characteristics of the long-acting insulin analogues
Insulin glargine has also been produced by altering the amino acid sequence of human insulin. The substitution of an asparagine amino acid by glycine and the addition of two arginine amino acids to the end of the insulin β chain results in the insulin becoming more soluble at acid pH. When the insulin is injected into the relatively alkaline, subcutaneous space the insulin glargine (Lantus, Sanofi-Aventis) forms microprecipitates. These tiny crystals are absorbed slowly and at a constant rate into the blood stream. The characteristics of its action profile are shown in Table 5.1. Insulin glargine has a ‘peakless’ action and is, therefore, ideal insulin to act as basal therapy.
Insulin detemir has a fatty-acid side chain added to the insulin molecule. This allows it to bind to albumin, which again slows its rate of release into the blood and produces a flat and ‘peakless’ blood concentration curve following injection. Insulin detemir has a shorter duration of action than insulin glargine (see Table 5.1) and may require to be injected twice daily for people with type 1 diabetes.
A further property of the long-acting insulin analogues is the reproducible blood profile from one injection to another. Insulin detemir has a lower coefficient of variation (23–27%) than insulin glargine (36–48%) and both are lower than isophane insulin (46–68%) (Vague et al 2003). (Coefficient of variation is a mathematical measure of variability.) People develop more confidence in their insulins when the effect on blood glucose levels becomes more reproducible from day to day.
Alternatives modes of delivery of insulin (without requiring painful, skin injections) have been sought for many years but with little success until recently. Insulin in a dry powder form, which allows the insulin particles to be delivered to the lung alveoli, has now been developed. The insulin thus inhaled can be rapidly absorbed across the thin alveolar walls into the circulation. Exubera (Pfizer and Sanofi-Aventis) is the first inhaled insulin to come to market.
When Exubera is inhaled, blood insulin levels rise rapidly and in a similar fashion when tested against the short-acting insulin analogue, insulin lispro. Its duration of action is, however, prolonged and simulates that of the older soluble insulins (Rave et al 2005). Several studies have now demonstrated the efficacy of inhaled insulin in people with both type I and type 2 diabetes (Hollander et al 2004, Quattrin et al 2004, Skyler et al 2005).
Inhaled insulin is contraindicated for people who smoke and should not be introduced until cigarette smoking has ceased for at least 6 months. It is also not suitable for people with poorly controlled asthma or chronic obstructive airways disease. Inhalation is associated with coughing at the time of inhalation but this does not appear to be a major problem. Inhaled insulin is also associated with deterioration in lung function as measured by forced expiratory volume in 1 second (FEV1) and carbon monoxide diffusing capacity (DLCO). However, these changes are small, of dubious clinical significance, and thought to be of a temporary nature.
Freemantle and colleagues (Freemantle et al 2005) have demonstratedincreased acceptability of inhaled insulin over conventional insulin delivery when offered to people with type 2 diabetes. It is likely that inhaled insulin delivery will prove popular with people who have diabetes. However, long-acting, background insulin will still be required to be given as subcutaneous injections.
FORMULATIONS OF INJECTED INSULIN
All conventional insulins are currently available in three broad types of formulation, classed according to their duration of action (see Table 5.1).
First there is unmodified or soluble insulin, which is short-acting and lasts for 5–8 hours when injected subcutaneously. These should be injected approximately half an hour before eating so that the peak action occurs at the same time as the postprandial rise in blood glucose. The peak action of soluble insulin is 1–6 hours.
Second are the intermediate-acting insulins (called isophane insulin in the UK and NPH insulin outside the UK). The action of this insulin is extended by complexing the insulin molecule with protamine (a large protein) and zinc. The mixture of neutral (or soluble) insulin with protamine and zinc was invented by Hagedorn and the point where this complex is chemically formed is termed the ‘isophane ratio’; hence the names, isophane or Neutral Protamine Hagedorn (NPH) insulin. The insulin–protamine–zinc complex is absorbed more slowly, extending its duration of action to between 18 and 24 hours. The peak action of isophane insulins is usually 2–12 hours.
Finally, there are the long-acting or lente insulins. If the insulin is mixed with zinc alone, it forms large insulin–zinc crystals, which are slow to dissolve. Thus the action of crystalline zinc insulins can be extended beyond 24 hours and peak action does not begin for at least 4 hours. The lente insulins have fallen out of favour, following the introduction of the long-acting analogues, and the only one remaining in the UK is Hypurin Bovine Lente.
INSULIN REGIMENS
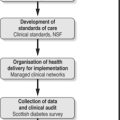
Insulin therapy aims to mimic the insulin response in people without diabetes (Fig. 5.1). It is evident that mealtimes are followed by immediate and sharp increases in insulin secretion as the pancreatic beta cells respond rapidly to rising blood levels of glucose. During the night and between meals there is a constant or basal secretion of insulin.
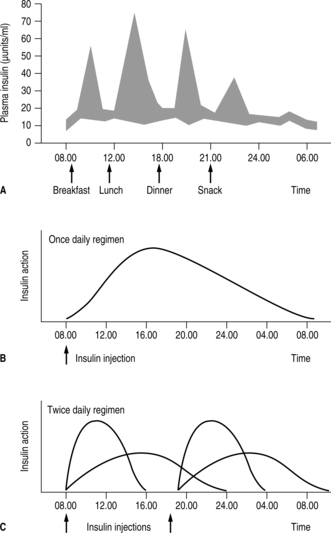 |
| Fig. 5.1(A) Insulin concentrations in the blood of normal non-diabetic subjects. Shaded areas represent one standard deviation above and below the mean for observations in six normal subjects. Arrows indicate timing of insulin injections for those with diabetes. (B and C) Insulin therapy: once-daily regimen and twice-daily regimen (arrows indicate insulin injections). |
Conventional insulin therapy has attempted in several ways to simulate this physiological process.
ONCE-DAILY INJECTIONS
A once-daily injection of either isophane insulin or a long-acting insulin analogue taken along with oral hypoglycaemic agents is becoming increasingly popular for people with type 2 diabetes. The insulin acts as a background while the oral agents are used to produce prandial rises in insulin or increased insulin sensitivity (see Chapter 4). Such a regimen would be unsuitable for people with type 1 diabetes. Those people on once-daily insulin injections will, however, represent a significant proportion of the caseload of community nurses who visit the frail and elderly with diabetes in their homes and administer their insulin injections.
TWICE-DAILY INJECTIONS
Twice-daily insulin regimens are appropriate for people who consider the number of daily injections an important issue in the quality of their lives. Similarly, people who find adherence to more complex regimens difficult will probably achieve better glycaemic control on this less rigorous regimen. In the twice-daily routine, the person injects a mixture of short-acting and intermediate-acting insulin before breakfast and also before the evening meal.
The profile of blood glucose levels produced by such a regimen differs significantly from physiological concentrations. Even with the newer insulin analogues, which can produce rapid rises in insulin in the postprandial period, the insulin profile between meals and during the night does not simulate normal physiology. Insulin concentrations are higher than required during the evening but gradually fall throughout the night following the evening dose of insulin. This means that most people on this regimen need an evening snack before going to bed to prevent nocturnal hypoglycaemia.
MULTIPLE INSULIN INJECTIONS
Alternative insulin regimens have been introduced in recent years. In particular, the system of multiple insulin injections has gained favour and is widely prescribed (Fig. 5.2). People inject short-acting insulin or insulin analogues before the three main meals of the day and usually take a further injection of isophane insulin or a long-acting insulin analogue just before retiring to bed. Those using short-acting analogues might need two isophane injections daily or a long-acting insulin analogue. This is because the duration of action of the short-acting analogue is such that pre-meal blood glucose levels might rise if not covered adequately by background insulin.
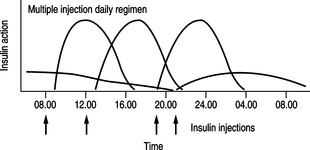 |
| Fig. 5.2Insulin therapy: multiple injection regimen (arrows indicate insulin injections). |
Isophane insulins peak during the night and wear off by the following morning. The introduction of long-acting insulin analogues, which provide a peakless profile of blood insulin levels, has resulted in improved glycaemic control overnight. Long-acting insulin analogues can be used when nocturnal hypoglycaemia or fasting hyperglycaemia is a problem.
The major advantage of multiple injection regimens is the flexibility that it provides. Mealtimes do not have to be adhered to rigidly. People can anticipate altered activity (they might want to take part in a sporting activity) and can plan if they are eating more or less than usual or at different times, by making suitable adjustments to their insulin dosage. The use of insulin pen devices has allowed people to carry their insulin with them in an acceptable form and to inject with relative convenience. Many people work complex shift systems and require the flexibility of a multiple injection regimen. An alternative to multiple insulin injection regimens is insulin pump therapy.
CONTINUOUS SUBCUTANEOUS INSULIN INFUSION (CSII OR ‘INSULIN PUMP THERAPY’)
James is a 52-year-old man with type 1 diabetes of 21 years’ duration. In the previous year, he had become prone to severe hypoglycaemia with no known cause. James would become disorientated and display bizarre behaviour whereby people thought he was either drunk or under the influence of drugs. James gave up his job and became depressed and withdrawn, especially when the efforts of the diabetes team to find a suitable insulin regimen were exhausted. Finally, James was offered insulin pump therapy, which had a transforming effect on his blood glucose profiles. He was able to reduce his 24-hour insulin dose by 50% and maintain blood glucose levels within the normal range without hypoglycaemia. His mood improved and he was able to return to work and lead a more normal life.
This case study demonstrates a rather dramatic example of the potential benefits of CSII. Insulin pump therapy was developed during the 1980s. Early pumps were difficult to use, bulky in size and unreliable. Sudden pump failure could precipitate severe diabetic ketoacidosis over a short time. This is due to the fact that there is no reserve of insulin in the subcutaneous space and blood insulin levels would fall rapidly if the pump failed to deliver insulin and hence blood glucose would rise quickly. For these reasons, the CSII fell out of favour in the UK. However, in recent years pump technology has improved and pumps are more sophisticated and easier to use; they are also smaller and more reliable. This has resulted in a resurgence of interest in pump treatment.
Studies that have randomly allocated people with type 1 diabetes to either multiple insulin injection regimens or CSII have demonstrated small improvements in glycaemic control in the CSII groups. HbA1c levels on average were about 0.6% lower using CSII. However, a meta-analysis of eight studies by the NICE Assessment Group showed that this improvement was not maintained at 6 months (NICE 2003).
People on CSII after 4 months of treatment required approximately 12 units/day less insulin than those using a multiple insulin injection regime. However, longer-term studies showed little difference in insulin dosage requirements.
Randomly controlled trials have failed to show any significant differences in the frequency of hypoglycaemic episodes between the two treatment modalities (Weissberg-Benchell et al 2003). However, observational studies that have reported on individuals chosen for CSII on clinical grounds have demonstrated significant reductions in the number of hypoglycaemic events in those people. This is clearly demonstrated in James’ case and would suggest that a subset of people who are susceptible to hypoglycaemia or diabetic ketoacidosis with conventional insulin treatments do particularly well with CSII (Rodrigues et al 2005).
CSII therapy demands a high level of commitment both from individuals with diabetes and from the diabetes team. However, individuals using pump therapy become expert in self-management and might require less input from the team over time.
For pump therapy to be successful, individuals will require to blood test at least four times per day, estimate carbohydrate consumption daily, move the cannula site every 2–3 days and learn how to programme the pump. The diabetes team should be trained to assess the person’s suitability for CSII and must be able to provide education and ongoing assistance to those people using pumps.
Failure of multiple injection therapy is considered to be failure to achieve target HbA1c without disabling hypoglycaemia occurring.
NICE has estimated that approximately 1–2% of people with type 1 diabetes may be suitable for CSII. This number may increase if pump therapy proves to be useful in children and if individual demand increases. Currently, pump use in the UK is restricted by cost factors and most people either buy their own pumps or are dependent on charity funding to sponsor the pumps. However, as a result of the recent NICE recommendations, most health authorities and health boards in the UK have made limited financial provision for CSII for those who need it.
HYPOGLYCAEMIA
Natasha is 25 years old and has had type 1 diabetes since she was four. Natasha’s diabetes was easily controlled during the first 12 months following diagnosis. This was because she entered a ‘honeymoon’ phase when there was some return of pancreatic function. However, her control deteriorated during early childhood when she refused to eat and her exercise activity was very variable. She started to menstruate at the age of 12 years and during her teenage years there was a further deterioration in metabolic control. However, over recent years, Natasha has taken much better care of her diabetes. Her HbA1c levels have fallen to 7.1–7.5% over the past 3 years.
At her most recent clinic visit, Natasha reported that she had had four recent severe hypoglycaemic episodes requiring another person to help her. On one occasion she awoke during the night to find the paramedics at her bedside. Her current insulin regimen is Human Mixtard 30, taking 24 units before breakfast and 18 units before her evening meal.
Natasha has begun to experience unexpected and severe hypoglycaemic episodes. This can be due to one of several causes:
▪ more insulin than is required
▪ not enough carbohydrate or abnormal absorption of carbohydrate from the gut
▪ exercise, especially exercise she is not used to
▪ alcoholism or binge drinking
▪ endocrine changes: pregnancy, Addison’s disease, hypopituitarism
▪ renal failure
▪ overused injection sites.
MALABSORPTION
Coeliac disease is slightly more common in people with type 1 diabetes and is often diagnosed late. The malabsorption of this condition can contribute to hypoglycaemia (Buysschaert 2003). Natasha should therefore be asked about symptoms of tiredness (caused by anaemia), abdominal bloating and discomfort and diarrhoea.
DELAYED GASTRIC EMPTYING
Impaired gastric emptying (gastroparesis) is present in 30–50% of people with long-standing type 1 diabetes and, for obvious reasons, might result in hypoglycaemic episodes.
EXERCISE
Exercise can affect glucose levels differently depending on blood insulin levels and the type of exercise. If the person lacks insulin, hepatic glucose production is increased and a progressive rise in blood glucose levels occurs. If the person has adequate levels of insulin in their system, hepatic glucose production is inhibited. Exercise enhances insulin sensitivity and peripheral uptake of glucose is increased resulting in hypoglycaemia if extra food is not taken. Prolonged moderate exercise involving mainly lower limbs, such as jogging, can result in hypoglycaemia during exercise or delayed hypoglycaemia that occurs typically 6–15 hours after exercise had ceased (Gallen 2005). Exercise that involves short bursts of anaerobic exercise can cause hyperglycaemia as can high intensity but short duration exercise (Gallen 2005).
Gallen (2005) suggests that in prolonged exercise long-acting analogues might increase the risk of hypoglycaemia and a switch to isophane insulin may be necessary. He recommends exercise should start with a blood glucose of around 7–10 mmol/L and that glucose be taken in regular small amounts when blood glucose starts to fall. Sports drinks contain around 6 g carbohydrate per 100 ml, as well as containing some sodium and potassium, which are useful for fluid replacement. Another factor contributing to hypoglycaemia might be the more rapid absorption of insulin from the limb involved in exercise. Injection of insulin into a subcutaneous site not involved in the exercise might be a safer option.
Levels of exercise vary. It is therefore important to know each person individually, as any increase in normal activity might be construed as exercise for that person and could result in apparent unexplained hypoglycaemia. As a young woman, Natasha might now have a partner and be engaging in sexual activity, which might be the cause of her nocturnal hypoglycaemia. Hence, education must be tailormade for the individual within the context of his or her lifestyle and significant others.
ALCOHOL
Alcohol inhibits gluconeogenesis and glycogenolysis, so that when alcohol is consumed in large amounts and food intake is inadequate hypoglycaemia can occur (van de Wiel 2004). The situation is especially serious if hepatic stores of glycogen are depleted. Under this circumstance, chronic alcohol abuse can lead to hypoglycaemia, which can sometimes be fatal. A modest intake of alcohol impairs the ability to perceive and interpret the symptoms of hypoglycaemia (Cheyne et al 2004). This is another reason why some people with type 1 diabetes experience hypoglycaemia after drinking alcohol.
ENDOCRINE CHANGES
Hypoglycaemia is common during the early weeks of pregnancy when insulin requirements may drop. The possibility of pregnancy should therefore be considered in any female of child-bearing age who presents with recurrent hypoglycaemic episodes or reducing insulin requirements.
Cortisol, adrenaline and growth hormone are hormones that antagonise the effects of insulin. If the blood levels of these hormones are reduced, insulin action will be unopposed and hypoglycaemia more likely to occur.
Addison’s disease, an autoimmune condition, is more commonly found in people with type 1 diabetes than the general population. This condition results in reduced secretion of cortisol from the diseased adrenal glands while people with hypopituitarism will have low circulating concentrations of cortisol and growth hormone. Although these conditions are fairly rare, Natasha should be examined for signs of Addison’s disease and hypopituitarism and if indicated she may require endocrine investigation to exclude hormone deficiency.
Hypothyroidism, or an underactive thyroid gland, is not uncommonly co-incidentally found in people with diabetes. Hypoglycaemic unawareness in theperson with type 1 diabetes should lead to hypothyroidism being excluded by biochemical testing. If found, it is readily treated with oral Levothyroxine.
LOSS OF WARNING SYMPTOMS OF HYPOGLYCAEMIA
The effect of tight glycaemic control
A fall in blood glucose stimulates the sympathetic nervous system, which in turn causes release of adrenaline from the adrenal glands. The combined effects of sympathetic nerve stimulation and increased blood levels of adrenaline cause the heart to beat faster and more powerfully, resulting in palpitations, increased sweating and tremor of the muscles. These symptoms contribute to hypoglycaemic awareness.
Hypoglycaemia also stimulates the release of glucagon, cortisol and growth hormone. These counter-regulatory hormones act to antagonise the action of insulin and help to restore normal blood glucose concentrations. A single episode of hypoglycaemia can reduce the neuroendocrine response to a subsequent episode occurring within 24 hours (Heller & Cryer 1991). Therefore, one hypoglycaemic episode makes a second episode within the following 24 hours more likely.
The blood glucose levels that trigger a counter-regulatory response and the onset of hypoglycaemia symptoms are not static. They are influenced by the prevailing standard of glycaemic control. The tighter the control, the lower is the blood glucose threshold that stimulates a neuroendocrine response (Widom & Simonson 1990). It is, therefore, not unusual for people with tight metabolic control to lose the warning symptoms of hypoglycaemia. This can lead to a three-fold increase in the incidence of severe hypoglycaemia (Gold et al 1994). In this situation, people usually regain their warning symptoms if they relax their glycaemic control and eliminate blood glucose levels of less that 4 mmol/L (Amiel 2001).
Autonomic neuropathy
For many years, autonomic neuropathy was considered to be the principal cause of impaired awareness of hypoglycaemia. However, several studies have now discounted this theory. People with autonomic neuropathy experience typical autonomic symptoms during hypoglycaemia (Hepburn et al 1993) and no relationship has been demonstrated between autonomic dysfunction and hypoglycaemic symptoms (Damholt et al 2001).
Renal failure
Deteriorating renal function is associated with decreased insulin requirement. This is because the kidneys are responsible for the excretion of insulin from the body and reduced function results in prolonged insulin action. This in turn can result in unexpected hypoglycaemic events. Other causes of the increased risk of hypoglycaemia in people with renal failure include anorexia and gastroparesis. Anyone with renal failure and diabetes would be referred to secondary care specialists for management.
TREATMENT OF HYPOGLYCAEMIA
For people with impaired awareness of hypoglycaemia, it is important to relax glycaemic control otherwise their lives can be blighted by frequent and severe episodes of hypoglycaemia. Relaxing control should result in a rise in the glucose level at which a counter-regulatory response occurs.
The management of acute hypoglycaemia depends on the severity of the episode (MacCuish 1993). When early symptoms of hypoglycaemia are recognised, the treatment is to eat carbohydrate in the form of glucose tablets or confectionary (sweets, biscuits or chocolates). Drinks with high glucose content are also suitable. At least 20 g of carbohydrate should be consumed.
When hypoglycaemia is more profound, the person might be unaware of his or her condition and refuse to eat or drink. Under this circumstance, a glucose gel such as HypoGel (Diabetic Bio-diagnostics) can be squeezed into the side of the mouth; jam or honey would be suitable alternatives. In the drowsy or unconscious person who cannot swallow, glucagon (GlucGen HypoKit 1 mg) can be given by subcutaneous or intramuscular injection and can be administered by family members or paramedics. Alternatively, when suitable personnel are available, dextrose (20–50 ml of 50% solution) can be administered intravenously. Cecilia was treated appropriately and was followed-up to ensure that she is aware of what causes hypoglycaemia and how she can prevent this occurring in the future.
Pancreatic and islet cell transplantation
Whole pancreas transplants
Transplantation of whole pancreases has been undertaken since the mid-1970s and this operation, in the right hands, can be associated with high success rates. Data from centres around the world now show that 82% of people remain free of insulin therapy 1 year after transplantation (Gruessner& Sutherland 1997). However, this is a major operation, which is associated with significant mortality. In addition, these people also require long-term immunosuppressive therapy to prevent rejection of the foreign pancreas. The side effects of immunosuppression are not inconsiderable. Pancreas transplantation is, therefore, never going to be a cure for most people with type 1 diabetes. In the UK, pancreas transplantation is undertaken at a few centres and is restricted to people with diabetes who are also undergoing, or have successfully undergone, kidney transplants.
Islet cell transplantation has distinct advantages over whole pancreas transplants. It is a fairly simple procedure; the islet cells are injected under local anaesthetic directly into the portal vein. Thereafter the cells travel to the liver where they seed themselves and grow.
The first reported islet cell transplant was in 1977. Early experience with islet cell transplants, however, was not encouraging with only a small number of people cured of their diabetes. In the early 1990s, people invariably needed to go back on insulin within 1 week of transplantation. It was not until the year 2000 that a Canadian doctor reported remarkable success rates in seven people who remained insulin free following islet cell transplantation (Shapiro et al 2000).
The success of Shapiro and colleagues is attributed to a number of factors. Shapiro realised that people were receiving insufficient numbers of islet cells and injected cells from two or three donor pancreases into each individual. Previous immunosuppressive regimes contained high doses of steroids, which are diabetogenic. Shapiro’s group uses more modern immunosuppressive drugs, which has allowed them to forego steroids. Finally, Shapiro recognised that chemicals that were previously used to extract the islet cells from the pancreas were, in fact, toxic to the cells and developed ‘more gentle’ techniques for isolating the islet cells.
It has been estimated that, with the number of donor pancreases available in the UK and the need for multiple donors for each person, only 90 individuals could be treated per year.
INSULIN ADJUSTMENT
People who inject insulin are encouraged to adjust their insulin depending on their day-to-day needs, which take into account diet, activity and information from blood glucose results as well as out-of-the-ordinary events such as illness, stress and exercise. Target blood glucose levels should be agreed between the clinician and the person with diabetes and this will guide the individual on what to aim for in terms of insulin adjustment. An understanding of how insulin works and knowing that insulin can work idiosyncratically in particular people is also helpful. For example a long-acting analogue insulin such as glargine can last 24 hours in some but only 18 hours in others. This will have implications for the timing of insulin injection.
Insulin adjustment is not in itself difficult but there seems to be reluctance by insulin-taking individuals to take on this responsibility. Part of the reason might be clinicians’ enthusiasm to adjust insulin, which leads to disempowerment of the individual. However, it is also been acknowledged that it has proved difficult for individuals to apply their knowledge of insulin adjustment and this will require more creative and sustained educational strategies for teaching this skill (Bonnet et al 2001). One of the other reasons for not adjusting insulin includes fear of hypoglycaemia (Reach et al 2005).
‘What would you do?’
This question will not only enable insulin adjustment skills to be demonstrated but facilitate the confidence required to maintain this skill. Development of confidence or self-efficacy is crucial in encouraging the individual to feel competent at adjusting his or her own insulin.
Before adjusting insulin, whether by the clinician or the individual taking insulin, it is important to consider the reasons why blood glucose levels may be outside the target range. Another question could be:
What do you think may be the reasons for your high/low blood glucose readings?’
or:
‘When you think about the way you manage your diabetes, is there anything you could do differently that would get these levels back to where you want them to be?’
This could lead to the individual considering other options to insulin adjustment, such as a change in dietary intake or activity levels. If no other changes can be made, then what are the considerations when adjusting insulin?
There is sometimes confusion around insulin adjustment when the dose of insulin is adjusted according to the blood glucose level at the time the insulin is due. This method of insulin adjustment leads to erratic results whereby frequent adjustment causes either hyperglycaemia or hypoglycaemia. Adjustment should occur only when a pattern of results is established ideally over 2–3 days and the insulin is thereafter adjusted to prevent the occurrence of either hyperglycaemia or hypoglycaemia and not in response to a single high or low result. The exception to this is if correction doses are recommended as part of an integrated package of self-management (see below).
The following points should be observed when considering insulin dose adjustment:
▪ When possible, insulin should be adjusted after a pattern of blood glucose results has emerged. For example, if the fasting blood glucose is high for three consecutive mornings then it is rational to increase either the basal insulin in a basal–bolus regimen or the evening insulin if the individual is on a twice daily fixed mixture of insulin.
▪ Traditionally, insulin has been adjusted by 2–4 units per dose, depending on type of insulin and level of blood glucose. If the individual takes large doses of insulin, e.g. 50 units or more at a time, then the adjustment might need to be 4 or more units to have any impact. Likewise, those on smaller doses, e.g. less than 20 units per day, will respond to smaller insulin dose changes, e.g. 1 unit at a time.
▪ Structured diabetes education programmes such as DAFNE (2002) match insulin doses precisely to carbohydrate intake with each individual learning his or her own insulin to carbohydrate ratio (see Chapter 11). Adjustment can take place proactively to accommodate varying meal sizes, exercise, alcohol or illness but patterns of blood glucose results over days are also observed to make sure that the matching has been accurate.
▪ Structured diabetes education programmes also include correction doses to be taken at normal times of insulin injections rather than being additional. If the blood glucose level is found to be higher than the target range then the individual can take additional insulin to achieve the target blood glucose. Again, this is done in a precise manner and not through guesswork. Correction doses should be a temporary measure because if the blood glucose is consistently above the target then the appropriate insulin taken at the previous mealtime or the basal insulin will be increased to achieve the target level.
NURSE PRESCRIBING
In the UK, following successful completion of the nurse prescribing course, a nurse can become an independent nurse prescriber and/or a supplementary prescriber. Independent prescribing is limited to around 110 medical conditions and there are about 240 prescription-only drugs listed in the Nurse Prescribers’ Extended Formulary. Independent prescribing is not appropriate for complex medical conditions or for individuals that have several medical conditions.
Supplementary nurse prescribing is more suited for those with chronic long-term conditions, including diabetes. Supplementary prescribing involves a voluntary partnership between the independent prescriber (medical) and the supplementary prescriber (nurse), who draw up a clinical management plan (CMP) that is agreed by the person with the condition. There are no legal restrictions on what conditions can be treated by a supplementary prescriber but the development of a clinical management plan for each person is a legal requirement. The CMP will include (Kyne-Grzebalski 2005):
▪ identification of the medicines, including the dosage, frequency and formulation. This can be drawn from existing protocols
▪ when to refer to the independent prescriber
▪ documentation of known drug sensitivities and how to report adverse reactions
▪ documentation of start dates and dates for review.
In the main, nurse prescribing has been shown to be beneficial both for individuals with a chronic condition and for clinicians, although further research is needed in this relatively new development in nursing (Latter & Courtenay 2004).
PEN DEVICES
Most people on insulin therapy in the UK are using pen injector devices. These are available on prescription and are usually manufactured by the insulin companies for use with specific insulins, although some independent pharmaceutical companies also manufacture insulin pen devices. Pen devices are available either prefilled, so that the entire device is discarded once used, or as reusable devices, where insulin cartridges are inserted.
Insulin pens will dial up ½-, 1- or 2-unit increments at a time and have a clicking mechanism that enables those with poor vision to dial up their dose by counting the clicks. Needles for insulin pens are also available on prescription and come in a range of sizes between 5 and 12.7 mm. Most popular are 6 mm and 8 mm. Insulin pens are easier to use than drawing up insulin into a syringe. However, many people still prefer to continue to use syringes.
INJECTION TECHNIQUE
Insulin should be injected into subcutaneous tissue at a 90-degree angle for optimal absorption. Skin preparation is unnecessary, other than making sure that the area of injection is visually clean. In those with little fat then the flesh might need to be pinched up, otherwise, whether the flesh is pinched or not is a matter of preference. The needle should be left in place for the count of 10 to allow the insulin to complete its flow through the pen.
DISPOSAL OF NEEDLES
Needles can be destroyed after use by the BD Safe-Clip device, which is available on prescription. Alternatively, used needles can be disposed of in a screw-top jar that is sealed and wrapped, or a ring-pull can that is crushed and disposed of in the normal way.
INJECTION SITES
Injection sites should be rotated between upper arms, abdomen, thighs and buttocks to avoid the development of fatty tissue growth, known as lipohypertrophy.
Lipohypertrophy, if it develops, will cause erratic absorption of insulin leading to unpredictable blood glucose levels, including hypoglycaemia. Overused injection sites should be avoided for at least a year or more. Individuals might not notice that sites have become overused and so part of the annual review should include a physical examination of injection sites.
Insulin not in use should be stored in a refrigerator but not allowed to freeze. Otherwise insulin is safe at room temperature for about 1 month. Exposure to heat will also reduce the potency of insulin action so care needs to be taken during warm weather and especially if on holiday in hotter climates.
The importance of tight metabolic control and the use of intensive insulin therapy and monitoring to prevent the long-term complications of diabetes are now well understood. The development of insulin analogues enabled the production of blood insulin profiles that more accurately simulate the natural secretion of insulin by the human pancreas. However, it is yet to be shown that these newer insulins result in significantly better diabetes control. The development of modern insulin pumps has allowed the introduction of more reliable continuous subcutaneous insulin infusion regimens. Intensive therapy also relies upon education and self-empowerment of individuals with diabetes. The DAFNE project, through education of individuals with diabetes, encourages people to more accurately match their insulin requirements to their diet and exercise schedule and so learn how to control their blood glucose levels. As the targets for HbA 1c levels are set lower and lower, the incidence of hypoglycaemic events increases. There is therefore a need for education of individuals, their families, friends and health professionals of the problems associated with hypoglycaemia. The value of increased nurse prescribing in diabetes is yet to be evaluated but is likely to lessen the burden on physicians and improve the knowledge-base of people with diabetes. The importance of the diabetes team for aiding the person with diabetes to achieve optimal health has never been greater.
REFERENCES
SA Amiel, Hypoglycaemia unawareness: a reversible problem? Diabetic Medicine 18 (suppl 1) (2001) 11–14.
C Bonnet, R Gagnayre, JF d’Ivernois, Difficulties of diabetic patients in learning about their illness, Patient Education and Counselling 42 (2001) 159–164.
M Buysschaert, Coeliac disease in patients with type 1 diabetes mellitus and auto-immune thyroid disorders, Acta Gastroenterologica Belgica 66 (3) (2003) 237–240.
EH Cheyne, RS Sherwin, MJ Lunt, et al., Influence of alcohol on cognitive performance during mild hypoglycaemia; implications for type 1 diabetes, Diabetic Medicine 21 (3) (2004) 230–237.
DAFNE Study Group, Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial, British Medical Journal 325 (7367) (2002) 746.
P Davey, D Grainger, J MacMillan, et al., Clinical outcomes with insulin lispro compared with human regular insulin: a meta-analysis, Clinical Therapeutics 19 (1997) 656–674 .
MB Damholt, NJ Christensen, J Hilsted, Neuroendocrine responses to hypoglycaemia decrease within the first year after diagnosis of type 1 diabetes, Scandinavian Journal of Clinical and Laboratory Investigation 61 (7) (2001) 531–537.
Diabetes Control and Complications Trial (DCCT) Research Group, The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus, New England Journal of Medicine 329 (14) (1993) 977–986.
N Freemantle, L Blonde, D Duhot, et al., Availability of inhaled insulin promotes greater perceived acceptance of insulin therapy in patients with type 2 diabetes, Diabetes Care 28 (2005) 427–428.
I Gallen, The management of insulin treated diabetes and sport, Practical Diabetes International 22 (8) (2005) 307–312.
AE Gold, KM MacLeod, BM Frier, Frequency of severe hypoglycaemia in patients with type 1 diabetes with impaired awareness of hypoglycaemia, Diabetes Care 17 (1994) 697–703.
D Gordon, CG Semple, KR Paterson, Do different frequencies of self-monitoring of blood glucose influence control in type 1 diabetic patients? Diabetic Medicine 8 (1991) 679–682.
A Gruessner, DER Sutherland, Pancreas transplantation in the United States (US) and non-US as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR), In: (Editors: JM Cecka, PI Terasaki) Clinical transplants 1996 (1997) UCLA Tissue Typing Laboratory, Los Angeles, pp. 47–67.
SR Heller, PE Cryer, Reduced neuroendocrine and symptomatic responses to subsequent hypoglycaemia after one episode of hypoglycaemia in non-diabetic humans, Diabetes 40 (1991) 223–226.
DA Hepburn, KM MacLeod, BM Frier, Physiological, symptomatic and hormonal responses to acute hypoglycaemia in type 1 diabetic patients with autonomic neuropathy, Diabetic Medicine 10 (10) (1993) 940–949.
PA Hollander, L Blonde, R Rowe, et al., Efficacy and safety of inhaled insulin (Exubera) compared with subcutaneous insulin therapy in patients with type 2 diabetes: results of a 6-month, randomized, comparative trial, Diabetes Care 27 (10) (2004) 2356–2362.
LN Jogensen, A Dejgaard, SK Pramming, Human insulin and hypoglycaemia: a literature survey, Diabetic Medicine 11 (10) (1994) 925–934.
D Kyne-Grzebalski, Nurse prescribing: the process, preparation and its impact on diabetes care, Practical Diabetes International 22 (8) (2005) 277–278.
S Latter, M Courtenay, Effectiveness of nurse prescribing: a review of the literature, Journal of Clinical Nursing 15 (17) (2004) 56–61.
AC MacCuish, Treatment of hypoglycaemia, In: (Editors: BM Frier, BM Fisher) Hypoglycaemia and diabetes: clinical and physiological aspects (1993) Edward Arnold, London, pp. 212–221.
SR Mudaliar, FA Lindeberg, M Joyce, et al., Insulin-Aspart (B28 Asp-insulin): a fast-acting analogue of human insulin, Diabetes Care 22 (9) (1999) 1501–1506.
National Institute for Health and Clinical Excellence (NICE), Technology appraisal guidance no. 57. Guidance on the use of continuous subcutaneous insulin infusion for diabetes. (2003) NICE, London .
National Institute for Health and Clinical Excellence (NICE), Type 1 diabetes: diagnosis and management of type 1 diabetes in adults. Clinical guideline no. 15. (2004) NICE, London ; www.nice.org.uk/pdf/CG015_fullguideline_adults_main_section.pdf; Online. Available:.
FS Nielsen, LN Jorgensen, M Ipsen, et al., Long-term comparison of human insulin analogue B10Asp and soluble human insulin in IDDM patients on a basal/bolus insulin regimen, Diabetologia 38 (1995) 592–598 .
T Quattrin, A Belanger, NJV Bohannon, SL Schwartz, for the Exubera Phase III Study Group, Efficacy and safety of inhaled insulin (Exubera) compared with subcutaneous insulin therapy in patients with type 1 diabetes. Results of a 6-month, randomised, comparative trial, Diabetes Care 27 (2004) 2622–2627.
K Rave, S Bott, L Heinemann, et al., Time action profile of inhaled insulin in comparison with subcutaneously injected insulin lispro and regular human insulin, Diabetes Care 28 (50) (2005) 1077–1082.
G Reach, A Zerrouki, D Leclercq, JF d’Ivernois, Adjusting insulin doses: from knowledge to decision, Patient Education and Counselling 56 (2005) 98–103.
IA Rodrigues, HA Reid, K Ismail, SA Amiel, Indications and efficacy of continuous subcutaneous insulin infusion (CSII) therapy in Type 1 diabetes mellitus: a clinical audit in a specialist service, Diabetic Medicine 22 (7) (2005) 842–849.
AMJ Shapiro, JRT Lakey, EA Ryan, et al., Islet transplantation in seven patients with Type 1 diabetes mellitus using a glucocorticoid free immunosuppressive regimen, New England Journal of Medicine 343 (2000) 230–280.
Scottish Intercollegiate Guidelines Network (SIGN), SIGN 55: management of diabetes. (2001) SIGN, Edinburgh .
JS Skyler, RS Weinstock, P Raskin, et al., Use of inhaled insulin in a basal/bolus insulin regimen in type 1 diabetic subjects. A 6-month, randomized, comparative trial, Diabetes Care 28 (2005) 1630–1635.
A van de Wiel, Diabetes mellitus and alcohol, Diabetes/Metabolism Research Reviews 20 (4) (2004) 263–267.
P Vague, JL Selam, S Skeie, et al., Insulin detemir is associated with more predictable glycaemic control and reduced risk of hypoglycaemia than NPH insulin in patients with type 1 diabetes on a basal-bolus regimen with premeal insulin aspart, Diabetes Care 26 (3) (2003) 590–596.
PH Wang, J Lau, TC Chalmers, B Zinman, Intensive blood-glucose control and diabetes: a meta-analysis, Annals of Internal Medicine 119 (1993) 71.
J Weissberg-Benchell, J Antisdel-Lomglio, R Seshadri, Insulin pump therapy: a meta-analysis, Diabetes Care 26 (4) (2003) 1079–1087.
B Widom, DC Simonson, Glycaemic control and neuropsychological function during hypoglycaemia in patients with insulin-dependent diabetes mellitus, Annals of Internal Medicine 112 (12) (1990) 904–912.