Lifespan and Cultural Modifications
Objectives
Key Terms
adolescence (ăd-ō-LĚS-ěns, p. 43)
culture (p. 53)
geriatric (jěr-ē-T-rĭk, p. 46)
infants (p. 44)
neonates (NĔ-ō-nāts, p. 43)
noncompliance (NŌN-cŏm-PLĪ-ăns, p. 54)
pediatric (pē-dē-ĀT-rĭk, p. 45)
regimen (RĔJ-ĭ-měn, p. 48)
teratogenic (TĔR-ă-tō-JĔN-ĭk, p. 49)
![]() http://evolve.elsevier.com/Edmunds/LPN/
http://evolve.elsevier.com/Edmunds/LPN/
Overview
As the nurse learns how to give medications, he or she will see there are many differences in the medications patients take. There are differences in the patients as well. For example, small infants cannot take the same medication dosages as adults. Older adult patients may have several diseases that require many drugs; their risk for drug problems increases with every new product given. What type of special information does the nurse need to know to care for patients from birth to death?
Patient variables, or differences such as age, weight, and other diseases or medications they may be taking, affect how a drug acts in the body. Many cultural and even religious beliefs may influence whether a patient is willing to even take medication. Helping the patient understand how important it is to take a medication and how to take it properly can be a challenge. Learning about patients’ backgrounds and the things that are important to the nurse will assist the nurse in helping patients to get well.
Patient Factors That May Affect Drug Action
Before a drug can be sold, a lot of research is done, and after people begin using the new drug, much additional information is gathered. Standards have been set up by the U.S. Food and Drug Administration (FDA) to require drug companies to provide certain information to people who may prescribe, administer, or take the drugs they manufacture. This information includes a description of the therapeutic response, side effects, and adverse effects of the drug, and a list of other drugs that may interact with this drug. Information must be printed by the manufacturer and put into the drug box (the “product package insert”).
General factors that influence drug activity help the nurse figure out what the response to the medication should be. Some of these patient factors or variables are listed in Box 5-1.
Special Considerations for the Pediatric Patient
The changes that occur as a child grows from birth to adolescence (12 to 16 years of age) have a huge or profound effect on drug action and effect. Some changes are obvious, but mild or subtle changes in their responses to drugs occur as children grow and develop.
The terms child or children cover a very broad category from neonates to 16-year-old adolescents. A very small amount of drug may have a big effect on neonates (less than 1 month of age) because of their small body mass, low body-fat content, high body-water volume, and increased membrane permeability (for example, the skin or the blood-brain barrier). Immediately after birth, several factors influence drug absorption: no gastric acid is present to help break down drugs, no intestinal bacteria or enzyme function is present to metabolize a drug, and the gastrointestinal (GI) transit time (the time it takes for a drug to move through the stomach and intestines) is slow. The systems that deactivate drugs in the liver are immature, and even the immaturity of the kidney and renal excretion system adds to the speed with which a drug might be eliminated in the neonate.
In infants (1 month to 12 to 24 months of age) and young children, the decrease in total body water, increase in body mass, decrease in membrane permeability, and changes in body fat produce less obvious changes in drug response. The infant has a high metabolic rate and a rapid turnover of body water, which result in relatively higher fluid, calorie, and drug dosage requirements per kilogram of body weight than those of the adolescent. Growth and development or maturation of drug-metabolizing systems and the development of the urinary tract also results in changes in drug response.
Absorption
Drug absorption in infants and children follows the same basic principles as in adults. However, three factors tend to be especially important in children. First, the physiologic status of the infant or child determines the blood flow at the site of intramuscular (IM) or subcutaneous drug administration. Factors that may reduce blood flow to muscular or subcutaneous tissues include cardiovascular shock, vasoconstriction caused by sympathomimetic agents, or heart failure. In these conditions, there would be reduced absorption of any drugs injected intramuscularly or into subcutaneous tissues. In premature infants with little muscle mass, the blood supply to these areas and the resulting absorption are very irregular. In older children, muscle size and circulation in the muscles affect how rapidly a medication is absorbed. There is more rapid absorption from the deltoid muscle (shoulder and upper arm) than from the vastus lateralis muscle (thigh), and the slowest absorption is from the gluteal (buttock) muscles.
Compared with older children and adults, the instability or immaturity of different body processes in premature infants is a second influence on drug absorption from IM sites. For example, toxic drug levels may occur if the blood supply to muscle or subcutaneous tissues suddenly increases, leading to greater absorption of medication and increasing the amount of the drug entering the blood. With some drugs, there is only a small difference between the level of drug that is helpful and the level of drug that is toxic and harmful. We say that these drugs have a narrow therapeutic margin. Examples of these drugs are anticonvulsants, cardiac glycosides, and aminoglycoside antibiotics. With these drugs, it would be easy for an infant to get too much medicine when absorption is variable.
A final factor in drug absorption is that the skin of premature and newborn infants has a greater ability to absorb some chemicals because of its greater hydration. That is, the outside stratum corneum of the epidermal barrier in the skin may allow more fluid to enter because the system is not well developed. The transdermal route may be used with some infants to reduce the unpredictability of some medications that are usually given orally or intramuscularly (for example, theophylline). However, transdermal dosage patches available for sale are not intended for pediatric patients and would deliver doses much higher than what is needed for infants and children. Instead, rubbing the drug into the skin, putting the drug in an oil base, or using an occlusive dressing (covering the skin on which the drug is placed by wrapping the area in plastic wrap) are all different ways that may increase the absorption of topical or skin products.
Distribution
Drug distribution is determined by two factors: (1) the chemical properties of the drug itself (for example, the molecular weight), which do not vary; and (2) the physiologic factors specific to the patient, including total body water, extracellular water, protein binding, and pathologic conditions modifying physiologic function, all of which vary widely in different patient populations.
Metabolism
The biotransformation of drugs in the body into usable substances involves chemical reactions that convert a drug to an inactive or less active compound. In general, drug metabolism in infants is much slower than that in older children and adults. Because most drug metabolism takes place in the liver, the fact that the levels of cytochrome P-450 enzymes of infants are only 50% to 70% of adult values is important in treatment of children. The amounts vary for the different enzymes, but the ability to increase production of all enzymes continues until the third or fourth year of life.
Because neonates have a decreased ability to metabolize drugs, they may be at increased risk for adverse effects as a result of slow clearance rates and prolonged half-lives, particularly when drugs must be given over long periods.
Excretion
As with metabolism, the growth and maturity of the child’s organs has an important effect on the child’s ability to excrete the end products of the drug reactions. Problems caused by the incomplete development of the renal excretion system, including glomerular filtration, tubular secretion, and tubular reabsorption, are slowly resolved as the child develops prior to birth. However, this system may still be very immature at birth and may only slowly develop to normal over the first year of life.
This process of normal development has implications for drug clearance, particularly of common drugs such as penicillin, aminoglycosides, and digoxin, for which clearance rates may fall to 17% to 34% of the adult clearance rate. If a child is sick enough to require these drugs, the glomerular filtration rate may not improve as predicted during the first weeks and months of life. This means that adjustments must be made in dosage and dosing schedules. The child will also require more careful monitoring, and dosages should be determined based on plasma drug levels determined at intervals throughout the course of therapy.
The growth spurt and the increase in adrenal steroid and sex hormone (estrogen in girls, androgens in both sexes) levels that occur before puberty affect drug response in children who are near puberty and in adolescents. The increase in male muscle mass, increase in female body fat, and stability of the body temperature in both sexes also affect adolescent drug response.
These facts about the drug-metabolizing system in pediatric (infants through adolescents) patients are important to remember in looking at a child’s sensitivity to medication. For example, infants and children require a total daily digoxin dose that is approximately twice that of an adult on a basis of the ratio of weight to dose. It is thought that this increased requirement for digoxin is the result of a greater binding strength of the child’s developing myocardial digoxin receptors for digitalis derivatives. Variations in the development of drug receptors may make a neonate very sensitive to anesthetics such as curare but resistant to other anesthetics such as succinylcholine.
Adverse Reactions
The risk for drug-drug interactions and adverse effects is increased in very ill children and infants. Children may be exposed to drugs in three major ways: (1) transplacentally, when the drug is given to the mother during pregnancy and delivery; (2) receiving the drug as a result of direct administration; and (3) getting the drug through breast milk if the mother has taken the drug. Fetal exposure to drugs through the placenta and neonatal exposure through breast milk share a common characteristic: These are the only stages in life in which one is exposed to and affected by drugs given to another person, the mother.
The number of adverse reactions in pediatric patients is unknown. Because young children are vulnerable, their diseases are often complex, their drug therapy is often complicated, and adverse drug reactions are unavoidable or hard to assess. However, studies have generally found that rates of adverse reactions in children are equal to those in adults. The rate may be as high as 5.8% of drugs administered to children, although the rate is higher if the child is hospitalized rather than at home. Adverse drug reactions may have a large and immediate, delayed, or long-term effect on the child’s neurologic and somatic development.
With younger children, it may be difficult to tell whether the child is having an adverse reaction, is just experiencing symptoms of the underlying illness, or is having a paradoxic reaction to a drug (e.g., hyperactive behavior with antihistamines or chloral hydrate, sleepiness with stimulants such as Ritalin). Over-the-counter preparations (particularly antihistamines and adrenergic drugs found in various cough syrups, cold remedies, decongestants, and nose drops) may also provoke adverse reactions in pediatric patients, and many of these medications have now been banned for this age group. A broad spectrum of reactions may be seen, varying from minor hypersensitivity reactions to more serious problems, including alterations in growth, damage to anatomic or physiologic systems, and numerous other problems.
Children are not just small adults who require a smaller dose of medication. Although we know that children do respond differently to drugs, less research has been done to determine the safety and efficacy of many specific drugs when used in children. It has only been since 1996 that the FDA has required drug companies to label all medications with specific information related to their use in different pediatric age groups. In many cases, the information gathered during research on a drug used in an adult population may be safely extended to pediatric patients. But in some cases, the FDA has required companies to file additional information about their products when used with pediatric patients. Very frequently, nurses find that drugs are labeled with “safety for use in infants and children not determined” when they look for pediatric information about a drug. Thus all drug use in very young children should be approached with caution because of their immature metabolic and elimination systems. Toxic effects may develop more quickly and stay around longer, so special dosages are required (see Chapter 9).
Special Considerations for the Geriatric Patient
Older adult patients also react differently to drugs. Medications are absorbed, metabolized, and excreted more slowly and less completely in older adults. In geriatric persons (adults older than 65), problems with medications are often due to a lack of understanding of the way drugs are processed in the aging body and the body’s changed response to drugs. To further complicate matters, people age differently, and their individual body systems may also age at different rates.
Absorption
The overall importance of changes in the absorption of drugs with aging is not completely clear. There may be some delay in the absorption process. Physiologic changes that affect the GI tract include a reduction in acid output, so there is a more alkaline environment, which may affect drugs that require an acid medium for absorption. Reductions in blood flow, enzyme activity, gastric emptying, and bowel motility may increase the delay in absorption of some drugs, although they probably have little if any effect on the extent of absorption. Compounds such as iron, calcium, and certain vitamins that depend on active transport mechanisms for absorption may be affected by the decreased blood flow in the aging patient’s GI tract.
Distribution
The distribution of drugs in the body may also be affected by the aging process and is linked to the chemical makeup of the agent involved. There is a decline in total body water and lean body mass with aging that may result in less movement or distribution of water-soluble drugs into some tissues. If the dose of these drugs is not decreased, the patient may develop higher serum concentrations, leading to an increased effect or toxicity. Thus the usual rule is to start drugs using a low dose and then increase the dose slowly in older adult patients. Drugs that are distributed into body water or lean body mass include digoxin, cimetidine, lithium, gentamicin, meperidine, phenytoin, and theophylline.
The distribution of fat-soluble drugs may also be changed by the aging process. With aging, there is usually a decrease in lean body mass but an increase in total body fat. Thus, lipid-soluble drugs may be stored in larger amounts in fat tissues and remain in the body for a longer time. Diazepam, chlordiazepoxide, flurazepam, thiopental, antipsychotics, and some antidepressants are lipid-soluble drugs that may require a lower dose and slow increases if used in the older adult population.
Another important concern that may exist with older adult patients is a decrease in serum proteins such as albumin. Albumin is the most common protein that binds to various acidic drugs, and a large decrease in albumin may result in a greater amount of unbound drug that may circulate freely. Highly protein-bound drugs that tend to bind quickly to albumin include phenytoin, warfarin, naproxen, theophylline, phenobarbital, and some antidepressants.
Metabolism
The effect of aging on liver function is difficult to determine because there is no good marker for measuring liver, or hepatic, function. Overall, a decrease in liver mass occurs with age, along with a reduction in hepatic blood flow. The result of lowered hepatic blood flow may be seen with drugs that are mostly broken down the first time they go through the liver (high first-pass metabolism). The extent to which these drugs are metabolized depends on how fast they go through the liver. When blood flow is reduced, as may occur with aging, less of the drug is metabolized, so increased amounts of the active form may remain the blood.
In an aging liver, there may also be changes in the specific pathways or phases of metabolism during which certain chemical and molecular changes occur to prepare the drug for metabolism. During phase I metabolism, drugs are generally made more water soluble so they may be excreted in the urine. Because of age-related changes in this process, drugs that are metabolized by phase I pathways may have decreased or unchanged clearance, so the drug may stay in the body and not be eliminated. Drugs that undergo phase I metabolism include diazepam, flurazepam, chlordiazepoxide, piroxicam, quinidine, and barbiturates. Such drugs should be used with caution and at lower doses in older adult patients, and the nurse will observe these patients carefully for adverse effects. If possible, these drugs should be avoided, and other drugs that are metabolized differently (phase II metabolism) should be used. No changes with aging have been reported with drugs that are metabolized by phase II metabolic processes, including conjugation, acetylation, sulfonation, and glucuronidation.
Drugs that are metabolized by the liver may have less or reduced metabolism because of other changes in the liver and also because of the influence of other diseases. The aging liver often gets smaller, has less blood flow, is affected by changes in nutritional status, and may become overloaded with fluid from diseases such as chronic heart failure. These factors may result in a loss of “hepatic reserve,” or the liver’s ability to handle all the different chemicals it must process. In this situation, the patient may have more risk of adverse effects when drugs are added to the existing treatment plan.
The important point in terms of giving medications to older adult patients is to use greater care in treating each patient individually and report patient response so that the dosage may be changed, if necessary.
Excretion
Kidney, or renal, function is the single most important factor that causes adverse drug reactions. Studies show that renal function varies with aging. Biologic changes in the aging kidney include decreases in the number of nephrons; decreases in renal blood flow, glomerular filtration, and tubular secretion rate; and an increase in the number of damaged glomeruli. In addition, damage to the arterial walls of blood vessels and lowered cardiac output reduce the amount of blood that flows to the kidneys by 40% to 50% between the ages of 25 and 65. The result of these changes may be a decrease in excretion of creatinine, which is reported to decrease 10% for each decade (10 years) after age 40 years.
Creatinine is a muscle by-product, and almost all of it is removed by the kidney, making it an excellent marker to measure kidney function (or renal clearance). A drug’s creatinine clearance rate is the amount of blood from which a drug is cleared per unit of time. Although creatinine clearance is used to measure renal function, it is important to note that it is only an estimate. A number of formulas can be used to determine the creatinine clearance rate, but the results may not be very accurate. Creatinine clearance can be assessed more accurately by collecting urine for 24 hours and directly measuring the amount of creatinine in it, although this may be difficult to do. However, any method of estimating creatinine clearance may not be accurate in older adult patients, who have very little muscle mass and produce very little creatinine. In addition to the normal slowing of kidney function that may occur with aging and be made worse by disease, problems leading to dehydration can also affect renal function and make the decision about how much drug to give the older adult patient even more complex.
The important factors to remember when caring for older adult patients who are taking drugs that will be excreted from the kidneys is that each patient may respond a little differently to the drug. The dosage ordered should have been adjusted based on the best creatinine clearance estimates, and low doses or longer intervals between doses are the norm if it is believed some kidney damage might be present. Drugs that depend on the kidneys for elimination include many antibiotics, some antivirals, antineoplastics, antifungals, analgesics, and many cardiac drugs.
Other kidney changes that occur with aging include a decrease in the ability of the kidney to remove only chemicals and not fluid (renal concentrating ability) and a tendency for the kidney to hold onto sodium (sodium conservation), which may affect patients on high-dose diuretics.
Adverse Reactions
Many older adults with chronic illnesses are required to take medications daily. These drugs are helpful in controlling disease, but they also present a very real hazard to older adult patients. Approximately 90% of older adults have adverse reactions to drugs, and 20% of these reactions require hospitalization. As many as 30,000 people may die each year as a result of adverse drug reactions.
Because many older adult patients take several drugs, interactions among these different drugs may also cause problems for them. These patients may see several specialists, each of whom may prescribe different medications. If the specialists don’t know about all the different drugs a patient may be taking at the same time, the patient may be at risk from combining drugs that have adverse interactions with each other.
All drugs have some risk or hazard, but the medications most dangerous to the older adult patient are tranquilizers, sedatives, and other drugs that alter the mind and change what the patient thinks he or she sees, or cause the patient to become dizzy or lose balance. Diuretics and cardiac drugs such as digitalis also pose special dangers and must be given with caution and careful observation of how the patient responds. Older adult patients may become dehydrated easily, thus allowing the amount of drug in the blood to increase. This places them at greater risk for side effects and toxicity with normal dosages. Results of research show there is also a high rate of alcohol use among many older people, both living at home and in nursing homes. Thus we are now becoming aware that drug-alcohol interactions are a serious concern in this age group.
Laboratory tests should be ordered regularly to look at kidney and liver function, and the nurse should look for side effects and signs of toxicity at every visit or encounter in the hospital or nursing home–entering the room in the hospital or long term care facility, passing the resident in the hall, greeting the resident in the dining room. If the nurse notices signs or symptoms of toxic reactions or adverse effects of drugs or observes behavior that might be a side effect, this should be reported immediately to the registered nurse. These signs and symptoms include changes in level of mental function, increased fatigue, restlessness, irritability, depression, weakness, dizziness, headache, and disorientation. These problems may interfere with appetite, balance, and energy, leading to dehydration, weight loss, falls, and immobility (not being able to move around). It is important to see that these often mild symptoms may be caused by drugs and should not simply be ignored as “typical” older adult behavior. For example, an older adult who becomes confused might have a urinary infection.
Patient Teaching Considerations
Some older persons may require special teaching about how to take their prescription medications and about the danger of taking nonprescription drugs at the same time. Failure of older adult patients to follow their medication plan, or regimen, may be because of many reasons: the cost of the drug, difficulty in getting it from a pharmacy, poor memory, lack of desire to take the drug regularly, depression, and feelings of being overwhelmed by the responsibility of taking care of themselves. These things all contribute to older adult patients failing to follow a medication regimen. In some cases, arthritis or another disease that causes physical disability may make it difficult to open bottle lids or use an inhaler. Poor eyesight may make it hard to draw up insulin or read the dose accurately. Many older patients also diagnose each other’s health problems and share medications, which may make it very difficult for the nurse to evaluate the effects of prescribed medications in a particular patient. Patients who struggle to pay for their medicines may cut pills in half or skip doses without realizing it may prevent the drug from helping them.
Women’s Health Issues
There are some drugs taken mostly by women for women’s problems. These include drugs to treat female genital tract infections and supplements used during the childbearing years. Other drugs taken by women may either prepare them for pregnancy, prevent pregnancy, or help their bodies recover from the loss of fertility-related hormones as a result of aging. Some women take these drugs faithfully, some take them only part of the time, and many women never have the chance to take the medications because of lack of information or money. But all of these medications may influence a woman’s quality of life.
One of the biggest problems women have faced, particularly with the increase in diets high in refined sugars, is recurrent vaginal Candida infection. Newer antifungal medications have cut the treatment time for vaginal fungal infections from 7 days to 1 or 2 days. Although these products were once only used by women, now men with acquired immune deficiency syndrome are also using these medications to treat the opportunistic infections that often occur with reduced immunity.
There is now a great deal of scientific data showing that eating more foods high in folate (citrus fruits, cereals, leafy greens, and whole grains) or taking a multivitamin that has folic acid protects against neural tube birth defects such as spina bifida and anencephaly. Folic acid may also reduce the risk of heart disease and stroke.
Iron supplements have long been known to be helpful for patients who suffer from anemia resulting from blood loss. Thus women of childbearing age are often placed on iron supplements. Most menopausal women would probably also benefit from a multivitamin containing 10 mg of iron or less.
Although oral contraceptive pills (OCPs) do not reduce the patient’s risk of getting a sexually transmitted disease, their use has cut both the birth rate and the abortion rate. For older women, the risks associated with taking OCPs are less than those of pregnancy. However, women who smoke and use OCPs are at an increased risk of adverse side effects such as stroke.
For many years, hormone replacement therapy (HRT) was routinely used at menopause to reduce uncomfortable symptoms, such as hot flashes, and prevent calcium loss from bones. However, research has now shown that HRT may lead to increased risk of stroke, heart attack, and breast cancer in certain women. HRT is still used, but only in some women and for short periods. Although these drugs may improve quality of life, women taking HRT are now closely monitored for cardiovascular problems.
Special Considerations for Pregnant and Breastfeeding Women
Pregnant and breastfeeding women may have both chronic diseases and acute problems, either of which may require drug treatment. Giving medicine to pregnant women poses a big challenge. In pregnancy, the drug is really going to two people, so you must consider how the drug may affect the growing fetus. The benefit of any drug to a pregnant patient must be carefully weighed against the possible (or potential) risk to the fetus. All mothers want to have perfect babies, so it is important for pregnant women to avoid as many drugs as possible, especially those drugs with teratogenic potential, or those likely to cause malformations or damage in the embryo or fetus. In addition, you must be aware of the changing body chemistry of the mother throughout the pregnancy, as well as that of the growing fetus, and how this will affect the action of the drug itself.
Since the reports in 1961 of severe fetal malformations caused by the drug thalidomide, which was given to control nausea and vomiting in pregnant women, greater precautions have been taken to consider the effect of medications on pregnant women. Medications that have been confirmed as teratogenic in humans include antithyroid compounds; aminoglycoside antibiotics; anticancer agents; androgenic hormones; tetracycline; thalidomide; warfarin (Coumadin) and other anticoagulants; lithium; diethylstilbestrol; penicillamine; vitamin A analogues; and many anticonvulsants, such as carbamazepine, primidone, valproic acid, and phenytoin. Alcohol, methadone, and cocaine also are known teratogens. The FDA has developed categories for classifying drugs according to their known level of risk to the fetus and to breastfed infants (Table 5-1; also see end of the textbook).
Table 5-1
| FDA CATEGORY | DEFINITION |
| A | Adequate, well-controlled studies in pregnant women have not shown an increased risk of fetal abnormalities. |
| B | Animal studies have revealed no evidence of harm to the fetus; however, there are no adequate and well-controlled studies in pregnant women. OR Animal studies have shown an adverse effect, but adequate and well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus. |
| C | Animal studies have shown an adverse effect, but there are no adequate and well-controlled studies in pregnant women. OR No animal studies have been conducted, and there are no adequate and well-controlled studies in pregnant women. |
| D | Adequate, well-controlled, observational studies in pregnant women have demonstrated a risk to the fetus. However, the benefits of therapy may outweigh the potential risk. |
| X | Adequate, well-controlled, observational studies in animals or pregnant women have demonstrated positive evidence of fetal abnormalities. The use of the product is contraindicated in women who are or may become pregnant. |
FDA, Food and Drug Administration.
From Meadows M: Pregnancy and the drug dilemma, FDA Consumer Magazine, 2001. Available online at www.fda.gov/fdac/features/2001/301_preg.html categories. Accessed July 2008.
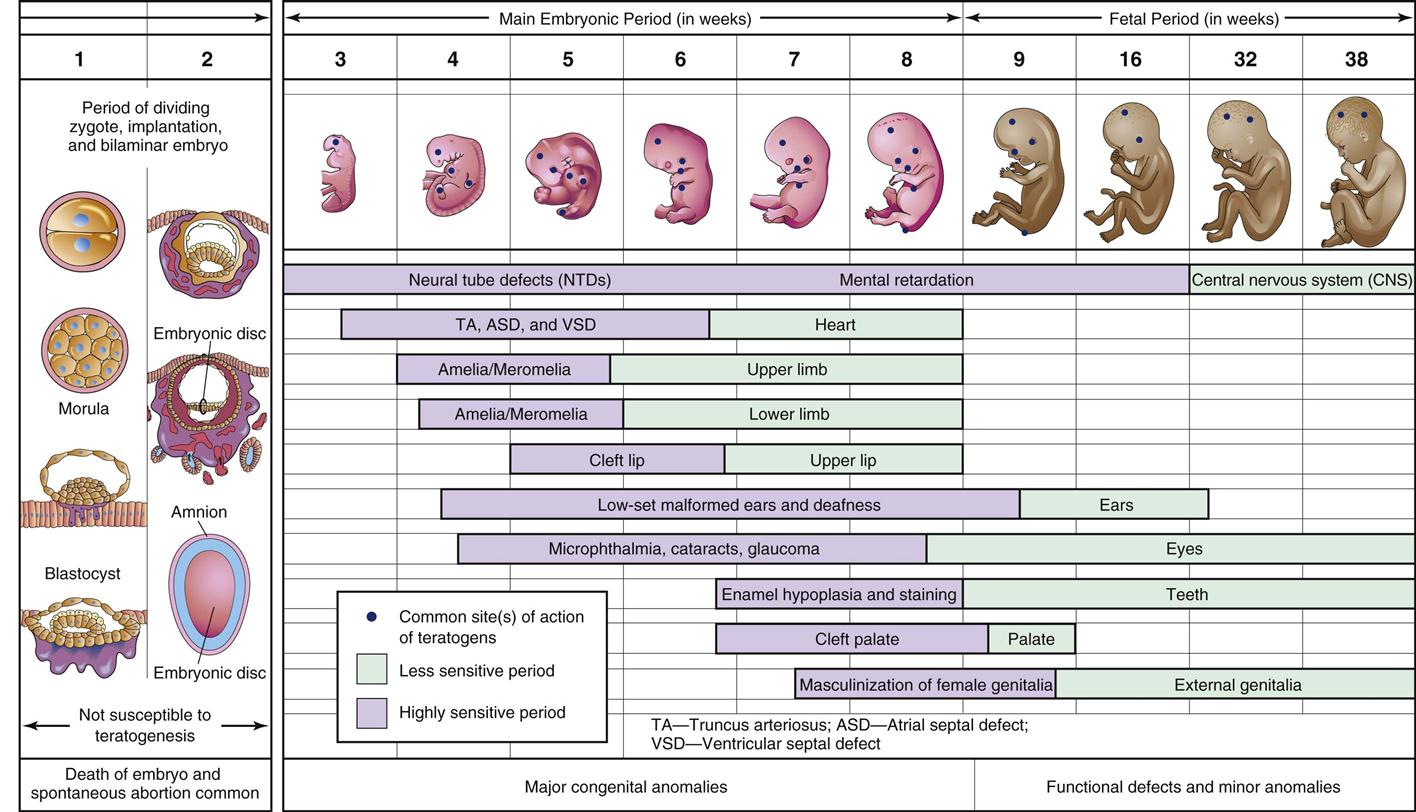
Factors such as what drug the mother takes, how much is taken, and the age of the fetus when the drug is taken are related to different types of malformations. Taking a drug during the first 2 weeks after conception (before implantation) results in an “all or nothing” effect. The ovum either dies from exposure to a lethal dose of a teratogen or recovers completely with no adverse effects. The critical period for morphologic, or structural, teratogenic effects in humans last from approximately 2 to 10 weeks after the last menstrual period (Figure 5-1). This embryonic period corresponds to the time of organ development (14 to 56 days), during which any teratogenic drug taken by the mother may produce major abnormalities in the embryo. Taking a teratogen later in the pregnancy during the fetal period (57 days to term) may result in minor structural changes, but abnormalities are more likely to involve problems with growth, mental development, and reproductive organ abnormalities. Clearly, it would be best if all women could stop taking any drugs before they got pregnant.
As the fetus grows, the placenta allows most drugs and nutritional products to cross from the mother to the baby. Thus it should be assumed that what the mother eats is also “eaten” by the fetus, with the exception of some drugs such as heparin and insulin. However, the reaction of a fetus to a medication is different from that of the mother. Because of an immature blood-brain barrier, many medications are able to pass into the brain of the fetus, and because of the immaturity of the hepatic enzymes, the liver of the fetus is not developed enough to metabolize drugs.
Because pregnancy causes symptoms, many pregnant women require medications. The top 10 chemicals or drugs that pregnant women take are analgesics, antacids, antibiotics, antiemetics, antihistamines, diuretics, alcohol, iron supplements, sedatives, and vitamins. Anyone giving medications will wish to read the latest information to make sure that every drug given to a pregnant woman is safe.
Drugs can pass into human breast milk, and this is also a major concern for the baby. Most information about the amount of drug that goes into breast milk has come from measuring the chemical content of the drug in the milk itself. Sometimes it is possible to see the effect of the drug in the baby, but not always. Drugs that should not be taken by breastfeeding mothers include bromocriptine, cyclophosphamide, cyclosporine, doxorubicin, ergotamine, lithium, methotrexate, phenindione, amphetamines, nicotine, cocaine, heroin, marijuana, and phencyclidine.
If a mother is given a prescription while she is nursing, she can lessen the infant’s drug exposure by taking the medication just before the infant is due to have a lengthy sleep period or right after a feeding. A bottle can then be substituted for the next scheduled feeding, and the affected breast milk can be expressed and discarded. Nevertheless, the infant should be watched for emotional changes, altered feeding habits, sleepiness, or restlessness. If short-term medication is required, the mother may need to consider stopping the breastfeeding for a short time and pumping and discarding her milk to maintain lactation until drug therapy is finished. Nursing mothers should not take sustained- or slow-release formulations or drugs with very long half-lives.
There is a growing body of knowledge about the influence of medication on breastfed babies. The FDA is currently proposing that drug manufacturers provide more information that helps identify the degree of risk to a baby from medication taken by a breastfeeding mother. The drugs are to be labeled with information about whether the drug will or will not be absorbed systemically and the degree of risk, how to minimize risk, and the data to support this information (Box 5-2).
Specific Products Used Throughout the Lifespan
Throughout the course of life, people may take many different types of medications. Some of these medications are used to help preserve health; others are given to help patients get well. Some of these common agents are discussed in this section.
Immunizations
The early immunization of children against diphtheria, pertussis, tetanus, chickenpox, measles, polio, and hepatitis is a national priority. Although many children are required to have their primary immunizations before beginning elementary school, the overall quality of the nation’s health would be better if these immunizations were given much earlier. Immunizations are one of the main things parents can do to protect the health of their children and one of the main things that have protected children from dying young. (See Chapter 21 for information on primary immunizations.) However, many children fail to receive these protective injections because of two factors. First, health care providers may not give immunizations because of the mistaken belief that they should be withheld if the child has a mild illness when examined. Second, parents may not have home-schooled children immunized or refuse immunizations for their children because of concern about possible adverse effects. Statistically, there is a greater chance that getting a disease will harm the child more than getting the immunization for that disease. Failure to immunize children places the whole community at risk. To encourage everyone to get immunizations, the U.S. Department of Health and Human Services created the National Vaccine Injury Compensation Program. This “no-fault” system provides payment to individuals or families of individuals who have been injured by childhood vaccines.
People who travel outside the United States, are in the military, or work in handling food are required to have immunizations against many diseases. To maintain protection against some diseases, patients must return for additional “booster” immunizations so their immunity will continue. We are learning that greater attention should be paid to immunizing more adult and geriatric patients against common diseases.
People at high risk, such as health care workers, older adults, and those who are immunocompromised, are encouraged to obtain yearly injections to help protect them against current strains of influenza. Children should also receive flu injections so that they avoid getting sick and bringing home infections to more vulnerable older adult or sick people at home.
Antidiabetic Agents
For many years, it was not clear if it was important for patients with diabetes to maintain strict blood glucose levels. But now, tight management of blood glucose levels has been proven to reduce organ damage in the diabetic patient. When the mother’s blood sugar level is controlled, there is less effect on the developing baby, who also responds to high glucose levels. These babies tend to gain more weight because their high sugar levels cause them to produce more insulin, and the blood glucose is then stored as fat.
Antihypertensive Agents
The latest findings from research on hypertension demonstrate that lowering the blood pressure below 120/80 mm Hg reduces the patient’s risk of myocardial infarction.
Cholesterol-Lowering Drugs 
It has been shown that lowering cholesterol levels helps reduce atherosclerosis and decreases the risk of heart attack and stroke.
Smoking-Cessation Products
Smoking has been linked to lung cancer and many other health problems. Both the smoker and those who are exposed to secondhand smoke (passive smokers) suffer. Fifty percent of cases of childhood asthma have been linked to the effects of passive smoking. It has been shown that the use of nicotine replacement products and drugs that reduce nicotine cravings, along with programs to change behavior, increase the chance that a person will be able to stop smoking. The risk of lung cancer and other adverse effects decreases in patients who are able to stop smoking.
Weight-Loss Drugs
Although they pose some risk, weight-loss drugs, along with exercise and behavior change, may increase a person’s ability to lose weight.
Antidepressant Medications
Evidence exists that many people who have depression because of chemical imbalances or lack of various neurotransmitters in the brain may be helped through the use of antidepressant medications and counseling.
Drugs for Erectile Dysfunction (Impotence)
Prescriptions for drugs to treat erectile dysfunction have broken all records in terms of numbers of prescriptions written per day. These drugs are reported to increase blood circulation to the penis, thereby producing an erection. Men who have taken the drug report an amazing response that has given them potency with few side effects. It is clear that the increased physical activity associated with the return of older adults to sexual activity will place some individuals at risk for myocardial infarction. Patients with coronary heart disease should not use these drugs if they are not healthy enough to have sex. The possible long-term effects of these drugs will have to be determined through study of patients who use them repeatedly for a long time.
Aspirin
The benefits of aspirin in some patients who have had cardiovascular problems are clear. Research studies have shown these benefits in both men and women with a wide range of prior cardiovascular disease, ranging from a past heart attack or occlusive stroke to angina—including former coronary bypass surgery and angioplasty patients. Current guidelines for treating patients who may be having a heart attack call for them to chew and swallow a 325-mg aspirin tablet as soon as possible. This may place them at risk for bleeding, but the benefit is seen as greater than the risk.
Caffeine
There is growing evidence that high levels of caffeine in pregnant women may be linked to a higher rate of miscarriages. Although mothers may continue to have some caffeine, the amount should be low.
Cultural Influences Related to Medications
Culture guides behavior for the members of a specific group and determines what is acceptable. The culture of a group represents the shared values, beliefs, customs, and behavior of the members. Each new generation learns the culture of the group through both formal teaching and informal life experiences but each new generation has different experiences that make them a unique subculture. Factors such as the roles of men and women, the need for privacy or personal space, the meaning of food and nutrition, religious beliefs, the significance of transitions from one stage of life to another, and the amount of economic and personal freedom all are part of the culture of the group. Changes in the group’s social and physical environment often lead to the development of different cultural practices. Subcultures may develop within the larger group based on ethnicity, when the subgroup has a common heritage, or on race, when the subgroup members share specific physical characteristics. As subcultures continue to live within the majority group, their ideas and values change, and they may grow to accept more of the practices of the dominant culture.
Over the years, cultural differences, or diversity, has increased among the citizens of the United States. There are many differences between the values and practices of the majority group of white, middle-class Americans and the minority subcultures that are growing in numbers. Some racial or ethnic group differences related to health care are obvious. People have different feelings, attitudes, and practices related to birth, death, and general health care. Some ethnic groups seem at risk for specific diseases. Culture often determines how they respond to suffering, pain, and loss. Culture may also direct standards of personal hygiene and need for privacy; acceptance of male and female children and tolerance of their behavior. People from different ethnic backgrounds may have differences in rate of growth and development of children; and how they adjust to life changes. The words used to talk about their feelings and attitudes, and the ideas related to health care behavior and treatments for illness, are quite different in each cultural subgroup and arise from the accepted values of the group. Good nursing care, whatever the setting, depends on the nurse having the ability to assess these differences among cultures and to adapt or change health care practices to better help the patient.
The importance of cultural diversity cannot be over- emphasized. There are frequent cultures among patients in a group, between patients and care givers. Different generations have different cultural norms. Because culture often dictates behavior, recognizing that a patient’s behavior may have meaning to them that is different than to you as a nurse is important. Nurses must ask rather than making assumptions about people’s beliefs.
Cultural assessment involves talking with a patient about differences and really listening to what they tell you about values, religion, dietary practices, family lines of authority, family life patterns, and beliefs and practices related to health and illness. There are usually strong cultural beliefs about important transitions in life, such as birth, marriage, and death. Patients may also have strong beliefs about such things as toilet training, common medical problems, and the use of herbs and other forms of therapy. Many of these individuals have already talked with friends, family, and religious leaders and may have incorrect notions about what is wrong with them and strong opinions about what should be done for them.
In the United States, health care workers are influenced by Western medical science and have been taught the values and beliefs of white, middle-class society. The nurses and physicians of many minority-group patients do not always share these same values and beliefs. Often today, the health care workers themselves come from a minority culture. Thus there are many challenges to talking with each other, setting priorities, and agreeing on solutions. For example, many of the health care beliefs shared by different cultural groups are based on “folk medicine” passed down through the generations of a culture. Many cultures have their own “healers” in the form of a medicine man, shaman, or curandera, whose services may be a blend of both medicine and religion. Members of the culture often seek the advice of such people before going to a Western or science-oriented health care provider. The various cures these healers suggest may be difficult to accept and include in Western health care. The fact that Western medicine has not been able to explain why some practices work does not mean they are harmful or not effective. Nurses must respect a person’s cultural beliefs in all areas if they want the patient to listen to their advice and teaching.
Attempt to accept and work with the cultural practices of patients as much as possible, and do not force patients to accept care that conflicts with their personal values. Forcing a patient to accept a particular type of care may even be harmful, because feelings of guilt and being separated from the religious or cultural group are likely to threaten the patient’s sense of well-being. Whether a patient is willing to take the medication provided by a nurse depends on what meaning the medication has to the patient and the individual’s beliefs about its helpfulness or harm. A great deal of research is available about subcultures within the United States. If the nurse works with minority groups on a regular basis, he or she will need to learn about these subcultures to provide good care to those patients. Usually, people are proud to tell someone about their background and beliefs. Because there is growing recognition of cultural diversity, many articles and texts are being published that also provide information helpful to health care workers. However, it is important not to assume that all African Americans, American Indians, or Hispanics are the same just because they are members of a specific group. Within these larger groups are many subcultures with different histories, beliefs, languages, and values. What may be seen as acceptable behavior by one segment of the culture may be offensive to another. Take care to ask minority-group patients about what they prefer.
It is now recognized that there is also health disparity, or inequality in health care, for many minority-group patients. Indeed, much has been written about the “culture of poverty” and its effects on health care. Many minority-group patients are at risk for severe health problems but get less health care because of discrimination against them. Through lack of money, insurance, knowledge, or other factors, it may be difficult for them to get better care. For example, many minority-group patients do not read English well enough to understand written instructions about their health problems, get prescriptions filled, or take drugs properly. The ability to read and understand this type of information is called health literacy. (See Evolve for information on health literacy considerations in patient teaching.) Some individuals are afraid to seek health care because they may be in the country illegally and do not wish to draw attention to themselves.
Finally, in addition to differences in health care beliefs, values, and attitudes, drug research has also shown important differences among racial and ethnic groups in their metabolic rates, clinical responses to drugs, and side effects. In particular, cardiovascular drugs and central nervous system drugs may produce varying clinical responses in various ethnic or racial groups, particularly the Chinese and other Asian groups.
Genetics
The mapping of the human genome and the research on genes and deoxyribonucleic acid (DNA) has shown that all individuals, no matter what race they belong to, are more similar than dissimilar. Research on individual races has concluded that African Americans are genetically the most heterogeneous (different) in their genetic profile. This may explain why this group as a whole has a greater rejection rate after organ transplantation, even with organs from living donors with similar tissue typing, and why they have particular unique responses to some types of medication. In people from some areas of the world, there are an unusually high number of cases of certain diseases, such as the thalassemia found in those of Eastern European and Mediterranean backgrounds. Research in genetics has shown that these diseases are passed down through families who carry certain genes. Hemophilia and sickle cell disease are other diseases that are the result of inherited traits in a family’s DNA.
The area of genetics and the response of groups to medications is a topic of increasing interest to researchers. It has been suggested that in the future, medications might be made specifically for different races and ages as we learn more about the role that heredity plays in both disease and treatment.
Spirituality and Religion
Regardless of basic ethnicity or culture, many individuals have a strong belief in a “higher power” that watches over or guides their lives, or may be asked for help in healing their illnesses. In times of sickness, people often think about religion or become more spiritual, and they try to find answers to why they have become sick or why they fail to get well. The idea of religious belief has at times been both controversial and unpopular, and many health care professionals are not comfortable talking with patients about their religious beliefs. However, it does not seem wise for a good nurse to ignore one of the most basic aspects of a person—one that affects how they view life and death. Often, all that is required is asking people about what they believe and then really listening or helping them find a religious leader to meet their spiritual needs.
Many research studies have been done about the influence of religion on health. The results suggest that people who pray have better symptom relief. People who have a strong social support network through their religion also seem to do better than those without such support. How a person of faith interprets symptoms, disease, and death, and how these beliefs influence the actions of medications, is still under study.
Why People Don’t Take Their Medicines 
The goal of patient teaching for drug treatment plans is to work with patients to help them make informed decisions about taking their drugs. Many of the variables of age, culture, and belief affect a patient’s willingness to take medications that are ordered. Patient difficulty with taking medications is a major unresolved problem. Compliance (cooperation) or concordance (agreement with) are the terms used to describe the patient who agrees with and follows the health care plan worked out with the health care provider. Drug noncompliance is when the patient does not follow the health care plan for taking medications. Patients sometimes use medications in ways that vary from the health care provider’s advice because they did not agree with the plan (nonconcordance). When patients decide what medications they are and are not going to take, they may not get well and may even take drugs that are harmful.
How does drug noncompliance begin? Often patients come to a health care provider almost as a last resort after having already tried a number of remedies for their symptoms. The patient’s age, sex, race, ethnic background, family, socioeconomic class, education, and past experience will have affected how the health problem is viewed and how the world in general is seen. Although today’s patients are more likely to be better informed about medical care than patients were in the past, they are also more likely to be skeptical or distrusting of the medical profession. They may have read about medical mistakes and successful lawsuits, heard about bad experiences in medical care from friends, or had bad experiences themselves. They may come from a lower socioeconomic class, be less educated, or have value systems different from those of the health care provider. Patients do have underlying fears and concerns and certain expectations for their care, but they are often reluctant to express these fears and expectations because they worry others will think they are foolish.
Health care workers may often assume patients’ value system is similar to their own and may not take the time to even determine the patient’s beliefs about and understanding of the illness. If this has happened, the patient may not have developed trust in the health care worker. So, many times a primary reason why patients are not cooperative is because of their past bad experiences with the health care system.
Most studies of drug compliance have been done with hospital-based patients. These studies have usually found that (1) patients are unfamiliar with their medications and how to take them, and (2) patients make errors in taking medications as much as 25% to 59% of the time. The nurse must ask questions and listen to what the patient is saying. If no one learns that the patient is not taking the medication as ordered, poor outcomes may be blamed on wrong dosage, failure of the drug plan itself, or incorrect diagnosis. Drug noncompliance often increases medical costs by leading to further hospitalization, by causing nursing-home placement for older adult patients, and by increasing the use of outpatient services. It is often difficult for the nurse to learn if the patient has not taken the drugs that were ordered; patients often wish to please health care workers and may not tell them the truth.
Reasons for drug noncompliance can be classified as errors of omission (a prescribed medication is not taken), errors of commission (a medication that has not been prescribed is taken), dosage errors (the wrong dose is taken), and scheduling errors (the medication is taken on the wrong schedule, for example, once daily instead of twice daily). There are six major reasons for patient noncompliance with drugs:
4. Poor understanding of instructions is a common cause of noncompliance.
6. People may not want to follow the treatment plan when there are unpleasant side effects.
Patients who have symptoms are more likely to take their medications than those who do not, especially if the symptoms are relieved by the medication. For example, patients will take pain medicine if they hurt but might not take high blood pressure medicine because they don’t feel bad. The patient’s age, sex, race, education, occupation, income, and marital status usually provide clues as to whether the patient will follow the instructions. People who have a stable support and family situation are more likely to follow treatment plans. For example, a husband who has a wife who will help cook special foods for the diet and remind him to take his medicine will have better success than a person who lives alone.
Although many people believe older adults are more likely not to cooperate with a treatment plan, most research indicates that aging does not affect compliance with prescribed medications. Middle-aged and younger patients are busy with careers, families, and the many activities they take part in each day and may forget about their medicines. Older adult individuals, however, typically have fewer things to do every day, and this helps them focus on taking medications as prescribed. Older adult patients have been shown to have difficulty opening some of the childproof drug bottles, and they may also experience difficulty with reading or understanding new instructions.
If the nurse establishes a good relationship with a patient, this may help the patient follow instructions. A good relationship means that the nurse and patient can share information and talk easily with each other. Factors that have been identified as helpful in having a good relationship with the patient include the following:
• Having a positive, confident approach
• Responding to patient complaints
• Encouraging patient questions
• Encouraging patients to become actively involved in their own care
• Seeking active patient participation rather than physician- or nurse-dominated decision making
• Working together to decide the plan of care
• Identifying and resolving things that make the patient less cooperative with the plan
Clearly, all these things take time, and the nurse may not always see the same patients over and over again in an office, clinic, or hospital. These positive activities can be included in the care plan as part of regular work with all patients.