CHAPTER 49
Lumbar Spondylolysis and Spondylolisthesis
Jennifer L. Earle, MD; Imran J. Siddiqui, MD; James Rainville, MD; John C. Keel, MD
Definition
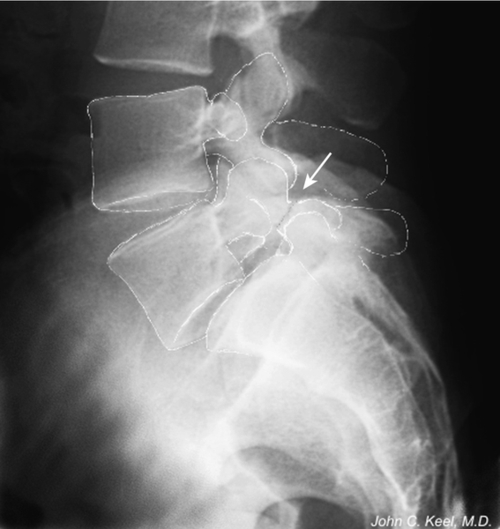
Spondylolysis refers to a bone defect in the pars interarticularis. Pars interarticularis translates to “bridge between the joints” and as such is the isthmus or bone bridge between the inferior and superior articular surfaces of the neural arch of a single vertebra (Figs. 49.1 and 49.2). When bilateral spondylolysis is present, the posterior aspect of the neural arch, including the inferior articular surfaces, is no longer connected by bone to the rest of the vertebra.
Spondylolysis is an acquired condition; it has never been found at birth [1]. Spondylolysis is most commonly acquired early in life [2,3] and is identified by lumbar radiographs in 4.4% of children 5 to 7 years of age [2,4]. By 18 years of age, 6% of the population has spondylolysis [2], with few additional cases thought to occur thereafter. The prevalence remains steady at approximately 6% in radiographic screening of adult spines [5,6]. Community population prevalence ranges from 5.7% to 11.5% when screening is performed by computed tomography (CT) scan [6,7].
Spondylolysis most commonly occurs at the L5 vertebrae, where about 90% of the cases are found. It is found with decreasing frequency at progressively higher lumbar levels [2,8,9]. It is more common in males than in females with roughly a 2:1 ratio (7.7%-9% vs 3.1%-4.6%) [6–8,10], can be unilateral (less common) or bilateral (more common), and has a suspected genetic predisposition [2,10,11].
The most likely cause of spondylolysis is a stress fracture of the pars that persists as a nonunion [12]. This is consistent with the higher incidence of spondylolysis suspected in adolescents and young adults who aggressively participate in sports requiring repetitive flexion-extension movements, such as gymnastics, throwing sports, football, wrestling, rugby, judo, dance, and swimming breast and butterfly strokes. Specifically among gymnasts, those with spondylolysis tend to be heavier, older, or training with more intensity. The incidence of spondylolysis is as high as 30% in professional soccer and baseball players in Japan [5]. Further supporting an acquired stress fracture as the cause is the lack of spondylolysis in the lumbar spines of individuals who have never walked [13].
Spondylolisthesis refers to displacement of a vertebral body in relation to the one below it. The most common type of spondylolisthesis, and the alignment abnormality that is implied when the term is used in this chapter, is an anterior displacement, also called anterolisthesis. Spondylolisthesis can also occur in a posterior direction, called retrolisthesis, or laterally, called laterolisthesis. Spondylolisthesis is an abnormal finding. Whenever spondylolisthesis is present, it is pathognomonic of structural and functional failure of the neural arch and facet joints, which are responsible for maintaining normal vertebral alignment.
Spondylolisthesis is classified by etiology and grade. There are five etiologic types (Table 49.1):
Table 49.1
Etiology of Spondylolisthesis
| Type | Etiology |
| Dysplastic | Congenital dysplasia of one or more facet joints |
| Isthmic (spondylolytic) | Bilateral pars defects (bilateral spondylolysis) |
| Degenerative | Degeneration of the facet joints and intervertebral discs |
| Traumatic | Fractures of posterior elements other than the pars |
| Pathologic | Malignant neoplasm, infection, or primary bone disease |
Dysplastic spondylolisthesis results from congenital dysplasia of one or more facet joints.
Isthmic or spondylolytic spondylolisthesis results from bilateral pars defects (bilateral spondylolysis).
Degenerative spondylolisthesis results from degeneration of the facet joints and intervertebral discs (most common at L4-L5 and with advancing age).
Traumatic spondylolisthesis results from fractures of posterior elements other than the pars, such as facet joints, laminae, or pedicles.
Pathologic spondylolisthesis results from pathologic changes in posterior elements due to malignant neoplasm, infection, or primary bone disease.
Isthmic spondylolisthesis is male predominant, whereas degenerative spondylolisthesis is more common in females [7]. This chapter is limited to discussion of spondylolytic (isthmic) spondylolisthesis.
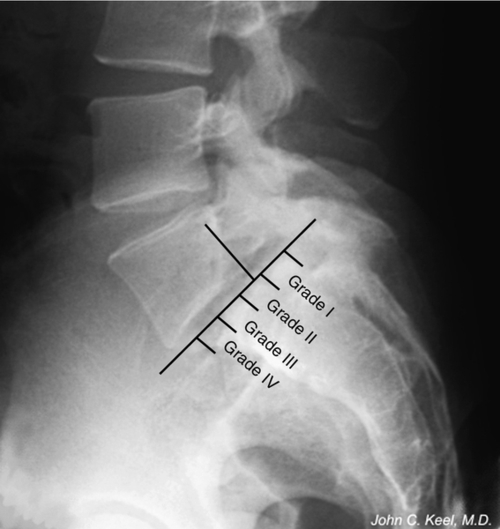
The grade of spondylolisthesis is rated by the percentage of slippage of the posterior corner of the vertebral body above over the superior surface of the vertebral body below. At least 5% slippage must be present for a diagnosis of spondylolisthesis to be conferred. Slippage can be further categorized into five grades: grade I indicates slippage from 5% to 25%; grade II is 26% to 50%; grade III is 51% to 75%; grade IV is more than 75% [14]; and grade V is complete dislocation of adjacent vertebrae, also called spondyloptosis. Most cases (60%-75%) are classified as grade I; 20% to 38% are classified as grade II; and less than 2% of all cases are graded III, IV, and V [8,15]. Slip of 15% or more is associated with increased risk of radicular pain or weakness [16] and is always associated with moderate to severe degeneration of the lumbosacral disc [10].
In children and adolescents with bilateral spondylolysis, spondylolisthesis is already present in 50% to 75% at the time of initial diagnosis of the spondylolysis [10,11,17,18]. The incidence of spondylolisthesis increases with age. After diagnosis, concern about the progression of spondylolisthesis is common but prognostic factors are lacking, making it difficult to predict which patients are at risk for progression [10,18]. However, participation in competitive sports has not been found to influence the progression of spondylolisthesis [10]. In addition, unilateral spondylolysis almost never progresses to spondylolisthesis [10]. Whereas spondylolysis is male predominant [7], spondylolisthesis and slippage are more common in females when bilateral spondylolisthesis is present on CT [19]. Typically, progression occurs before and during the early teenage years [2], and only minor progression occurs after skeletal maturity [10]. Advancing age may lead to slight additional progression of spondylolytic spondylolisthesis, which is usually attributed to progressive degeneration of the disc and facet joints [20]. There is a positive correlation between the percentage of slip and the degree of degenerative change but no correlation with disc herniation [10].
Symptoms
Most people with spondylolysis and spondylolisthesis are asymptomatic. Less than 5% of children diagnosed with spondylolysis or spondylolisthesis have back pain before the age of 18 years [10]. Whereas the incidence of back pain increases with age, the incidence of back pain in those with spondylolysis or spondylolisthesis is similar to that of the general population [2,8,10,16]. In addition, the reverse is true: those with and without back pain have nearly an identical incidence of spondylolysis [21]. Furthermore, the degree of spondylolytic spondylolisthesis is not associated with the prevalence of back pain [10], and no study has linked progression of spondylolisthesis with pain symptoms. Disability because of back pain is no more prevalent in the population with spondylolysis and spondylolisthesis than in the general public [10,17]. Ultimately, in patients with established spondylolysis, with or without spondylolisthesis, it is difficult to attribute pain symptoms to these abnormalities.
One exception is the child or adolescent who initially presents with acute back pain, in whom reactive changes to the bone marrow from spondylolysis or spondylolisthesis likely contribute to the patient’s symptoms. For these patients and others with back pain from associated spondylolysis or spondylolisthesis, no distinct pain characteristics have been found to distinguish their pain from that experienced by others with common degenerative back disorders [2,17]. The back pain can range from mild to severe and is frequently described as a dull, aching pain in the back, buttocks, and posterior thigh [2].
Spondylolysis with spondylolisthesis combined with disc degeneration may result in significant narrowing of the neuroforamina at the affected level. This can cause compression or irritation of the exiting spinal nerve, resulting in radiating pain and neurologic sequelae in the lower limb, often in dermatomal or myotomal distribution. Because spondylolytic spondylolisthesis most commonly involves the L5-S1 level, the L5 nerve is most often affected by this problem.
Physical Examination
The physical examination in spondylolysis and spondylolisthesis has few specific or sensitive findings. Painful trunk range of motion is often noted with children and adolescents with symptoms from acute spondylolysis. It is suspected that pain with trunk extension may be common in acute spondylolysis as this motion shifts load to the posterior vertebral elements and thus through the region of the pars [22]. Indeed, limited range of motion for trunk extension has been observed [15,23]. However, the precision of painful trunk extension has not been determined, and these findings are common to other spinal disorders. Palpation of the back may reveal local tenderness at the lumbosacral junction, the level at which spondylolysis is most common [15].
Detection of spondylolisthesis on physical examination is difficult except in the rare cases of grade III or greater slips. Here, a “step-off” of the spinous processes can be seen or palpated at the level of the spondylolisthesis. In grade I and II spondylolisthesis, the step-off is much more difficult to detect and has never been shown to be a reliable finding. Neurologic deficits and positive results of straight-leg raising tests are rarely found in cases of spondylolytic spondylolisthesis, including cases with sciatica [15]. When neurologic deficits are noted, they usually involve the L5 roots, which can become irritated within their neuroforamina, presenting as an L5 radiculopathy (weakness of the extensor hallucis longus and hip abductors as well as sensory loss on the dorsum of the great toe) [15,24,25].
Diagnostic Studies
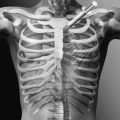
The lateral oblique radiograph may reveal the classic “collar on the Scottie dog” finding of spondylolysis (Fig. 49.3). This represents the bone defect between the inferior and superior articulating processes. The sensitivity of this view approaches only 33% because the plane of the pars defect must be close to the plane of the radiographic image to be clearly visualized [26]. The lateral radiograph reveals the presence and grade of spondylolisthesis.
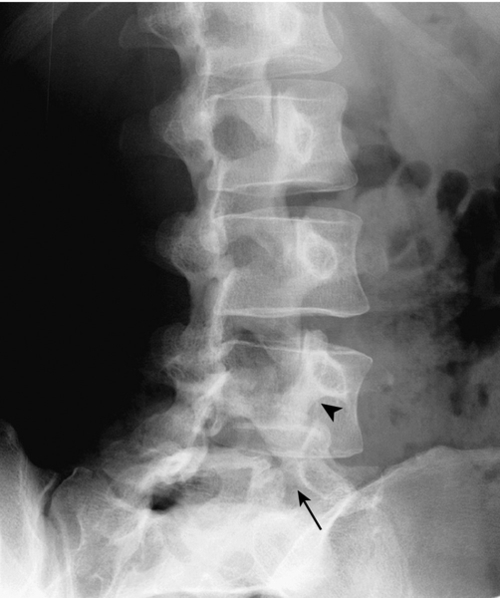
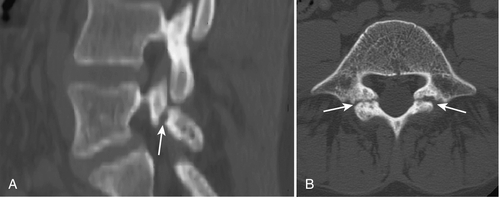
Because of its ability to clearly visualize bone, CT is considered superior to magnetic resonance imaging (MRI) for direct visualization of the pars interarticularis, and some advocate CT axial images as the test of choice (Fig. 49.4) for identifying spondylolysis [27]. Even with CT, pars defects can be difficult to identify because they can simulate the adjacent facet joints. However, they usually lack the regular cortical surfaces of the facets [28]. Also useful is trying to identify an intact cortical ring for each vertebra, including the vertebral body, the pedicles, the pars, and the posterior neural arch. If the intact ring is not found in any of the cuts through the levels of the pedicles, a defect in the ring at the appropriate position suggests diagnosis of spondylolysis [29]. If spondylolysis is suspected when CT is ordered, scanning can be done with thin sections or reverse gantry angle to ensure optimum visualization of the pars [27].
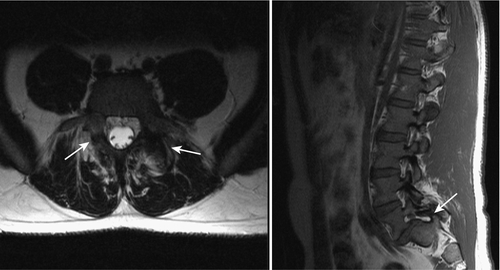
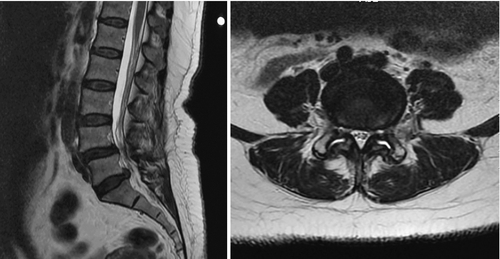
For children and adolescents with acute spondylolysis, MRI with fat saturation technique can identify subtle bone marrow edema of early stress injury to the pars interarticularis [30]. Identifying the bone defect of the pars is more challenging with MRI. Defects are most commonly noted as a lack of bone continuity between the superior and inferior articulating process on sagittal T1 images. MRI has a sensitivity of 57% to 86% (Fig. 49.5) [31]. Axial T2 MRI demonstrating increased fluid in the facet joints is also sensitive for early detection of spondylolisthesis (Fig. 49.6) [32]. Combining both of these MRI methods increases the sensitivity for detection of spondylolisthesis to 94%. MRI is also useful for grading spondylolisthesis and for visualizing the neuroforamina. As spondylolisthesis progresses, the neuroforamina become horizontally oriented, resulting in a position of the exiting spinal nerve that is between the pedicle above and the uncovered disc below. As the disc degenerates and loses height, foraminal narrowing is exacerbated, which may entrap the exiting spinal nerve. This is well visualized on T1 sagittal and axial images as the high fat signal that normally surrounds the spinal nerve becomes obliterated [25].
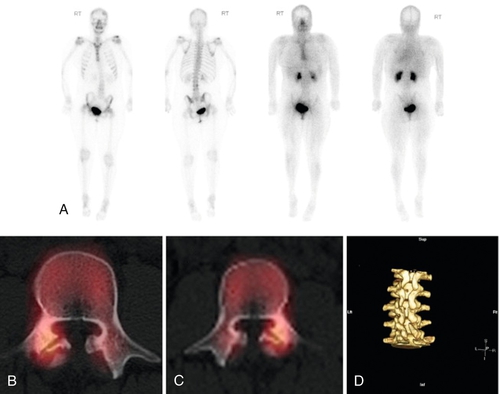
Bone scintigraphy uses radioisotopes that accumulate in metabolically hyperactive bone. Acute spondylolysis will reveal the bone activity of attempted healing. Long-standing spondylolysis with an established nonunion reveals no such activity [33]. Single-photon emission computed tomography (SPECT) improves the localizing ability of bone scintigraphy (Fig. 49.7). SPECT creates a series of slices through the target structure, permitting spatial separation of overlapping bone. Because of these abilities, SPECT imaging has established its place for the evaluation of adolescent athletes with back pain [34,35]. Nonetheless, MRI is considered a first-line imaging modality, given that it demonstrates a high level of agreement with SPECT in diagnosis of juvenile spondylolysis [36] without exposing the patient to radiation.
Treatment
Initial
In children or adolescents with acute symptomatic spondylolysis as suggested by bone scintigraphy, SPECT, or MRI, attempts to induce or to aid healing are warranted. Nonoperative treatments for spondylolysis include bracing, activity restriction, and therapeutic exercises [37]. Rigid antilordotic bracing for 3 to 6 months (often for 23 hours a day) has been advocated to reduce stress on the pars interarticularis [38]; however, a more recent meta-analysis found that although 83.9% of nonoperatively treated patients clinically improve, there is not a difference between those treated with and without bracing [37]. Activity modification is usually recommended until symptoms subside, and physical therapy for trunk muscle strengthening has been used. With this approach, symptoms improve in about 75% of children, and many lytic defects are noted to heal [38]. Unilateral defects are more likely to heal than bilateral defects [37]. With nonoperative treatment of spondylolysis, children and young adults return to a pain-free or nearly pain-free state in unrestricted activities 84% of the time [37].
A similar approach is advocated in children and adolescents with concurrent spondylolisthesis. However, once spondylolisthesis is present, healing of the pars defect becomes improbable. In this situation, the goal is symptom reduction, and good results are observed in many patients. Nonoperative treatment does not specifically lead to a bone union in terminal defects but may alleviate symptoms [37]. To date, there is no evidence to support bracing for the prevention of slip progression. In cases of incidental findings of spondylolisthesis, there is no need for treatment or restriction of activities, including aggressive sports [12,39].
In adults with spondylolysis, with or without spondylolisthesis, back pain complaints are treated like other nonspecific back pain disorders. Such treatment includes education, analgesics, nonsteroidal anti-inflammatory drugs, exercise, avoidance of bed rest, and rapid return to activities [40–42].
Rehabilitation
Exercise has received some study as a treatment of symptomatic spondylolysis and spondylolisthesis. One study has advocated flexion over extension exercise [43], although methodologic shortcomings limit the strength of these recommendations. A spinal stabilization exercise program was found to be superior to uncontrolled treatment at short- and long-term follow-up [44]. Aggressive physical therapy programs for those with back pain including spondylolysis or spondylolisthesis have been shown to provide significant short-term benefit in terms of flexibility, strength, endurance, pain tolerance, and level of disability. This type of program consists of stretching and high-intensity, progressively resistive exercise training, performed in a non–pain-contingent manner [45,46]. Stretching targets the hip flexors, hamstrings, quadriceps, and gastrocnemius-soleus muscles. Strengthening targets the abdominal and various back muscles. Active individual therapy and functional restoration program participation both improve work ability and hasten return to sports and leisure activities; the functional restoration program participation improves endurance in treatment of chronic low back pain [47].
A randomized controlled trial comparing fusion surgery with intensive rehabilitation for chronic low back pain, including patients with spondylolisthesis, found that both approaches led to symptom improvement and suggested there was no clear evidence that surgery is more beneficial [48]. With the complications and cost of surgery, this minimal difference is worth considering in choosing a treatment approach [48].
For lumbosacral pain in general, in addition to exercise, cognitive interventions allowing patients to feel safe while being active lead to improvements in fear-avoidance beliefs and forward bending ability in patients with chronic back pain [49]. It has been suggested that the specific type of exercise may be less important than the general message exercise conveys—that normal use of the back is not harmful [50]. A randomized trial of rehabilitation methods after spinal fusion has demonstrated that early postoperative implementation of a combination of motor training exercise and cognitive-behavioral therapy is safe and results in superior functional outcomes compared with a customary exercise program of strength, flexibility, and cardiovascular exercises, including in patients with spondylolisthesis [51].
Alternative therapies such as massage [52] and therapeutic horseback riding [53] have been considered potentially beneficial treatments at the case report level. Modalities including ultrasound and electrical stimulation have not been shown to improve symptoms and are generally of limited value.
Procedures
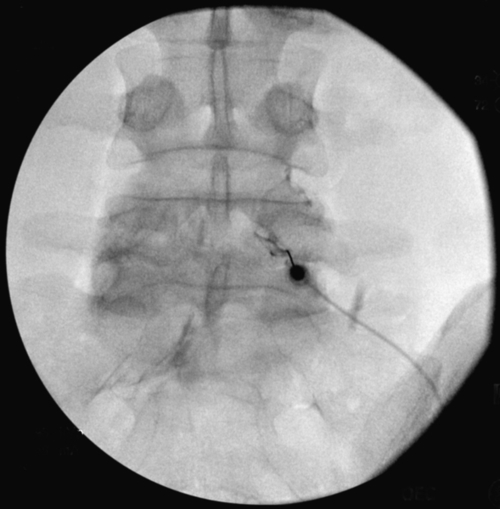
Various types of spinal injections are given with use of fluoroscopy to ensure proper needle placement (Fig. 49.8). Anesthetic agents may be used for short-term pain relief, usually for diagnostic purposes. Injection of steroids may be tried for longer term therapeutic purposes in adults with symptomatic spondylolysis or spondylolisthesis. For radicular pain, epidural injections may be used if the site of nerve root compression lies within the spinal canal, whereas selective nerve root blocks may be used if compression lies within the transforaminal space. For axial pain, facet injections may be used if the facet joints themselves are thought to be the source of pain.
Surgery
Referral to a surgeon is indicated in cases of spondylolisthesis causing progressive neurologic deficit; cauda equina compression with leg weakness, sensory loss, or bladder or bowel incontinence; neurogenic claudication; and persistent and severe back and leg pain despite aggressive conservative treatment. However, the patient’s preference, age, and comorbidities must be considered before surgery because rates of reoperation range from 7% to 15% with a perioperative mortality of 0.5% to 1.3% [54–56]. In general, lumbar spinal decompression or fusion is usually indicated only when imaging studies correlate well with the history and physical examination findings. Surgical outcomes are generally favorable [57].
Potential Disease Complications
Most cases of spondylolysis and spondylolisthesis are asymptomatic and remain that way [58]. The intervertebral disc and facet degeneration that occurs naturally with age is accelerated in the presence of spondylolysis. Because of this, spondylolytic spondylolisthesis can progress and result in spinal nerve compression or spinal stenosis [59].
Potential Treatment Complications
Nonsteroidal anti-inflammatory drugs can cause gastric bleeding and renal and hepatic toxicity. Acetaminophen, in large amounts, can cause hepatic toxicity. Narcotic analgesics are potentially addictive, and because studies of the low back pain population have not found significant differences between narcotics and nonsteroidal anti-inflammatory drugs in terms of pain relief, extreme caution should be used beyond the short term [60]. Exercise can irritate spinal tissue already inflamed. Spinal injections may result in a temporary increase in pain, spinal headache, infection, spinal nerve damage, or spinal cord damage. Surgical decompression or fusion can lead to infection, failed fusion, persistent back pain, spinal nerve damage, or spinal cord damage. Lumbar stabilization can accelerate adjacent level degeneration [61].