CASE 47 Patent Foramen Ovale Closure for Recurrent Stroke
Cardiac catheterization
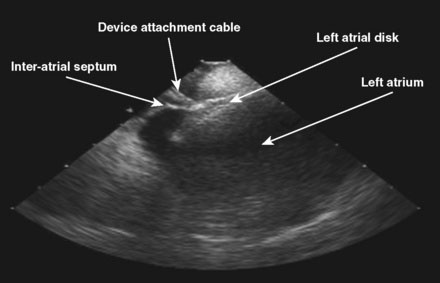
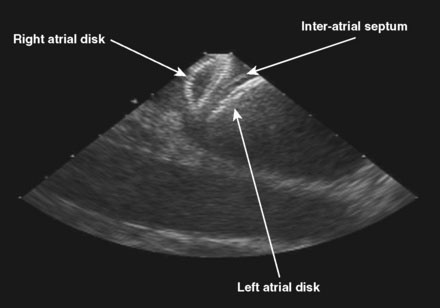
The patient was referred for percutaneous closure of the patent foramen ovale. The operator attained venous access in the right femoral vein using two vascular sheaths, 11 Fr and 9 Fr respectively. No contrast was used during this procedure, and the PFO closure was done using fluoroscopic and intracardiac echocardiographic guidance. An ACUSON AcuNav™ intracardiac ultrasound catheter was advanced through the 11 Fr sheath to the right atrium. The ICE catheter was rotated clockwise to attain views of the tricuspid valve and, subsequently, of the aortic valve. The catheter was retroflexed to view the septum (Video 47-1). After the intraatrial septum was well visualized and the absence of a thrombus attached to the PFO was confirmed, the patient was given 75 U/kg of unfractionated heparin. Subsequently, a 6 French multipurpose A2 catheter was passed to the right atrium through the 9 French sheath. The catheter was rotated posteriorly, easily crossing the patent foramen ovale into the left atrium with minimal manipulation. A 0.035 inch J-tip Amplatzer exchange length guidewire was passed into the left atrium and the multipurpose catheter removed. Stable wire position in a left pulmonary vein was confirmed by respiratory, but not cardiac, motion of the wire. A 9 French, 45 degree curved Amplatzer delivery sheath preloaded with a 25 mm Amplatzer Cribiform Septal Occluder was advanced across the interatrial septum and the left atrial disk was delivered under fluoroscopic and intracardiac echocardiographic guidance (Figure 47-1 and Video 47-2). The delivery catheter was pulled back until the left atrial disk was snugly apposed to the septum, and the right atrial disk was delivered out of the sheath but remained attached to the delivery cable while positioning was confirmed both by fluoroscopy and by intracardiac echocardiography (Figure 47-2 and Video 47-3). Once a stable position was confirmed and each disk was observed by intracardiac echo in appropriate position with the interatrial septum appearing between the left atrial and right atrial disks, the Septal Occluder was released by rotating the delivery cable in a counterclockwise direction until the delivery cable was disconnected from the Occluder. The position was again confirmed by both fluoroscopy and by intracardiac echocardiography, including injection of agitated saline into the femoral vascular sheath to confirm absence of bubble contrast moving from right to left (Video 47-4).
Discussion
1 Ogara P.T., Messe S.R., Tuzcu E.M., et al. Percutaneous device closure of patent foramen ovale for secondary stroke prevention. J Am Coll Cardiol. 2009;53:2014-2018.
2 Meissner I., Khandheria B.K., Heit J.A., et al. Patent foramen ovale: innocent of guilty? Evidence from a prospective population-based study. J Am Coll Cardiol. 2006;47:440-445.