CASE 46
Anne is 20 months of age and has been hospitalized with a fever, irritability, and moderate dehydration. Blood work suggests a bacterial infection, with elevated neutrophils (see Appendix for reference value), although chest radiograph and urinalysis are normal. A lumbar puncture taken yesterday showed an elevated white blood cell count, and preliminary culture results today suggest growth of Neisseria meningitidis. She has been receiving intravenous antibiotics for 30 hours, and there is some improvement in her condition. Physical examination indicated that Anne was below the 50th percentile for weight, had recurrent diarrhea, and had seborrheic dermatitis, more commonly referred to as “cradle cap.” Detailed question of Anne’s clinical history revealed that she had had recurrent infections, but none had ever been this bad. Detailed family history reveals that a distant male cousin and an aunt both died at an early age (<2 years) of meningitis. What further tests might you order? What is the significance of the history and findings?
QUESTIONS FOR GROUP DISCUSSION
RECOMMENDED APPROACH
Implications/Analysis of Clinical History
Anne’s immediate problem is the Neisseria meningitidis infection, which is the cause of the overwhelming sepsis, now referred to as systemic inflammatory response syndrome (SIRS). Although deficiencies in B cells, phagocytes, and complement are considerations in patients with severe bacterial infections, the fact that this is a Neisseria infection suggests that we should be focusing our attention on possible deficiencies in the terminal pathway of complement. Patients with deficiencies in the proteins that compose the membrane attack complex are particularly susceptible to infections with Neisseria species (Fig. 46-1).
Additional Laboratory Tests
Although one could request a measure of each of the terminal pathway components, Anne’s clinical history and results of the physical examination suggest that she may have a deficiency in C5. Patients with deficiencies in C5 present with Leiner’s disease, which is characterized by recurrent diarrhea, seborrheic dermatitis (which often manifests as cradle cap), and failure to thrive. Anne’s serum sample was sent to a specialized laboratory to test for a defect in C5. (C5 is only measured in specialized laboratories.)
THERAPY
ETIOLOGY: SIRS
Inflammation
Inflammation occurring in response to trauma or infection is complex, involves a number of cells, adhesion molecules, cytokines, chemokines, complement proteins, and coagulation proteins. Many of the processes occurring in inflammation are triggered following the release of tumor necrosis factor (TNF) and IL-1 from activated macrophages. Infections with Gram-negative bacteria lead to the activation of immunologic processes that lead to lysis of bacteria and release of lipopolysaccharide (LPS/endotoxin) from the cell wall outer membrane and subsequent release of TNF from activated macrophages. Release of TNF from activated macrophages occurs after binding of endotoxin-MD-2 complex with toll-like receptor-4 (TLR-4). Complex formation and signaling via TLR-4 occurs only if endotoxin undergoes interactions with, and transfer from, LPS-binding protein (LBP) and soluble CD14. Whether these sequential bindings and transfer modify endotoxin so that it is able to form a complex with MD-2 and TLR-4 is unknown. Signaling via TLR-4, however, activates biochemical pathways that lead to the synthesis and secretion of TNF. Therefore, it is not surprising that TLR-4 mutations in humans make individuals more susceptible to Gram-negative infection. (See Case 7)
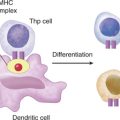
Differentiation of Thp to Th1 or Th2 Cells
Regulation of Type 1 and Type 2 Cytokines
Type 1 and type 2 cytokines reciprocally regulate cytokine secretion. Therefore, high levels of IL-10 would lead to a decrease in type 1 cytokine production (i.e., inflammatory cytokines), supporting studies in which anti-inflammatory agents did not rescue patients with SIRS. Further support for a positive role for proinflammatory cytokines comes from studies in which mononuclear cells from patients with sepsis (secondary to burns or trauma) have reduced type 1 and increased type 2 levels of cytokines and reversal of the dominant cytokine profile using IL-12 reduced mortality. IL-12 activates natural killer cells to secrete IFNγ, which promotes differentiation of Thp to Th1 cells secreting type 1 cytokines that downregulate IL-10 production.
New Approaches to Therapy
Immunotherapy Regimens
A number of other immunologic therapies are under consideration. IFNγ is a potent macrophage activator and has been reported to improve survival in a subgroup of patients with SIRS, possibly by restoring macrophage human leukocyte antigen (HLA)-DR expression (induction of Th1 activation) and TNF production. Similarly, IL-12, another Th1 inducer, reduced mortality from subsequent sepsis when administered after burn injury. In a different approach, anti–complement-activation product (anti-C5a antibodies) decreased the frequency of bacteremia, prevented apoptosis, and improved survival in SIRS patients. Not unexpectedly, given the previous discussion, strategies that block apoptosis of lymphocytes or gastrointestinal epithelial cells (both profoundly increased in almost all models of SIRS and in patient populations) have improved survival in experimental models of sepsis. This is consistent with evidence that mice with sepsis that are deficient in poly-adenosine diphosphate-ribose polymerase 1 (PARP-1), a key molecule in the proapoptotic pathway, have improved survival and that administration of a PARP-1 inhibitor was beneficial in pig models.