CHAPTER 44 Renal Function and Anesthesia
1 Describe the anatomy of the kidney
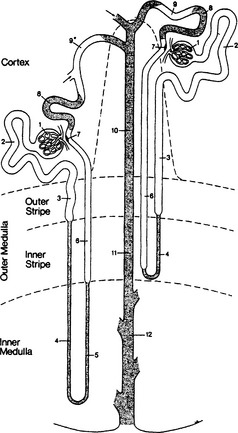
On cross section of the kidney, three zones are apparent: cortex, outer medulla, and inner medulla (Figure 44-1). Eighty percent of renal blood flow is distributed to cortical structures. Each kidney contains about 1 million nephrons. Nephrons are classified as superficial (about 85%) or juxtamedullary, depending on location and length of the tubules. The origin of all nephrons is within the cortex.
4 Review the site of action and significant effects of commonly used diuretics
| Drug (Example) | Site of Action | Action and Side Effects |
|---|---|---|
| Carbonic anhydrase inhibitors (acetazolamide) | Proximal convoluted tubule | Inhibits sodium resorption; interferes with H excretion; hyperchloremic, hypokalemic acidosis |
| Thiazides (hydrochlorothiazide) | Cortical diluting segment (between ascending limb and aldosterone-responsive DCT) | Inhibits sodium resorption; accelerates sodium-potassium exchange (hypokalemia); decreases GFR in volume-contracted states |
| Potassium-sparing diuretics (spironolactone, triamterene) | Competitive inhibition of aldosterone in DCT | Inhibiting aldosterone prevents sodium resorption and sodium-potassium exchange |
| Loop diuretics (furosemide, bumetanide, ethacrynic acid) | Inhibit Cl− resorption at thick ascending loop of Henle | Potent diuretic; acts on critical urine-concentrating process; renal vasodilator; hypokalemia; can produce hypovolemia |
| Osmotic diuretics (mannitol, urea) | Filtered at glomerulus but not resorbed; creates osmotic gradient in tubules; excretion of water and some sodium | Hyperosmolality reduces cellular water; limited ability to excrete sodium; renal vasodilator |
DCT, Distal convoluted tubule; GFR, glomerular filtration rate.
* With the exception of osmotic diuretics, all diuretics interfere with sodium conservation.
8 Discuss the major causes of perioperative acute renal failure
“Acute Kidney Injury” is now increasingly replacing the term “Acute Renal Failure.”
10 Comment on various laboratory tests and their use in detecting acute renal dysfunction
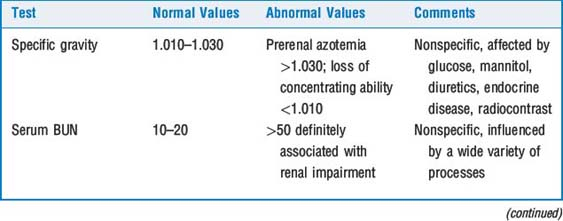
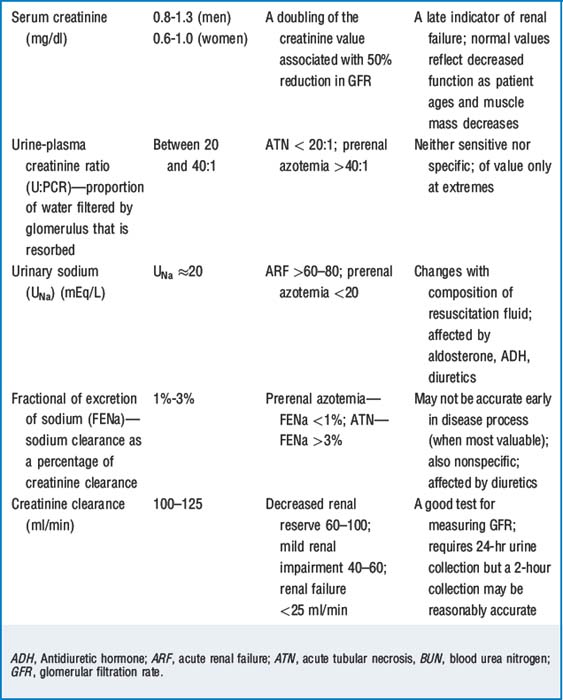
Creatinine clearance (Ccr) is a sensitive test of renal function. Creatinine is filtered at the glomerulus and not resorbed. There is also some secretion of creatinine with the tubules, and this results in about a 15% overestimation of Ccr. (In actuality, clearance of inulin, a polyfructose sugar, is the gold standard for measurement of GFR because it is filtered at the glomerulus and neither resorbed nor secreted at the tubules.) It has been a long held belief that measurement of Ccr required 12- to 24-hour urine collections. In fact, if Scr is rapidly changing, calculations of Ccr based on 24 hours of urine collection and a single creatinine measurement may be inaccurate. In addition, this method requires meticulous urine collection, and failure to accomplish this is a common source of laboratory error. Two-hour spot tests are thought to be reasonably accurate, and serial 2-hour spot tests may be particularly valuable when renal function is acutely deteriorating. Table 44-2 describes various tests of renal function.
12 At what point is renal reserve lost and do patients develop laboratory evidence of renal insufficiency?
15 Does dopamine have a role in renal preservation?
There is now considerable evidence that dopamine is not renoprotective (i.e., improves renal perfusion) nor does it improve splanchnic perfusion (see Chapter 15).
17 What is the best relaxant for patients with renal insufficiency?
Atracurium undergoes spontaneous degradation under physiologic conditions (Hofmann degradation and ester hydrolysis) and is preferred. Because atracurium is water soluble, patients with altered body water composition may require larger initial doses to produce rapid paralysis but smaller and less frequent doses to maintain paralysis. Succinylcholine may be used in patients with renal insufficiency if the serum potassium concentration is <5 mEq/L (Table 44-3).
18 How are patients with renal insufficiency managed perioperatively?
KEY POINTS: Renal Function and Anesthesia 
1. Devabeye Y.A., Van den Berghe G.H. Is there still a place for dopamine in the modern intensive care unit? Anesth Analg. 2003;98:461-468.
2. Moitra V., Diaz G., Sladen R.N. Monitoring hepatic and renal function. Anesthesiol Clin. 2006;24:857-880.
3. Playford H.R., Sladen R.N. What is the best means of preventing perioperative renal dysfunction. In: Fleisher L.A., editor. Evidence-based practice of anesthesiology. Philadelphia: Saunders; 2004:181-190.
4. Wagener G., Brentjens T.E. Renal disease: the anesthesiologist’s perspective. Anesthesiol Clin. 2006;24:523-547.