CASE 43 Slow Reflow After PCI for Acute ST-Segment Elevation Myocardial Infarction
Cardiac catheterization
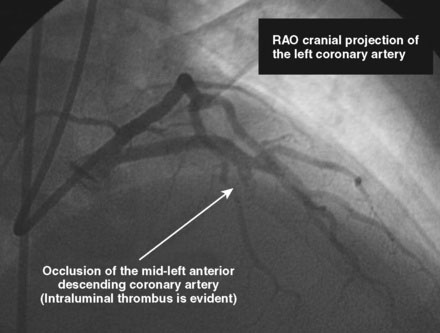
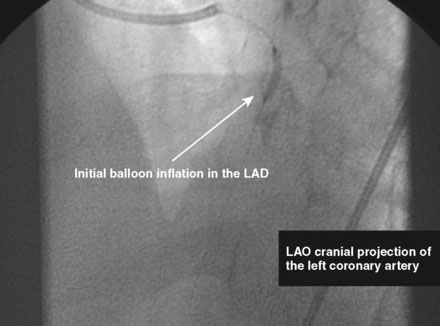
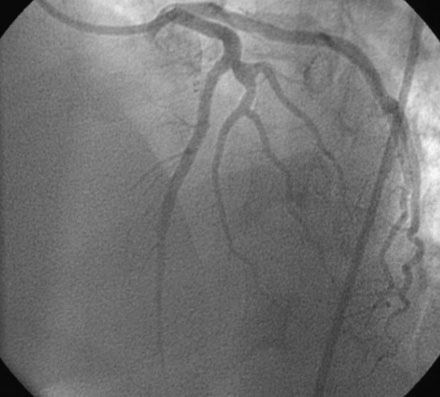
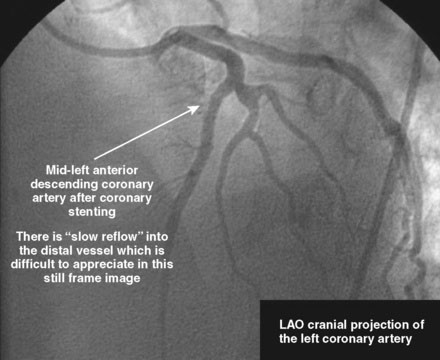
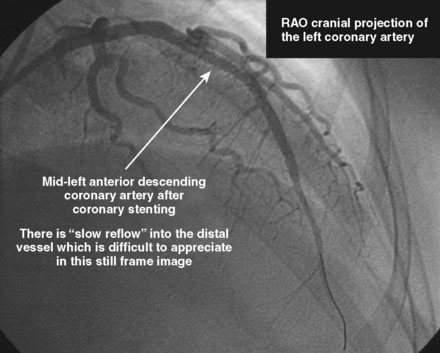
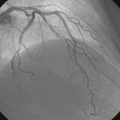
The initial coronary angiogram showed occlusion of the left anterior descending (LAD) coronary artery with the appearance of intraluminal thrombus (Figure 43-1 and Video 43-1). The circumflex and right coronary arteries were without significant obstruction. The operator decided to proceed with immediate PCI of the LAD. The ACT was adjusted with additional boluses of unfractionated heparin to maintain an ACT between 250 and 300 seconds, and eptifibatide was administered as adjunctive therapy (two intravenous boluses, each of 180 mcg/kg, and an infusion of 2.0 mcg/kg per minute continued for 14 hours). The left coronary artery was engaged with a JL4 guide, and the lesion was easily crossed with a 0.014 inch floppy-tipped guidewire. In this case, a thrombus extraction catheter was not used. The operator centered a 2.5 mm diameter by 15 mm long compliant balloon on the lesion and inflated it to 6 atmospheres of pressure (Figure 43-2). Following balloon angioplasty, there was a good angiographic result with resolution of the stenosis and thrombus, but “slow reflow” was noted in the LAD, with significantly slower filling of the distal LAD compared with the circumflex artery (Figure 43-3 and Video 43-2). To treat the slow flow, the operator administered boluses of intracoronary adenosine via the guiding catheter, beginning with boluses of 50 mcg and increasing to 80 mcg as tolerated. Flow improved, although it remained abnormal. At this point, the operator treated the lesion with a 3.0 mm diameter by 18 mm long paclitaxel-eluting stent and further dilated it with 16 atmospheres inflation pressure using a 3.5 mm diameter by 15 mm long noncompliant balloon. Flow worsened after stenting (Figures 43-4, 43-5 and Videos 43-3, 43-4). Again, the operator administered multiple intracoronary boluses of adenosine via the guiding catheter, beginning with boluses of 50 mcg and increasing to 80 mcg as tolerated, until a total of 700 mcg had been administered. At the conclusion of the case, LAD flow was improved, although it had not returned to normal. The patient reported resolution of chest pain and remained hemodynamically stable, although persistent ST elevation was noted on the monitor and on a subsequent 12-lead ECG. The right femoral access sheath was removed with manual compression after 4 hours when the ACT measured less than 180 seconds but while the eptifibatide infusion was continuing.
Postprocedural course
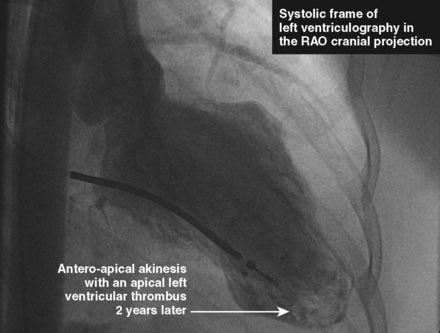
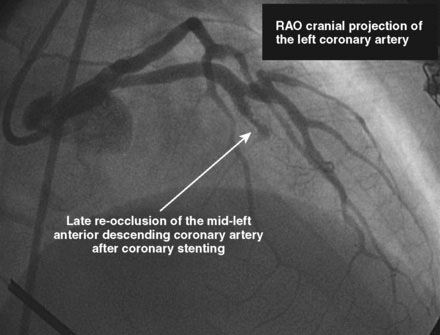
She had no further cardiac symptoms during follow-up. Two years later, she developed end-stage renal failure from lupus nephropathy and required long-term dialysis. After the onset of end-stage renal failure, she began to report shortness of breath, and her left ventricular function appeared worse, with an estimated ejection fraction of 25%. Repeat cardiac catheterization showed total occlusion of the LAD at the site of the previous drug-eluting stent and severe left ventricular dysfunction (Figures 43-6, 43-7 and Videos 43-5, 43-6). She was treated with an implantable defibrillator for primary prevention of ventricular arrhythmias and her medications for congestive heart failure were optimized.
Discussion
The terms “no-reflow” or “slow-reflow” describe the phenomenon when there is an apparent reduction in coronary blood flow seen by angiography following a percutaneous coronary revascularization procedure, despite the presence of a widely patent lumen and in the absence of a mechanical complication such as a dissection.1 As seen in this case, no-reflow can initially occur immediately after balloon angioplasty but is often exacerbated by stenting, presumably due at least in part to distal embolization of atherothrombotic material during stent expansion (“cheese-grater effect”). No-reflow can occur in up to 5% of PCI procedures and is more commonly seen with acute coronary syndromes, during rotational atherectomy, and in saphenous vein bypass graft interventions. In the last, distal protection devices can significantly reduce the frequency of no-reflow. No-reflow is more commonly seen during acute PCI for acute ST-elevation MI as in this case.2,3 Other predictors of the no-reflow phenomenon include the presence of bulky and large plaque burden, intracoronary thrombus, lipid pools (seen on intravascular ultrasound), and total coronary occlusion on the presenting angiogram. In a study comparing coronary aspirates from patients with normal flow to those with no-reflow, the latter contained more atheromatous plaque, more platelets and thrombotic material, more macrophages, and more cholesterol crystals.4
The initial approach to primary PCI reperfusion for acute ST-elevation myocardial infarction has been an evolving field. Based on recent randomized controlled trials, there is increasingly compelling data to suggest that routine performance of thrombus extraction with a simple aspiration catheter improves clinical outcomes prior to stenting for acute ST-elevation MI.4,5 The procedure in the case presented here was performed prior to the routine use of these devices and this may have contributed to the no-reflow that complicated her intervention.
1 Kloner R.A., Ganote C.E., Jennings R.B. The ‘no-reflow’ phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974;54:1496-1508.
2 Ito H., Tomooka T., Sakai N., et al. Lack of myocardial perfusion immediately after successful thrombolysis: a predictor of poor recovery of left ventricular function in anterior myocardial infarction. Circulation. 1992;85:1699-1705.
3 Neumann F.J., Blasini R., Schmitt C., et al. Effect of glycoprotein IIb/IIIa receptor blockade on recovery of coronary flow and left ventricular function after the placement of coronary artery stents in acute myocardial infarction. Circulation. 1998;98:2695-2701.
4 Svilaas T., Vlaar P.J., van der Horst I.C., et al. Thrombus aspiration during primary percutaneous coronary intervention – The TAPAS Trial. N Engl J Med. 2008;358:557-567.
5 Sardella G., Mancone M., Bucciarelli-Ducci C., et al. Percutaneous coronary intervention improves myocardial reperfusion and reduces infarct size. The EXPIRA (thrombectomy with export catheter in infarct-related artery during primary percutaneous coronary intervention) Prospective, Randomized Trial. J Am Coll Cardiol. 2009;53:309-315.