CHAPTER 42
Tumour markers
CHAPTER OUTLINE
Evaluation of the clinical utility of tumour markers
Tumour marker requests and the responsibilities of the clinical laboratory
TUMOUR MARKERS IN THE MANAGEMENT OF SPECIFIC CANCERS
Gastrointestinal stromal tumours (GIST)
Gestational trophoblastic neoplasia
Hepatocellular carcinoma (primary liver cancer)
INTRODUCTION
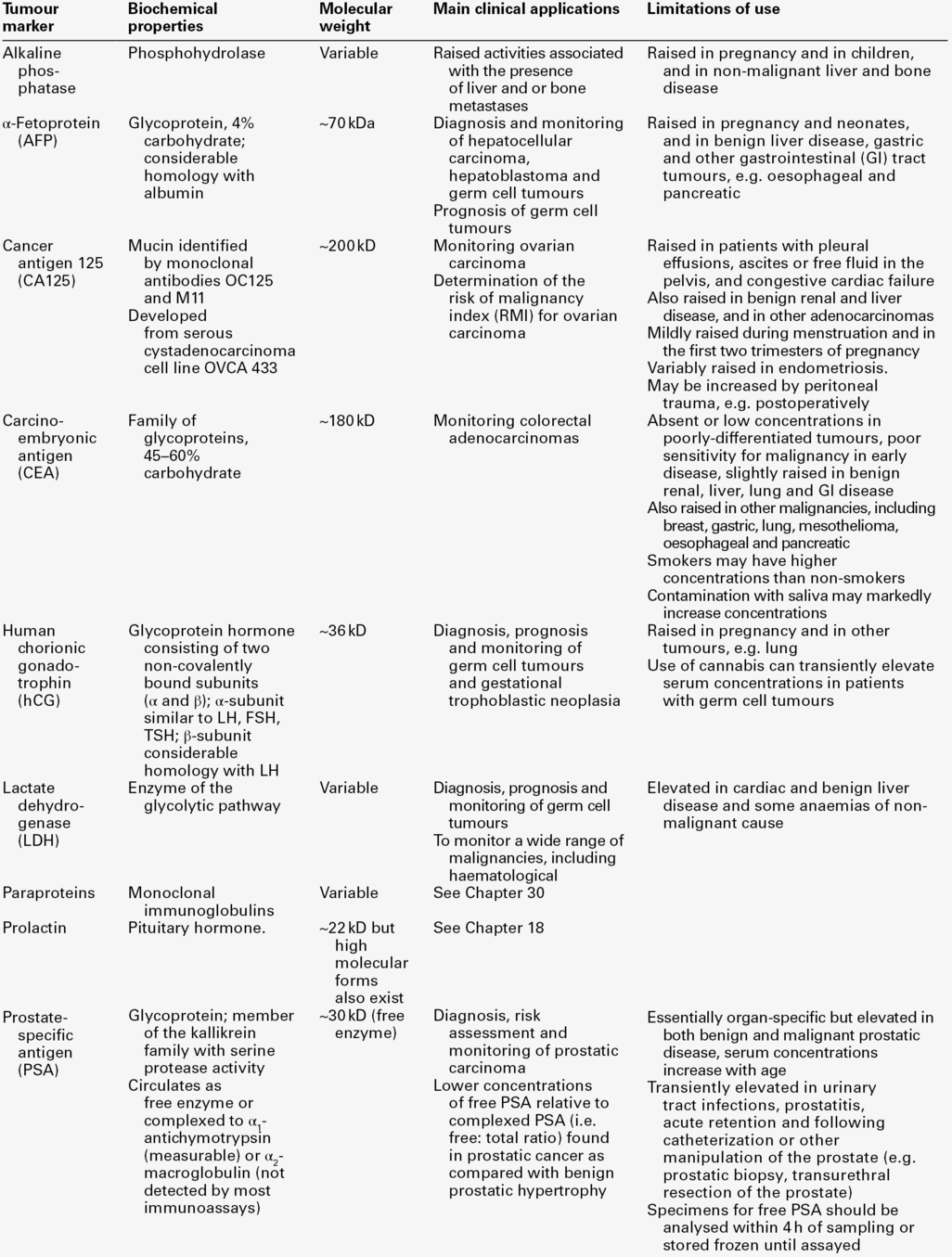
Tumour markers are substances (often proteins, enzymes or hormones) that are present in body fluids or tissues and whose measurement provides information about the presence, progression or remission of tumours. They may be tumour-derived (produced within the tumour by malignant or stromal cells) or tumour-associated (produced by non-malignant cells as a metabolic consequence of tumour presence). Some are tumour-specific (produced by cancerous but not normal tissue), while others are present in normal tissue but are produced at higher concentrations in body fluids or malignant tissue from cancer patients. A few are organ-specific but many are produced by a variety of different cancers.
Histopathological identification of tumour markers expressed at the tumour cell surface and detectable in biopsy specimens can provide both diagnostic and prognostic information, with genetic tests becoming increasingly important in predicting which patients are most likely to respond to costly new therapies. Secreted tumour markers, present in blood or other body fluids, are measured quantitatively, often by immunoassay. While knowledge of circulating marker concentrations may assist in diagnosis and prognosis, their most important clinical use is in monitoring the success of treatment.
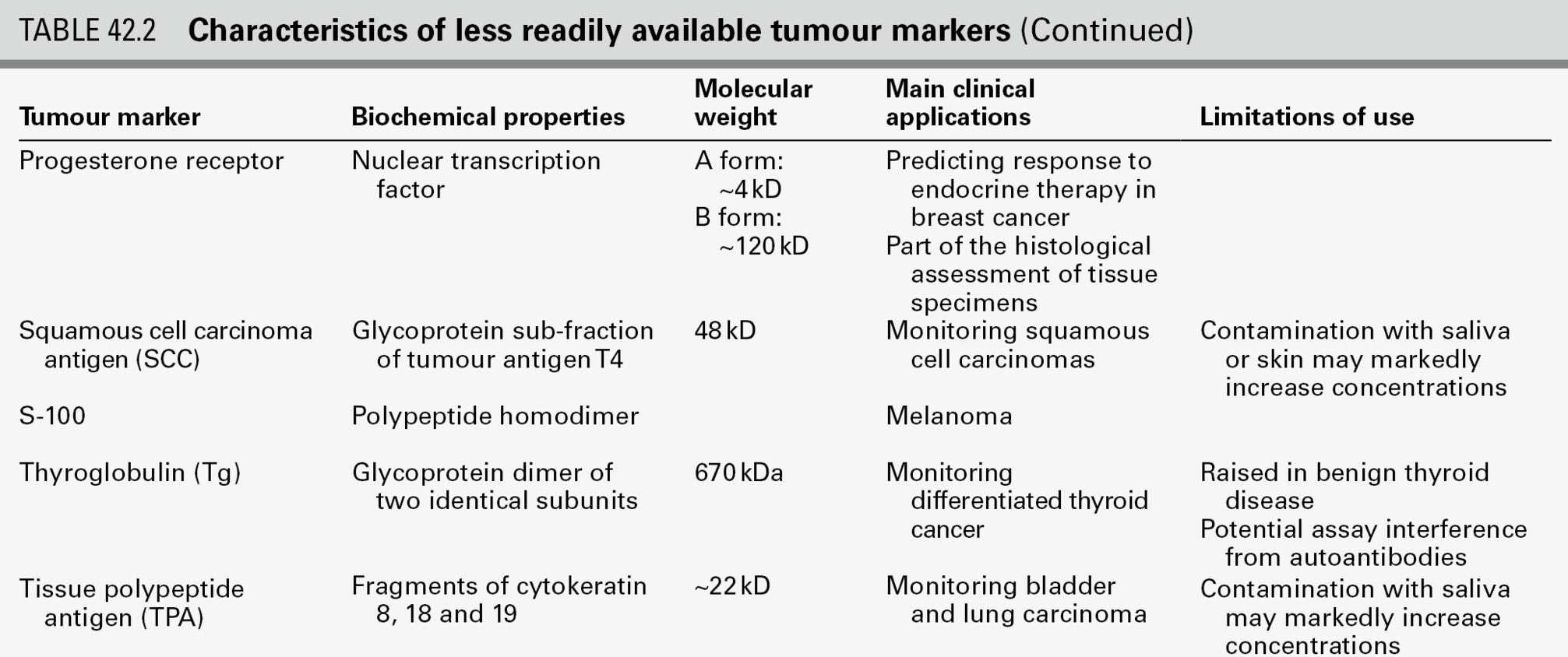
This chapter focuses primarily on the properties and clinical applications of the tumour marker tests that usually fall within the remit of clinical biochemistry laboratories (Tables 42.1 and 42.2), with the aim of enabling readers to provide advice about which tumour marker tests are most likely to be helpful, to recognize which results require immediate action, and to develop a high-quality interpretative tumour marker service. Before considering these aspects, however, it is useful to review some recent developments relevant to the introduction of tumour markers into routine clinical practice. Abbreviations used in the chapter for the names of individual tumour markers are explained in Tables 42.1 and 42.2.
Evaluation of the clinical utility of tumour markers
Although hundreds of potential tumour markers have been investigated, with more than 200 000 papers describing them, the number that contribute significantly to the management of cancer patients is remarkably small, as is evident in Tables 42.1 and 42.2. Historically, over-enthusiastic reporting of small, poorly designed studies of new markers has led to disillusionment when the results have not been confirmed in larger studies or in other centres. Together with the difficulties inherent in comparing different studies reported in non-standard form, this has made objective assessment of the utility of many tumour markers very difficult and has meant that the recommendations made by expert panels about their clinical use tend to be conservative.
The three critical factors that should be considered when evaluating a tumour marker are its clinical utility, the magnitude of the benefit of its use and its reliability. Tumour marker measurement can provide information at multiple stages of diagnosis and treatment (Table 42.3) but, until appropriate studies have been performed, it can be difficult to know how best to use a tumour marker in particular circumstances. Having identified a specific use (i.e. utility), the clinical value (i.e. magnitude) of using the tumour marker for that application needs to be assessed, by evaluating the difference in outcome between marker-positive patients and marker-negative patients, ideally in a randomized controlled trial designed for the purpose. Clinical precision and accuracy (i.e. reliability) then need to be established, since the marker will only be useful if results are reproducible. In this context, it is important to note that, although it is necessary to demonstrate a statistically significant difference in tumour marker concentrations between patient groups to show that a marker may have potential utility, this is not in itself sufficient evidence that a marker is of clinical benefit (i.e. that it should be used), but merely suggests that the differences observed are not likely to occur by chance. It is also essential to ensure that biochemical analysis of the marker is reliable and reproducible, that assays are standardized and that their analytical and clinical performance is tested objectively in appropriately designed and well-conducted studies.
TABLE 42.3
Examples of applications of tumour marker measurements at different stages of diagnosis and treatment
| Application | Examples |
| Assessment of risk | PSA: used with or without digital rectal examination to assess risk of prostate cancer and determine the need for biopsy |
| Screening | HCG: screening of women who have had a previous molar pregnancy and who are at high risk of developing choriocarcinoma |
| Differential diagnosis | CA125: together with menopausal status and ultrasound findings, contributes to calculation of the risk of malignancy index in the differential diagnosis of women with pelvic masses |
| Prognosis: prediction of relapse or progression: in primary disease in metastatic disease |
CEA: measurement at three-monthly intervals following curative surgery in patients to assess need for further treatment Thyroglobulin: following ablation of the thyroid, increasing serum concentrations suggest an alternative site of production |
| Prognosis: prediction of response to therapy: in primary disease in metastatic disease |
Oestrogen and progesterone receptors: their presence or absence in breast cancer tissue determines whether endocrine therapy is likely to be effective AFP, hCG and LDH: in patients with germ cell tumours, concentrations of these markers are used to assess prognosis |
| Monitoring course of disease To detect relapse in patient with no evidence of disease post-therapy To follow detectable disease |
AFP, hCG and LDH used in the follow-up of patients treated for germ cell tumours CA125 used to monitor ovarian cancer patients |
The Tumor Marker Utility Grading System (TMUGS), developed some years ago, provides a useful framework for such evaluation and it is encouraging that the recommendations made therein have been implemented for some analytes, e.g. by improvements in the standardization of immunohistochemical assays for HER-2 and in the analytical accuracy and equimolarity of serum assays for PSA. The TMUGS also describes levels of evidence for grading the clinical utility of tumour markers, an approach that has been adopted by the US National Academy of Clinical Biochemistry (NACB), which has developed Laboratory Medicine Practice Guidelines for the use of tumour markers in the clinic. Other initiatives include development of Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK), which provide guidance about study design, pre-planned hypotheses, patient and specimen characteristics, assay methods and statistical analysis methods and complement the broader statements on Consolidated Standards of Reporting Trials (CONSORT) and Standards for Reporting of Diagnostic Accuracy (STARD). These should help to encourage improved design and publication of tumour marker studies in the future.
Tumour marker requests and the responsibilities of the clinical laboratory
Reasons for requesting tumour markers
Knowledge of tumour marker results can contribute useful clinical information relating to different aspects of patient management (Table 42.3). For use in screening and diagnosis, the ‘perfect’ tumour marker would be absent in all healthy subjects (100% specificity), would be raised in all patients with a single tumour type (100% sensitivity) and its serum concentration would accurately reflect tumour size. Unfortunately, the ‘perfect’ marker does not exist and the extent to which currently available markers meet this ideal is very variable. Consequently, the predictive value of a positive or negative result also varies and is highly dependent on the population considered (see Chapter 2 for detailed discussion).
There is increasing interest in improving the specificity of some markers (e.g. AFP, CA125 and PSA) by using serial measurements to assess the rate of change in the concentration of the marker with time in individual patients. Such measurements may contribute to diagnosis, but in practice, are more likely to aid post-treatment monitoring, which is the most established clinical role for tumour markers. Whether such monitoring improves patient outcome depends on the availability of further treatment options, should increases in marker concentrations indicate progressive disease. If potentially curative treatment is available, as for example is frequently the case for patients with recurrent germ cell tumours, it is essential to identify patients with rising tumour marker concentrations promptly; failure to do so may constitute a critical clinical error. However, if alternative therapy is not available, knowledge of progressive disease before it is clinically evident may not benefit the patient and can have adverse psychological consequences, in which case it may be advisable to discontinue tumour marker monitoring. Serial monitoring is also desirable for patients with cancers that are unlikely to progress and for which treatment is not necessary or can be delayed: active surveillance (sometimes called ‘watchful waiting’) programmes, including serial PSA measurements, are appropriate for some men with indolent prostate cancers.
Choice of tumour marker test
In practice, the clinical biochemistry laboratory receives three types of tumour marker requests: those for patients with diagnosed malignancy who have already been referred to specialist centres; those for patients being investigated for suspected malignancy in secondary care, and those for patients presenting to their general practitioners. Requests in the first group are the most likely to be appropriate and are usually made to clarify the patient’s diagnosis or to monitor response to treatment and/or detect recurrence.
Non-specialist users, whether in hospital or general practice, should be encouraged to consider carefully whether knowledge of a tumour marker result is likely to be helpful before requesting it. Requests such as ‘tumour marker screen’ or ‘?malignancy’, particularly from emergency departments and other receiving units, should be actively discouraged. Since it is not usually practicable for requests to be scrutinized prior to analysis, laboratories should provide comprehensive advice about appropriate choice of tumour markers, as well as reminders of their limitations. Most tumour markers do not have sufficient sensitivity or specificity, particularly for early stage disease, to be regarded as diagnostic tests, although they may contribute to diagnosis. Importantly, whatever the malignancy or tumour marker, a result within the reference interval never excludes malignancy or progressive disease.
Requestors should be aware that the converse is also true. Raised tumour marker concentrations do not necessarily indicate malignancy, as they may be increased in a number of benign conditions. Increased concentrations may be associated with more than one tumour type, since, with few exceptions, tumour markers are not organ-specific. Clinical biochemists themselves should also think carefully before requesting any additional testing which may lead to a diagnosis of malignancy and before doing so should seek the agreement of the doctor managing the patient. General advice that can be readily disseminated to non-specialist users has recently been prepared by Pathology Harmony UK.
Pre-analytical requirements
Although the timing of blood sampling is not usually critical, a pre-treatment specimen is helpful when interpreting subsequent results. Specimens should always be taken before any investigative procedure, since some of these may cause transient releases of tumour markers into the circulation (e.g. increases of PSA following insertion of a urinary catheter or prostatic biopsy, of CA125 following abdominal surgery, and of CEA following colonoscopy). Possible conditions, which can transiently affect tumour marker concentrations (e.g. marked increases of PSA in men with active urinary tract infections or of CA19.9 in patients with cholestasis) should be excluded. Failure to recognize that misleadingly high results can be obtained if sampling times are inappropriate may cause undue distress to the patient, as well as decreasing confidence in laboratory testing. Some additional examples are listed in Tables 42.1 and 42.2.
Analytical requirements
Manufacturers’ instructions should always be followed when performing tumour marker measurements, with carefully defined internal quality control (IQC) and external quality assessment (EQA) procedures in place to monitor performance. Internal quality control and EQA specimens should closely resemble patient sera and be of clinically relevant concentrations, including those near important decision limits. Regular assessment of reproducibility and stability of results with time is particularly important for all tumour markers, as these are often monitored over long periods. Ensuring good reproducibility at low concentrations is critical where treatment may be instituted solely on the basis of a relatively small increase in tumour marker concentration, as is the case for AFP and hCG in germ cell tumours and for PSA following prostatectomy for prostate cancer.
Major international efforts are being directed towards improved comparability of tumour marker methods by encouraging accurate calibration against the relevant international standards where these exist (Table 42.4), by producing reference reagents to enable improved characterization of assays, by organizing collaborative workshops to identify the most clinically appropriate antibody specificities and by encouraging use of equimolar assays where relevant (e.g. PSA assays that recognize free and complexed PSA equally well). Nevertheless, the molecular heterogeneity of most tumour markers (as illustrated by the number of hCG-related molecules in Table 42.4) means that results obtained with different methods are not interchangeable. Clinical biochemists should be aware of the characteristics of the methods used in their own laboratory. They should also be familiar with the vulnerability of their methods to potential interferences such as the high-dose hook effect, specimen carry-over and interference from heterophilic or human anti-mouse antibodies (Box 42.1).
TABLE 42.4
International standards (IS), reference preparations (IRP) and reference reagents (IRR) for tumour markers
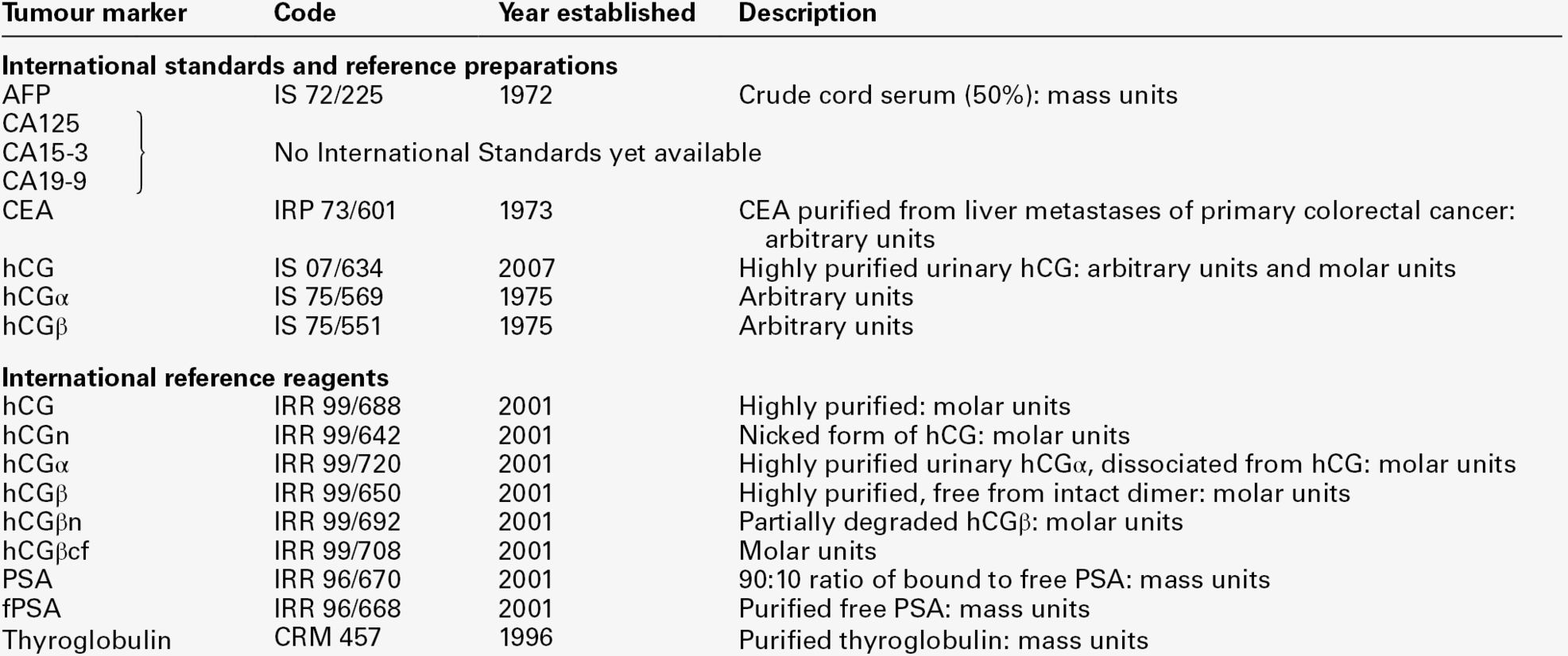
Most currently available immunoassays are calibrated against the relevant standards where they exist. The lack of international standards for the CA antigens is a major hindrance to improved between-method comparability.
hCGα, hCG α-subunit; hCGβ, hCG β-subunit; hCGn, nicked hCG; hCGβn, nicked hCG β-subunit, hCGβcf, hCG β-core fragment.
International Standards and Reference Reagents are available from the National Institute for Biological Standards and Control, Potters Bar, Herts, UK: http://www.nibsc.ac.uk/catalog/standards/preps/sub_endo.html
Reporting of tumour marker results
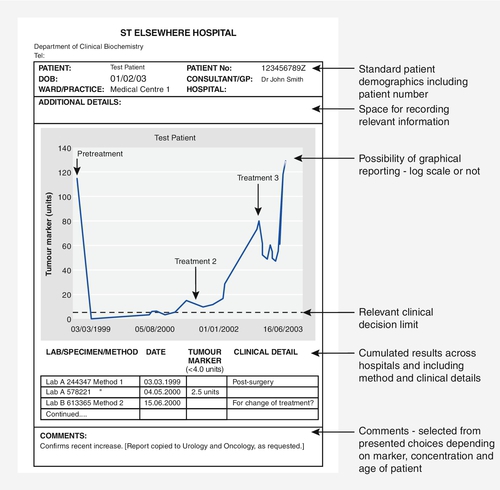
Cumulative and/or graphical reporting of serial results can identify trends in marker concentrations, which are generally more informative than single values, and may alert the laboratory to unexpected results (e.g. sudden changes) that require further investigation. Recording brief clinical information (e.g. ‘postoperative’), preferably both in the laboratory computer and on any printed reports, can be helpful both in identifying results that are out-of-accord and when interpreting results.
In view of method-related differences in tumour marker results, it is recommended that the method used is stated on the clinical report. If there has been an intervening method change, it is highly desirable that the laboratory also indicates whether this is likely to have affected interpretation of the trend in results. Reference intervals specific to the method used should be provided, although for serial monitoring, the patient’s own baseline provides the most important reference point for future results. Laboratory and clinical staff should engage in active dialogue about appropriate clinical decision points (e.g. when using PSA measurements to select patients for biopsy) and should know how these limits were derived. Laboratories can increase the value of their reports by adding short interpretative comments relating to the analytical results and preferably individually tailored to the requestor (e.g. omitting these for specialist users). Tumour marker half-lives, which are defined as the time to 50% reduction of circulating tumour marker concentrations following complete removal of tumour tissue, provide an important measure of the efficacy of therapy, particularly for germ cell tumours, and it may be helpful for the laboratory to calculate these for AFP and hCG (see p. 833 for further information). Providing advice about whether a change in marker concentration is likely to be significant or not is also helpful and should take into account both biological and analytical variation. A confirmed increase of 30%, or two serial increases of 20–25%, are often considered to be of clinical significance. Recommendations about the need for confirmatory specimens and the desirable frequency of tumour marker measurements are also helpful.
The clinical biochemist should identify urgent results that may be required for immediate patient management and ensure that these reach the relevant clinician promptly, telephoning results when appropriate. Results in this category include those which can be used to diagnose advanced disease in critically ill but treatable patients (e.g. AFP in hepatoblastoma; hCG in choriocarcinoma; AFP and hCG in non-seminomatous germ cell tumours; PSA in men with advanced prostate cancer that may respond to endocrine therapy). Provision of a proactive and high-quality tumour marker service helps to encourage good communication between laboratory and clinical staff, and is likely to facilitate both appropriate use of tumour marker tests and early identification of any results that are not in accord with the clinical picture. An example of a laboratory report meeting many of these requirements is shown in Figure 42.1.
FIGURE 42.1 Possible template for a clinical laboratory report for tumour markers that fulfil current reporting recommendations.
TUMOUR MARKERS IN THE MANAGEMENT OF SPECIFIC CANCERS
Regularly updated national guidelines on the management of the majority of tumours are now widely available, often provided electronically on the worldwide web. Regional cancer networks are also a valuable source of information and have often developed modified versions of national guidelines tailored for local use. The optimal use of tumour markers for assessment of prognosis, monitoring treatment and detecting recurrent disease has been studied in most detail for choriocarcinoma and germ cell tumours, relatively rare diseases for which tumour marker measurements are mandatory for clinical management. Measurements of serum tumour markers also contribute significantly to the management of some of the more common malignancies (e.g. ovarian, colorectal and prostate), while they are less widely used in others (e.g. bladder, breast and lung). In the following sections, the extent to which tumour markers currently contribute to the clinical management of a number of important malignancies is briefly reviewed.
Bladder cancer
The most common symptom of bladder cancer is intermittent haematuria, which is present in 80–85% of patients. The majority of bladder cancers are transitional cell carcinomas but adenocarcinomas, squamous cell carcinomas and sarcomas also occur. In some patients, urine cytology is positive for tumour cells, but the diagnosis is usually established by cystoscopic evaluation.
Urine cytology is very effective in detecting high-grade bladder cancers but will miss the majority of papillary urothelial neoplasms of low malignant potential. Commercially available assays for two tumour markers present in urine have been approved by the United States Food and Drug Administration (FDA) for the detection of recurrent bladder cancer. The BTA (bladder tumour associated antigen) -Trak™ and -Stat™ tests detect complement factor H and related proteins, which are involved in the regulation of the alternative pathway of complement activation to prevent complement-mediated damage to healthy cells. A point of care version of this test is also available. The nuclear matrix protein 22 (NMP22™) test is a quantitative measure of the nuclear mitotic apparatus protein, a component of the nuclear matrix which is over-expressed in bladder cancer. Both these tests are more sensitive than cytology in detecting low grade bladder cancers but are less specific, and their high false positive rates limit their clinical application. Better specificity has been achieved with a fluorescence in situ hybridization assay (UroVysion™), which detects bladder cancer-associated aneuploidy of selected chromosomes and which has been approved by the FDA for screening patients for recurrent bladder cancer.
Breast cancer
Breast cancer is by far the most common cancer affecting women worldwide with approximately one million new cases diagnosed each year. The main presenting features in women with symptomatic breast cancer include a lump in the breast, nipple change or discharge and skin contour changes.
Screening and diagnosis
Currently available blood-based biomarkers are of no value in the early diagnosis of symptomatic or asymptomatic breast cancer, the latter being addressed by national screening programmes using mammography. Individuals who are at increased risk of breast cancer because they are carriers of the BRCA1, BRCA2 or TP53 genetic mutations and those with a strong family history of breast cancer (e.g. close relatives diagnosed with breast cancer at a young age) may be eligible for screening with magnetic resonance imaging (MRI), as is recommended in the UK by the National Institute for Health and Care Excellence (NICE). Definitive diagnosis requires biopsy and histopathology.
Prognosis
Measurement of oestrogen-binding receptors (ER) and progesterone-binding receptors (PR) in tumour biopsy tissue obtained at diagnosis is mandatory in order to determine the likely response to endocrine therapy of both early and advanced (metastatic) breast cancer. Early ligand-binding and enzyme-linked immunosorbent assay methods for measuring ER and PR have been superseded by immunohistochemical assessment, which can be performed on paraffin-fixed tissue sections of the smaller tumours now detected by screening. Immunohistochemical or fluorescence in-situ hybridization tissue measurement of HER-2 (or its gene), a glycoprotein that controls cell growth and is amplified in ~ 30% of early-stage breast cancers, is now essential in all newly diagnosed patients with breast cancer. Patients with tumours that do not produce HER-2 are not likely to benefit from treatment with trastuzumab (Herceptin®), a humanized monoclonal antibody against HER-2, either administered on its own or in addition to other chemotherapy treatment. An assay is available for the measurement of the soluble shed form of HER-2 in serum and has potential value, both for prognosis and for monitoring trastuzumab therapy in patients with advanced breast cancer. However, the serum assay is not widely used.
Monitoring
CA15-3, a high molecular weight mucin, and similar MUC-1 based glycoprotein markers (e.g. BR27-29) may be used to monitor response to treatment, rising serum concentrations providing early indication of progression in some patients. Their routine use is not currently recommended, since there is as yet no evidence that therapeutic intervention prior to radiological or clinical detection of recurrent tumour is beneficial. However, in individual patients, measurement of CA15-3 may help to determine whether bone symptoms are due to benign or malignant disease, as high concentrations are frequently associated with metastatic disease. CA15-3 may also be useful in monitoring response to therapy in advanced disease if there are no other indicators of response and if rising concentrations would be an indication to stop all but palliative treatment. As with other tumour markers, the low sensitivity of CA15-3 means that results within the reference interval do not exclude active disease or progression.
Cervical cancer
Worldwide, cervical cancer is the major cause of death from gynaecological cancer, with reported incidence rates in developing countries much higher than those in developed countries. Since cervical cancer progresses slowly from pre-invasive cervical intraepithelial neoplasia to invasive cancer, screening asymptomatic women with regular smears provides an effective means of early detection. The addition of human papilloma virus (HPV) testing appears to improve the effectiveness of screening as certain types of HPV are involved in the development of cervical cancer.
Women who have not been screened may present with symptoms of abnormal vaginal bleeding and, in advanced cases, pelvic pain and pressure symptoms relating to the bowel or bladder. Treatment of early stage disease usually requires radical hysterectomy and pelvic lymphadenectomy, with adjuvant radiation therapy if disease has spread to the lymph nodes. Surgery and concomitant chemoradiation or neoadjuvant chemotherapy followed by radical surgery are options for bulky disease. Measurement of plasma CEA and CA125 may have clinical utility in patients with cervical adenocarcinomas, which constitute 10–15% of all cervical cancers. The majority of cervical cancer patients (~ 85%), however, have squamous cell cervical carcinomas, for which the potentially most useful serum tumour marker is squamous cell carcinoma antigen (SCC), a serine protease inhibitor.
Screening and diagnosis
The low sensitivity and specificity of SCC, particularly for early stage disease, preclude its use in screening or diagnosis, which requires immunohistological evidence. Although 60% of patients with cervical cancer will have elevated concentrations of SCC at diagnosis, raised values are also found in other squamous cell carcinomas (e.g. lung, oesophagus and head and neck) as well as in benign diseases (e.g. psoriasis, eczema, sarcoidosis).
Prognosis
Some studies suggest that a raised concentration of SCC is an independent risk factor for poor survival but other studies contradict this. It has also been suggested that pre-treatment SCC concentrations may be helpful in stratifying patients at greater risk of recurrence and therefore requiring more intensive therapy, but formal trials will be necessary to confirm this.
Monitoring
Various studies have confirmed that SCC shows a strong correlation with the clinical course of cervical cancer, with a lead time of up to 14 months in detecting progression prior to the onset of clinical symptoms, but whether earlier detection improves treatment outcome is not known. As only 10% of patients with recurrent disease can be cured and most patients with recurrent disease have clinical symptoms, until treatment options improve it is unlikely that SCC measurement will be widely adopted.
Choriocarcinoma
See Gestational trophoblastic neoplasia, below.
Colorectal cancer
Worldwide, colorectal cancer (CRC) is the third most common cancer, with more cases in the developed world than in Africa and Asia. Its incidence increases as populations adopt a western style diet. The risk of recurrence and subsequent death from CRC is closely related to the stage of the disease at the time of the primary operation. Although the treatment of colon and rectal cancer differ, they can be considered together in terms of tumour marker measurement.
Screening
Individuals at high risk of developing CRC (e.g. those with hereditary non-polyposis colon cancer (HNPCC, Lynch syndrome), familial adenomatous polyposis (FAP) or a strong family history of CRC (e.g. first degree relative with CRC diagnosed before age 45) should be referred to a clinical genetics unit for risk assessment and endoscopic screening. Mismatch repair gene mutations (e.g. MLH1, MSH2) predispose individuals to additional tumours, including those of the endometrium, ovary, genitourinary tract, small bowel and biliary tract. Individuals with FAP are at increased risk of developing duodenal tumours and thyroid tumours.
Early detection of CRC using faecal occult blood testing (FOBT) has been shown to reduce mortality in several randomized controlled trials. A number of countries now provide screening programmes for defined age groups (often > 60 years old in the general population and at younger ages in high-risk groups) based on FOBT. Such screening is undertaken biannually in the UK, currently using a guaiac test, which detects the pseudoperoxidase activity of either intact or free haemoglobin. The test has many limitations, including relatively low clinical sensitivity and specificity for CRC; lack of specificity for human haemoglobin; vulnerability to interference from some foodstuffs and medications and difficulties associated with automating it. Screening using guaiac-based methods is therefore gradually being replaced by faecal immunochemical tests, which detect the globin component of haemoglobin, can be used quantitatively with an adjustable cut-off concentration and are generally superior. All FOBTs lack specificity, and positive screens must be followed-up with colonoscopy. Markers based on DNA detection are potentially more specific than FOBT and may ultimately replace them, provided a clinically cost-effective panel of markers can be identified. At present, however, the cost and technical difficulties associated with these assays preclude their adoption.
Diagnosis
Although CEA is the most frequently used marker for CRC, depending on the cut-off point chosen, serum CEA will be raised in only 30–50% of CRC patients at the time of diagnosis. Its specificity is also low, since CEA concentrations can be raised in benign liver and kidney disease as well as in other malignancies (e.g. breast, gastric, lung, mesothelioma, oesophageal and pancreatic cancers), and may also be raised in smokers. Therefore, CEA cannot be used in isolation to diagnose even advanced CRC. However, raised concentrations can aid in diagnosis in certain clinical circumstances (e.g. indicating a high probability of malignancy in a frail elderly patient who cannot undergo invasive investigations) and confirmed markedly raised concentrations (serum CEA > 40 μg/L) are suggestive of metastatic disease.
Prognosis and staging
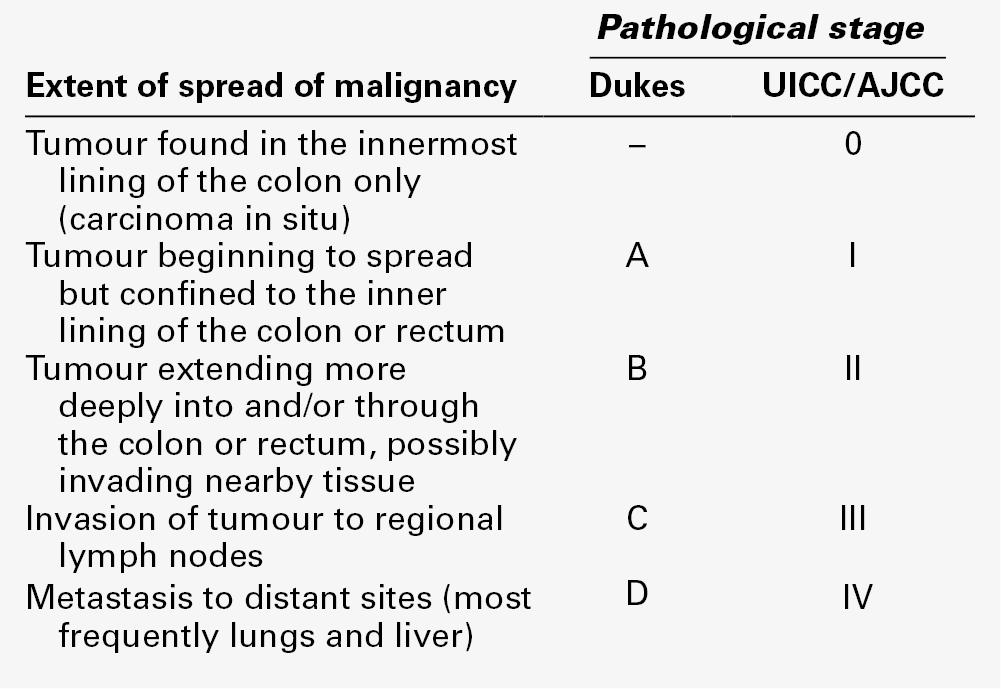
The strongest predictor of recurrence of CRC is the histopathological stage of disease, as assessed using the Dukes staging system or one of its modified versions (Table 42.5), which take into account local tumour invasion, involvement of regional lymph nodes and presence of distant metastases. The five-year survival for patients with Dukes Stage A disease is > 90% but that for those with Stage D disease is < 10%.
High preoperative CEA concentrations are associated with poorer prognosis, while those within the reference interval are generally associated with better outcomes. An Expert Panel of the American Joint Committee on Cancer has suggested that CEA concentrations should be incorporated in the TNM staging system for CRC, but this proposal has not yet been formally adopted. Supporting this, a College of American Pathologists Expert Panel has ranked preoperative serum CEA as a Category I prognostic marker for CRC. In practice, pathological examination of resected tumour currently determines prognosis and the need for adjuvant therapy but preoperative CEA concentrations may provide additional prognostic information complementing histopathology in newly diagnosed CRC patients. Further information may also be obtained from new prognostic markers, e.g. microsatellite instability and gene expression profiling. Further evaluation of these is required prior to their routine implementation but it is likely that measurement of CEA will continue to be both simpler and cheaper.
Monitoring
Serum CEA is a reliable and validated marker for the surveillance and detection of disease recurrence in CRC. However, it is important to consider whether this would be likely to benefit the individual patient. Monitoring of the disease should be undertaken only if it were likely to lead to clinical action that would either improve the survival or quality of life of the patient, e.g. by early detection of recurrence that is amenable to treatment.
Meta-analyses of a number of trials confirm that intensive follow-up results in 20–30% reduction in all cause mortality, but variation in follow-up strategies and reporting makes it difficult to draw conclusions about the best combination of tests and their timing. Nevertheless, results suggest that inclusion of regular CEA measurements in intensive follow-up regimens is necessary to achieve significant improvement in survival. Intensive follow-up is a cost-effective strategy, with measurement of CEA being one of the least expensive tests performed, estimated to be approximately half the cost of a chest X-ray and less than one-tenth the cost of a colonoscopy in the USA.
A number of expert panels have issued broadly similar recommendations stating that CEA should be measured in patients with Dukes stage B or C CRC who may be eligible for further intervention (e.g. resection of liver metastases or systemic treatment) if their disease recurs. It should be measured at baseline and then every 2–3 months for at least three years after diagnosis and approximately every six months for a further five years. Measurement of CEA following resection of solitary liver metastases provides helpful information, with CEA falling rapidly following successful resection. Unfortunately, while CEA is a relatively sensitive marker for liver metastases, this is not the case for lung metastases.
Increasing CEA concentrations in an asymptomatic patient pose a difficult dilemma. The National Comprehensive Cancer Network (NCCN) recommends that if CEA is increasing in the presence of normal imaging, computed tomography (CT) scans should be repeated every three months until disease is detected or CEA concentrations stabilize or decrease. Exploratory surgery based solely on an increase in CEA is not recommended by the NCCN; neither is the use of CEA-radiolabelled scintigraphy in patients with negative imaging. It is important to note that low serum CEA results do not necessarily exclude progression and other confirmatory tests (e.g. CT scan, X-ray, colonoscopy or possibly CA19-9 measurement) may be required if this is clinically suspected.
Monitoring of advanced disease
Patients with metastatic CRC are likely to receive a fluoropyrimidine (e.g. fluorouracil) in combination with oxaliplatin and/or irinotecan. Biological treatment with therapeutic antibodies (e.g. bevacizumab, cetuximab and panitumumab) is also increasingly used. While radiology remains the ‘gold standard’ method for evaluating response, studies have demonstrated good concordance between radiological response and CEA response in > 90% of patients being treated for isolated CRC liver metastasis, leading to the conclusion that CEA is as accurate as CT imaging for assessing response to chemotherapy. Caution in interpretation is, however, required, particularly in the first few weeks following chemotherapy, as transient rises in CEA may occur in 10–15% of patients. Interestingly, these increases appear to be associated with a favourable outcome and are thought to be due to necrosis and/or apoptosis caused by the cytotoxic therapy. A confirmed increase during treatment may lead to a change of treatment or withdrawal of ineffective therapy.
Cautions and caveats
It is essential that, whenever possible, CEA concentrations in individual patients are monitored using the same method, since method-related differences in recognition of CEA mean that results may differ sufficiently to influence the interpretation of serial measurements. As for all serum tumour markers, when a change of method is unavoidable, the laboratory should establish a new baseline value for each patient.
An increase of > 30% from the previous value of CEA is considered to be significant and should be confirmed by a second sample taken within a month. Smaller increases (e.g. 15–20%) demonstrated over at least three consecutive assays at minimum intervals of two weeks, may also prompt intervention. Patients with symptoms of recurrence require additional investigation such as CT scanning. CEA results within the reference interval do not exclude recurrence. Conversely, increasing CEA concentrations following curative surgery may reflect factors that are not associated with the primary tumour (e.g. lung nodules, liver lesions, ovarian masses and mediastinal lymphadenopathy).
Genetic pre-screening for hereditary non-polyposis colon cancer
Considerable progress has been made in understanding genetic influences on the development of CRC, and genetic testing is likely to become increasingly important. Pre-screening for HNPCC, which accounts for approximately 3% of all CRC patients and which predisposes to development of other cancers, is currently performed by testing for microsatellite instability (MSI), a surrogate marker for DNA mismatch repair (dMMR) gene dysfunction, which is present in > 90% of affected patients. Further testing for mutations in the BRAF gene (proto-oncogene B-Raf) may be desirable in some cases, as it may determine sensitivity to specific anti-tumour drugs (see p. 837). Studies have suggested that cancer rates and mortality are both decreased by close surveillance of patients with HNPCC, but there is still much debate about whether routine measurement of MSI/dMMR is desirable in all patients with CRC. Reports also suggest that MSI/dMMR status may have prognostic relevance in CRC, with the presence of MSI or defective MMR activity associated with favourable outcomes.
K-RAS mutation detection
Colorectal cancer patients with specific activating mutations in either codon 12 or 13 of K-RAS rarely respond to treatment with anti-epidermal growth factor receptor (EGFR) antibodies, which include cetuximab and panitumumab, both of which bind to the extracellular domain of EGFR. Expert groups have therefore recommended that patients should be tested for K-RAS mutations before treatment, to identify individuals unlikely to benefit from these relatively expensive drugs.
Gastric cancer
The second most common gastrointestinal cancer worldwide, gastric cancer, is frequently diagnosed only when it is at an advanced stage. Plasma concentrations of CEA and CA19-9 are raised in 20–50% of patients with advanced disease and AFP in 20–25%, but these markers are increased in < 20% of patients with early stage disease. None of these has the sensitivity or specificity required for screening or diagnosis of gastric cancer, although some studies suggest that post-treatment monitoring of patients using serum CEA or CA19-9 may provide early detection of recurrence in a proportion of patients. Further data are required, together with evidence that early detection improves clinical outcome, before monitoring with either can be recommended.
Results of a recent phase III trial suggest that trastuzumab (Herceptin®), in combination with chemotherapy, should be implemented as standard treatment for the 15–20% of patients with HER-2-positive advanced gastric cancers. Testing for HER-2 to identify gastric cancer patients who over-express HER-2 is likely to become increasingly important, particularly as there are newer forms of anti-HER-2 therapy (e.g. lapatinib, pertuzumab and trastuzumab-TM1), which may prove more efficacious.
Gastrointestinal stromal tumours (GIST)
Gastrointestinal stromal tumours (GIST) are rare tumours that occur in the stomach, small bowel, large bowel, oesophagus or omentum. At a molecular level, they are characterized by the presence of KIT protein (also known as CD117 antigen), whose measurement in serum is recommended by a number of expert panels. The mutational status of the KIT proto-oncogene is also important in predicting which patients will benefit from imatinib, a tyrosine kinase inhibitor, whose availability has dramatically improved the treatment of patients with GIST.
Germ cell tumours
Germ cell tumours are more common in males than females and can be benign or malignant. Although most frequent in young adults, they can occur at any age. They may be seminomatous germ cell tumours, non-seminomatous germ cell tumours (NSGCT) or combined tumours (tumours with both seminomatous and non-seminomatous elements). Germ cell tumours often originate in the gonads, but they also occur elsewhere, particularly in the mediastinum, retroperitoneum or pineal gland (i.e. along the ‘midline’). Testicular germ cell tumours are increasing in incidence and ~ 1500 new cases are diagnosed each year in the UK. Plasma concentrations of AFP and/or hCG are elevated in 80–85% of men with NSGCT, whereas < 25% of those with seminomas have raised hCG and none have raised AFP. Methods used in oncology for the measurement of hCG should recognize both intact hCG and its free β-subunit (‘total βhCG’ assay) since a significant proportion of hCG in patients with seminomas may be present as the free subunit rather than the intact molecule. Placental alkaline phosphatase (PLAP) is another promising tumour marker in seminoma, but lack of commercially available assays and the significantly increased concentrations of PLAP observed in smokers have thus far limited its application.
Although germ cell tumours are usually aggressive neoplasms, they are highly sensitive to treatment with surgery and, when appropriate, chemotherapy and/or radiotherapy. The anticipated cure rate is > 90%, although patients presenting with advanced disease have a lower five-year survival of 50–60%.
There are well-accepted clinical guidelines relating to the management of patients with germ cell tumours, for which measurements of AFP, hCG and LDH are an integral part. In the UK, patients are referred to a regional centre serving a population of two to four million for further assessment and chemotherapy. All patients should be discussed at multidisciplinary team meetings attended by a clinical biochemist. Tumour marker results should be reviewed along with histological immunostaining and radiological results and any inconsistencies noted when treatment decisions are considered.
Screening
The relatively low sensitivity and specificity of AFP, hCG and LDH for germ cell tumours, together with the low prevalence of these cancers in the general population, means that tumour markers cannot be used for screening.
Diagnosis
The possibility of a germ cell tumour should be considered in any patient with a poorly defined epithelial malignancy, particularly young individuals with mid-line masses. Plasma concentrations of AFP and hCG should be measured in any male with a suspicious lump in the testis and in any patient under 50 years of age with a malignancy of unknown origin. Young patients with germ cell tumours and very high serum hCG concentrations may present with thyrotoxicosis as hCG shares structural similarities with TSH. Patients with markedly elevated concentrations of AFP and/or hCG and clinical findings consistent with a germ cell tumour (e.g. testicular lump, lung metastases, abdominal mass) should be discussed urgently with the consultant responsible for managing germ cell tumours at the regional cancer centre. Immediate referral for chemotherapy may be appropriate if surgery is likely to be required later to remove residual tumour.
Distinguishing teratomas from seminomas is essential, as their treatments differ. Production of AFP is associated with yolk sac elements in germ cell tumours and therefore AFP is not found in pure seminomas (which never contain yolk sac elements), while the presence of hCG is associated with syncytiotrophoblast tissue. Differentiated teratoma tissue does not produce either AFP or hCG. Marker measurements can sometimes modify histopathological diagnoses, e.g. raised AFP in a patient diagnosed with pure seminoma suggests that yolk sac elements may have been overlooked or that the AFP is not related to the tumour (e.g. may be of liver origin). Before surgery for a suspected germ cell tumour, measurement of both AFP and hCG is essential to allow the rate of fall of the markers to be monitored post treatment. Some patients with pure seminoma histologically may have a preoperative AFP of 5–30 kU/L (6–36 μg/L) that remains stable following surgery. Provided that the AFP concentration remains unchanged before and after orchidectomy and is stable when re-checked four weeks after surgery, the AFP may be considered to be ‘normal’ for that individual and not related to the tumour.
Rarely, if primary or secondary (metastatic) disease of the central nervous system (CNS) is suspected, measurement of hCG in cerebrospinal fluid (CSF) may contribute both to diagnosis and to treatment monitoring. A CSF:serum hCG ratio of > 2% is predictive of CNS involvement. In practice, measurement of tumour markers in the CSF has been largely superseded by radiological investigations.
It is important to remember that tumour marker concentrations within reference intervals do not exclude malignancy since up to 25% of NSGCT do not produce AFP or hCG, and only a small proportion of seminomas or dysgerminomas (the female equivalent of seminomas) produce hCG. The possibility of false positive results must also be considered and account should always be taken of clinical and radiological findings. Plasma AFP can be increased in benign liver disease and primary hepatocellular carcinoma as well as in other cancers, including gastric and oesophageal tumours. Similarly, hCG can be increased in other non-germ cell tumours (e.g. bladder, lung) and both AFP and hCG are significantly raised in pregnancy.
Prognosis
Tumour marker measurements contribute significantly to the prognostic assessment of germ cell tumours. Criteria developed by the International Germ Cell Cancer Collaborative Group (IGCCCG) classify patients with metastatic NSGCT as belonging to one of three prognostic groups (Table 42.6). The lowest tumour marker concentration reached post surgery, the primary tumour site, and the sites of metastatic disease all contribute to this prognostic classification.
Whether AFP and hCG measurements made prior to primary surgery are prognostically useful is less clear, since patients with large primary tumours but without metastases may have very high AFP and hCG concentrations that return to normal following surgery.
Monitoring
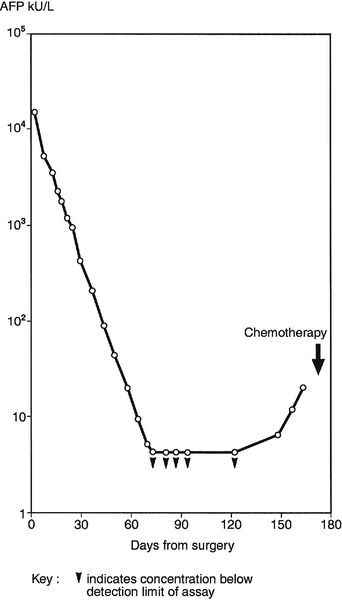
Tumour markers should be measured before and after surgical excision of germ cell tumours. Ideally, if disease is limited to the testis or ovary, serum AFP and/or hCG should decrease to normal with an apparent half-life of 5–6 days for AFP and 1–2 days for hCG. This is illustrated in Figure 42.2, which shows the decrease in AFP observed in Patient A, a patient with good prognosis following surgical treatment of a malignant teratoma. The apparent half-life (t1/2) of the tumour marker can be calculated using the equation:
FIGURE 42.2 Serum α-fetoprotein (AFP) concentrations in a patient treated by surgery for malignant teratoma.
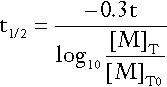
where [M]T and [M]T0 are tumour marker concentrations at time T and T0, respectively and t is the difference in days between T and T0. As indicated in Figure 42.2, in which AFP concentration is plotted on a logarithmic scale against time on a linear scale, the apparent t1/2 of AFP for Patient A is six days, i.e. at the upper limit of the expected half-life of AFP in normal subjects. This suggests possibly complete tumour removal. If AFP or hCG remains elevated post surgery or if metastatic disease is identified radiologically, further treatment with chemotherapy or radiation is required.
The importance of continued monitoring with tumour markers following surgery is also illustrated in Figure 42.2, where, although the measured AFP became undetectable by day 65, the concentration subsequently increased, first becoming detectable on day 151 (AFP 7 kU/L or 8.4 μg/L). Such findings should be confirmed within two weeks, after which further clinical investigations should be instituted immediately. In the absence of residual tumour or scan evidence, other causes of raised AFP and/or hCG, including the possibility of analytical interference, must be excluded, but provided this is done, Patient A would be a candidate for chemotherapy to combat progressive disease. Patients with very high marker concentrations before chemotherapy are more likely to have undetected micrometastases, as was probably the case for Patient A.
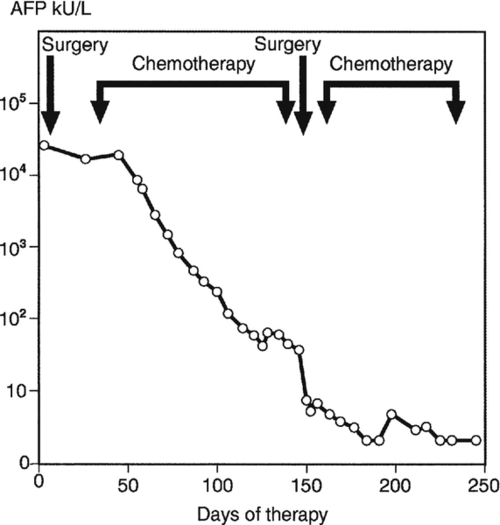
All chemotherapy regimens for germ cell tumours include platinum-based drugs, but the regimen selected will depend both on prognosis and on the results of clinical trials appropriate to that prognostic category. Following chemotherapy, AFP and/or hCG concentrations should decrease linearly when plotted as described above, but their apparent half-lives will usually be somewhat longer, i.e. up to seven days for hCG and ten days for AFP. A somewhat different pattern of AFP results is seen for Patient B (Fig. 42.3), a patient with a metastatic teratoma that did not respond to initial surgery. In some patients, AFP and/or hCG may continue to rise for up to three weeks following the start of chemotherapy, although concentrations usually start to decrease by ten days (as for Patient B). They should then fall linearly with time when plotted as described above. However, if marker concentrations plateau, as is also illustrated in Figure 42.3 (at about day 130) this is cause for concern, suggesting either that the chemotherapy used is failing to penetrate the target tissue (which may be a large mass) or that the patient has developed drug resistance. A change in chemotherapy may then be implemented or further surgery undertaken if the tumour is considered resectable, as was the case for Patient B. Timing of surgery is important, since the outcome is likely to be most favourable if surgery is performed when the tumour marker concentration is at its nadir (i.e. the lowest concentration that can be achieved with chemotherapy).
FIGURE 42.3 Serum α-fetoprotein (AFP) concentrations in a patient with metastatic teratoma that was initially unresponsive to surgery.
It is estimated that with an hCG concentration of only 1 U/L, up to 100 000 tumour cells may persist. Chemotherapy should therefore be continued for some time after AFP and hCG both become undetectable, so that as many tumour cells as possible are eradicated. In some patients, results may be difficult to interpret, since chemotherapy may damage the liver (e.g. causing increased serum AFP concentrations in patients with purely hCG-producing tumours). This is particularly relevant in children.
The availability of serum tumour markers for the majority of NSGCT patients has facilitated clinical trials designed to minimize treatment toxicity for patients with good prognosis disease. By considering the rate of change of tumour marker concentrations it is possible to identify subtle differences in the effects of treatment within days, much more rapidly than the weeks it may take for significant radiological changes to become apparent.
Long-term surveillance
Tumour markers should be measured regularly following treatment, according to defined clinical protocols. Time intervals will depend on prognostic category and treatment. As is clear from Figure 42.2, any analytically significant increases in tumour markers should be reported immediately to the relevant clinical team.
Seminoma patients at low risk of recurrence who have remained well for five years can be discharged from follow-up, as can those with NSGCT who have remained well for ten years. Separate monitoring of cardiovascular risk in these patients may, however, be desirable as there is increasing evidence of an excess of cardiovascular events following chemotherapy with platinum-based drugs.
Gestational trophoblastic neoplasia
Gestational trophoblastic neoplasia (GTN) form a group of several diseases associated with pregnancy, usually involving abnormal growth of cells within the uterus. Rare and once fatal, these now highly curable tumours develop in the trophoblast cells that surround the embryo immediately after conception. Gestational trophoblastic neoplasia may develop after a molar pregnancy, a non-molar pregnancy or a live birth and should be considered in any woman developing acute respiratory or neurological symptoms or persistent abnormal vaginal bleeding after any pregnancy. In the UK, the Royal College of Obstetrics and Gynaecology has developed guidelines for management of GTN (available at: www.rcog.org.uk).
Since measurement of hCG and related molecules is fundamental to the successful management of these diseases, it is useful briefly to review their characteristics.
Hydatidiform moles
The most common type of GTN is hydatidiform mole (also termed molar ‘pregnancy’, although a normal baby cannot be produced), which is not cancerous. The moles are villi that have become swollen with fluid and then grow in clusters resembling bunches of grapes. Hydatidiform moles may develop when a sperm fertilizes an ‘empty’ egg containing no nucleus or DNA (‘complete’ mole) or when two sperm fertilize a normal egg (‘partial’ mole). There is no fetal tissue present in complete moles, as all the genetic material comes from the father’s sperm. Relatively few patients with partial moles need further treatment after initial surgery and these moles rarely become malignant. Up to 20% of patients with complete moles will need further surgery or chemotherapy; a small percentage develop into choriocarcinoma, a malignant form of GTN.
Invasive moles
Invasive moles develop in about 20% of women who have had a complete mole removed by curettage of the lining of the uterus. Invasive moles penetrate the muscular wall of the uterus (the myometrium) sometimes leading to heavy bleeding. In about 15% of patients, the tumour metastasizes to other sites, most frequently the lungs. The risk of developing invasive mole is increased if more than four months elapse between cessation of periods and treatment, if the uterus has become very large, if the woman is over 39 years old or if she has had a previous GTN.
Choriocarcinoma
Choriocarcinoma is a malignant form of GTN that most often develops from a complete hydatidiform mole but can occur after normal pregnancy or after early fetal loss in pregnancy. Choriocarcinomas are more likely to metastasize to distant organs than invasive moles, but treatment with chemotherapy is highly effective.
Placental site trophoblastic tumours
These are rare forms of GTN that develop where the placenta remains attached to the uterus, often after a normal pregnancy or abortion. They do not usually metastasize but are not sensitive to chemotherapy and must be completely removed surgically.
Screening
All patients in the UK who have had a previous molar pregnancy are automatically registered with the National Hydatidiform Mole Registry. Screening for choriocarcinoma using hCG in this highly selected group of women provides the single best example of a successful screening programme using a tumour marker, since ~ 8% of these women will develop the disease (i.e. the prevalence in the population studied is high) and early detection and treatment improves outcome. The logistics are also convenient as hCG, which has approximately 99% sensitivity and specificity for choriocarcinoma, can be measured reliably in urine specimens that are sent by post by the patient to laboratories in specialist referral centres, thereby facilitating early detection of disease and prompt treatment. These laboratories use broad-spectrum hCG methods that are designed to detect hCG and its major isoforms.
Diagnosis
Increasing use of ultrasound in early pregnancy has probably led to earlier diagnosis of molar pregnancy, but measurements of hCG may also contribute as concentrations may be higher than in normal pregnancy.
Prognosis
Prognosis is assessed according to the International Federation of Gynaecology and Oncology staging for GTN, the hCG concentration prior to treatment being one of a number of factors scored. Low risk patients receive different treatment from those classified as high risk.
Monitoring
Women with persistent GTN are treated with appropriate chemotherapy at specialist centres. About 15% of patients with complete moles require chemotherapy and 0.5% after partial moles. Treatment is continued until the hCG concentration has returned to normal and then for a further six weeks. Women should be advised not to attempt to conceive until the hCG concentration has been normal for six months. After any further pregnancy, hCG should again be measured to exclude disease recurrence.
Hepatocellular carcinoma (primary liver cancer)
Hepatocellular carcinoma (HCC) is rare in the developed world but is common in China, South-east Asia and sub-Saharan Africa, and is the fifth most common cause of cancer death worldwide. In most parts of Africa and Asia, infection with hepatitis B virus is a major causative factor, as is ingestion of the fungal toxin, aflatoxin B1, from contaminated food. The higher incidence observed in Europe during the last decade probably reflects the increased frequency of hepatitis C infection and alcoholic liver cirrhosis, both of which are strongly associated with development of HCC.
Although many potential tissue and serum tumour markers are increased in HCC, AFP is at present the most clinically useful. Normally produced during gestation by the fetal liver and yolk sac, AFP is increased in the maternal circulation during pregnancy and is also markedly elevated in newborns, with concentrations declining over the first year of life. An oncofetal antigen, AFP appears inappropriately in adults with malignancy, most frequently in hepatocellular carcinoma and germ cell cancers, and also in some benign conditions, particularly those associated with liver damage and/or regeneration. Circulating AFP concentrations range from within the reference interval to as high as 8.3 × 106 kU/L (10 × 106 μg/L), but in the UK, up to 50% of patients with HCC may have normal concentrations of AFP.
Screening of high-risk groups
Screening by six-monthly measurement of serum AFP together with abdominal ultrasound, is now recommended for early detection of HCC in high-risk populations. There is increasing evidence that such screening (when compared with no surveillance) detects HCC of smaller size and enables a greater proportion of patients to be cured, thereby leading to improved long-term survival and cost-savings. In the UK, it is suggested that such screening be restricted to patients with liver cirrhosis secondary to hepatitis B or C or genetic haemochromatosis, and to men with primary biliary cirrhosis (PBC) and alcoholic cirrhosis (if abstinent). (Available data suggest that women with PBC or alcoholic cirrhosis have a lower risk of developing HCC.) Validation of optimal follow-up protocols, when AFP is raised or when suspicious nodules are detected, is now in progress. Sequential measurement of serum AFP provides useful information and is undergoing validation for routine clinical practice. An elevated AFP concentration detected by a single measurement may be transient (e.g. arising from an inflammatory flare of underlying viral hepatitis). Elevated but stable concentrations decrease the likelihood that HCC is the causative agent, while a steadily rising AFP concentration should always be rigorously investigated.
Diagnosis
Plasma concentrations of AFP up to 1245 kU/L (1500 μg/L) may occasionally be associated with benign conditions, with 20–40% of adult patients with hepatitis or liver cirrhosis having AFP > 8.3 kU/L (10 μg/L). An AFP result within the reference interval does not exclude a diagnosis of HCC. Rapid increases (a doubling time of < 5 days) are suggestive of acute liver damage rather than malignancy. As with screening, a steady rise in AFP is suggestive of HCC, while stable or decreasing results make HCC less likely. Histopathology of appropriate biopsy material is essential for definitive diagnosis of HCC since AFP may be raised in malignancies other than HCC, including germ cell tumours and stomach, biliary tract and pancreatic cancers.
Prognosis
Large multivariate analyses confirm that raised AFP concentrations predict poor prognosis when compared with AFP-negative patients, some studies indicating that patients with larger tumours tend to have higher AFP concentrations. Together with tumour size and extent, AFP appears to be an independent predictor of survival, patients with serum AFP > 8300 kU/L (10 000 μg/L) doing less well than those with AFP < 166 kU/L (200 μg/L). α-Fetoprotein doubling time may also be an important prognostic factor.
Monitoring
Following complete surgical removal of HCC, AFP concentrations typically decrease with a half-life of 3.5–4 days, incomplete resection being associated with a longer half-life and poorer outcome. α-Fetoprotein concentrations that fail to return to normal suggest incomplete resection or severe liver damage.
Serum AFP measurements can also be used to monitor HCC patients following treatment with chemotherapy or radiotherapy. Decreases in tumour markers may in some cases reflect tumour regression more accurately than CT scans, since interpretation of the latter may be complicated by residual fibrosis and other factors. It is important to note that recurrence is possible even when AFP is stable or within reference limits, presumably from micrometastases too small to produce measurable AFP concentrations in serum, and that recurrent tumour may not secrete AFP even if the original tumour did so.
Lung cancer
Lung cancer is the cancer with both the highest incidence and the highest number of deaths in the world. There are two major histological types: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Non-small cell lung cancer, which accounts for 75–85% of lung cancers, consists of several subtypes, predominantly squamous cell carcinoma, adenocarcinoma and large cell carcinoma. Surgery is the only curative treatment for NSCLC. Small cell lung cancer accounts for 15–25% of lung cancers, often has neuroendocrine elements and is primarily treated with chemotherapy and/or radiotherapy. Small cell lung cancer is an aggressive tumour characterized by a short doubling time and early development of metastatic disease. Many lung cancers are mixed tumours containing both small cell and non-small cell components.
A large number of tumour markers have been tested in lung cancer, different markers being required to identify the response to treatment of the different cell types present. Some of the more promising markers and their associated cell types are listed in Table 42.7.
TABLE 42.7
Tumour markers of potential utility in different types of lung cancer
| Tumour marker | Type of cancer | Cell type |
| Cyfra 21-1 | NSCLC | Squamous cell |
| Carcinoembryonic antigen (CEA) | NSCLC | Adenocarcinoma Large cell |
| Squamous cell carcinoma antigen (SCC) | NSCLC | Squamous cell |
| Neuron specific enolase (NSE) | SCLC | Small cell (neuroendocrine) |
| Progastrin releasing peptide (ProGRP) | SCLC | Small cell (neuroendocrine) |
NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer.
Screening
No markers are suitable, either singly or in combination, for screening either in the general population or in specific high-risk groups, such as smokers.
Differential diagnosis
The pattern of elevated serum concentrations of CEA, Cyfra 21-1, NSE, SCC and ProGRP may suggest which histological subtype of lung cancer is present but further work is required before measurement of tumour markers in this situation becomes standard practice. Where inoperable lung cancer is suspected but no histology is available, raised serum NSE and especially ProGRP concentrations are highly suggestive of small cell lung cancer, while raised serum SCC concentration is suggestive of squamous cell cancer.
Prognosis
Studies have suggested that Cyfra 21-1, CEA, NSE and LDH (lactate dehydrogenase) may all provide prognostic information in NSCLC, whereas only NSE and LDH act as prognostic indicators in SCLC.
Monitoring
Although tumour markers can be used in individual lung cancer patients to monitor their response to therapy (using NSE and/or ProGRP for SCLC, and CEA and/or Cyfra 21-1 for NSCLC), in view of the limited range of treatment options currently available, this is controversial. However, serial determinations of the appropriate marker following surgery may help to assess the completeness of tumour removal and provide early indication of recurrence.
In patients receiving systemic treatment, measurements of tumour markers may assist in assessing response to therapy and to document progressive disease, although reliable criteria for ‘biochemical progression’ have yet to be developed. There is currently no evidence that monitoring with tumour markers improves patient outcome.
Epidermal growth factor receptor and K-RAS mutation analysis
A number of expert panels recommend determining EGFR mutation status in patients with advanced NSCLC prior to administration of the tyrosine kinase inhibitors (TKI) gefitinib or erlotinib. Patients lacking the mutation are unlikely to benefit, while 65–70% of those with specific activating mutations in the EGFR gene respond to these therapies. Similarly, there is a significant correlation between the presence of K-RAS mutations and an absence of response to the TKIs. Combining the tumour mutation status of K-RAS with that of EGFR can therefore be helpful both in identifying patients with advanced NSCLC who are likely to respond to treatment with TKIs and the likelihood of resistance to specific TKIs.
Melanoma
Melanoma is a malignant tumour of melanocytes (cells that are derived from the neural crest), with a rising incidence worldwide. Most melanomas occur in the skin, but they may also develop at mucosal surfaces or other sites to which neural crest cells migrate. Early stage disease can be cured by surgery but the prognosis associated with metastasis to distant sites is poor.
Immunohistochemical staining with antibodies to S100 is the method of choice for diagnosing malignant melanoma in pathological specimens, but further studies are required to define the role of S100 measurement in serum. Measurement of S100B lacks sensitivity in early disease. Rising concentrations of S100B are specific and sensitive for tumour progression in patients with advanced disease, but measurement is only appropriate if further treatment options are available. Measurement of a tumour-associated glycoprotein antigen (TA90-IC) shows promise as a prognostic indicator and for monitoring patients but requires further evaluation. Lactate dehydrogenase, although a very non-specific marker, can be useful for monitoring patients with melanoma and may have prognostic value in patients with advanced disease.
BRAF mutation analysis
About 40–50% of patients with disseminated disease, but without brain metastases, may respond to treatment with the BRAF gene inhibitor vemurafenib, provided they have a V600 mutated BRAF gene. BRAF gene status therefore needs to be confirmed prior to treatment.
Neonatal and paediatric tumours
Cancer is the second leading cause of death in children < 15 years old, but major advances in treatment mean that > 70% of children diagnosed with cancer are now cured. Most childhood solid tumours are of mesenchymal or embryonal origin. Tumour markers can contribute significantly to the management of childhood neuroblastomas, malignant hepatic tumours and germ cell tumours. While the general principles of marker use in these cancers are the same as for adult malignancies, some additional points should be noted.
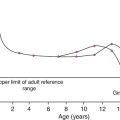
When interpreting AFP, it is important to remember that plasma AFP is markedly raised at birth and then declines steadily to adult concentrations by 6–12 months. α-Fetoprotein is higher in children born prematurely and may remain elevated for longer in children with delayed development. Appropriate gestational and age-related reference intervals therefore need to be used for infants. Serial concentrations are often more useful than isolated results. This is particularly relevant in neonates, in whom acute hepatocellular damage may result in marked increases in AFP concentrations. As with adults, other possible causes of raised AFP need to be considered, including hereditary tyrosinaemia and ataxia telangiectasia. The very high concentrations of tumour markers that may be seen in some childhood cancers mean that particular care must be taken to minimize the risk of the high-dose hook effect (Box 42.1). Since AFP and hCG requests for young children are relatively infrequent, it would be eminently feasible, and highly desirable, to assay all such samples at more than one dilution.
Germ cell tumours in childhood
As in adults, AFP and hCG are often elevated at the time of diagnosis and their measurement is mandatory. Yolk sac tumours are the most common pure malignant germ cell tumours in children. Seminomas rarely occur in infants or young boys, but dysgerminomas are the most common pure malignant germ cell tumour occurring in the ovary and central nervous system in girls, who may present with precocious puberty.
Hepatoblastoma
Hepatoblastoma and hepatocellular carcinoma (HCC) are the most frequent malignant hepatic tumours of childhood. More than 80% of hepatoblastomas, which are embryonal tumours, are diagnosed in children aged < 3 years, with 45% of patients diagnosed during the first year of life. Most patients (98%) have a raised AFP at presentation, often to extremely high concentrations (e.g. 106 kU/L or 1.21 × 106 μg/L), which can assist in diagnosis. Thereafter, serum AFP can be used to monitor therapy and follow-up. Children with hepatoblastomas that secrete hCG may develop isosexual precocious puberty. Complete surgical resection is the treatment of choice, with chemotherapy also playing an important role. Overall survival rates are > 60%, or > 80% if complete resection is achieved.
Hepatoblastomas must be differentiated from HCCs, 50% of which also produce AFP. Positive hepatitis B serology is present in some children with HCC, with other laboratory abnormalities including anaemia and hyperbilirubinaemia. Complete surgical resection is the treatment of choice for HCC. Aggressive chemotherapy has not significantly improved outcome and most children with HCC die within 12 months of diagnosis.
Neuroblastoma
Neuroblastoma is a malignant embryonal tumour that accounts for 8–10% of all childhood cancers, with 80% of cases occurring before the age of four years. The clinical behaviour is varied, some tumours undergoing spontaneous regression and others exhibiting extremely malignant behaviour. Treatment includes surgery, chemotherapy and radiotherapy. Urinary catecholamine metabolites are increased in > 90% of patients and are helpful to confirm the diagnosis and monitor progress (see Chapter 38). Significant elevations of serum NSE, LDH and/or ferritin tend to be associated with a poorer outcome.
Ovarian cancer
Ovarian cancer is the fourth most common cause of death from cancer in women in the UK. Early diagnosis is key to successful treatment, but the absence of symptoms in early stage disease means that many ovarian cancers present late. Standard treatment for ovarian cancer is surgical, involving bilateral oophorectomy and pelvic clearance, usually followed by chemotherapy. New chemotherapeutic agents have significantly improved the five-year survival rate.
About 15% of malignant ovarian tumours are germ cell tumours, for which AFP, hCG and LDH are the markers of choice as previously described, or sex cord stromal tumours, two-thirds of which are granulosa cell tumours. Inhibin is the marker of choice for granulosa cell tumours of the ovary. An inhibin method, which detects all forms of inhibin, including A, B and pro-αC, is required.
However, most malignant ovarian tumours (80–85%) are surface epithelial carcinomas. These occur in five histologically distinct subtypes: serous, mucinous, endometrioid, clear cell and transitional. They exhibit different clinical behaviours, tumorigenesis and pattern of gene expression, which should be taken into account when evaluating the clinical utility of tumour markers in ovarian cancer. The most widely used tumour marker for epithelial ovarian cancer is CA125. Most sensitive in serous adenocarcinomas, it is also used in other histological types of epithelial cancer despite its poorer sensitivity.
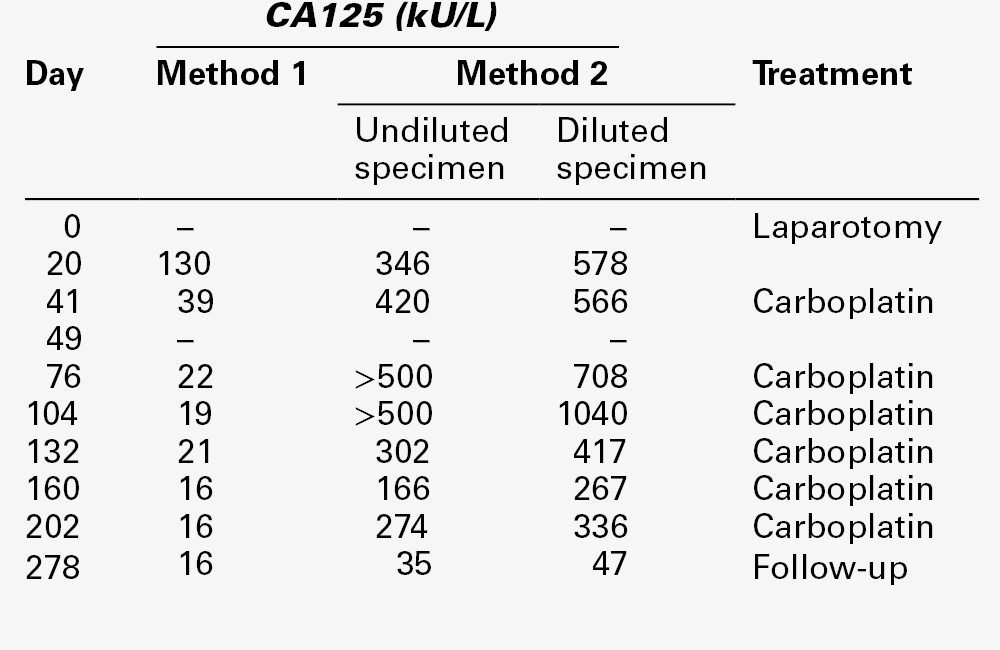
Interpretation of CA125 results is particularly challenging, and it is essential to be aware of the many benign conditions in which the marker can be significantly elevated, sometimes transiently (Table 42.1). These conditions include during menstruation and the first two trimesters of pregnancy. Serum CA125 may be raised in any patient with ascites (occasionally to > 5000 kU/L) or pleural effusion (usually 200–300 kU/L) and is also raised in patients with a wide variety of tumours, especially adenocarcinomas. Method-related differences in results can also be problematic and some methods are more vulnerable to interference, such as from heterophilic antibodies, as illustrated in Table 42.8.
Screening
In view of the absence of early symptoms, a reliable screening test for ovarian carcinoma would be highly desirable. Variations in results in premenopausal women (including increases during menstruation) mean that single CA125 measurements lack the sensitivity and specificity essential in a screening setting. However, use of CA125 for screening in postmenopausal women is the subject of several trials. In a large controlled trial (the UK Collaborative Trial of Ovarian Cancer Screening), patients have been randomized into three groups: an unscreened control arm; a group screened annually with CA125, with repeat CA125 and ultrasound follow-up if positive, and a group screened annually with ultrasound, with repeat ultrasound in 6–8 weeks if positive. Until this trial has reported in 2015, screening of the general population cannot be recommended.
There is, as yet, no evidence that using CA125 to screen women at high risk of ovarian cancer (e.g. those with a strong family history) is effective. However, a US National Cancer Institute Panel has recommended annual CA125 determinations, in addition to pelvic and ultrasound examinations, in women with a history of hereditary ovarian cancer, who have an estimated lifetime risk of 40%. Such investigations should always be carried out in specialist units.
Diagnosis
Ultrasound assessment is essential in women with suspected ovarian cancer. This is likely to identify a pelvic mass and may suggest the presence of metastatic disease. If no obvious source is identified, determining whether the pelvic mass is likely to be malignant can be problematical but is important, as it will influence plans for surgery. Prognosis in ovarian cancer correlates strongly with the extent of surgical clearance of malignant tissue.
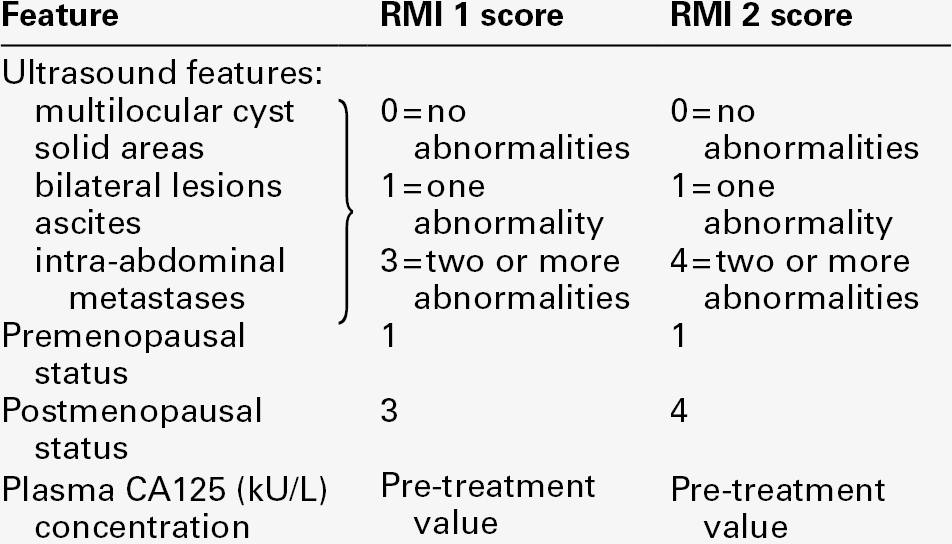
Cut-off concentrations (e.g. CA125 > 95 or > 65 kU/L) have been used with some success in postmenopausal women to distinguish between malignant and benign pelvic masses. However, a risk of malignancy index (RMI) scoring system, incorporating CA125, ultrasound findings and menopausal status, is more widely used (Table 42.9). The two scoring systems (RMI 1 and RMI 2) are similar but RMI 2 gives greater weight to ultrasound findings and menopausal status than does RMI 1. A positive predictive value for malignancy of about 80% is obtained using the RMI 2 scoring system and a cut-off value of 200.
It is clear from Table 42.9 that whichever score is used, the result depends critically on the numerical CA125 value and therefore also on the reliability of the assay, particularly in the range of 10–25 kU/L. If there is a change in method the cut-off value used may need to be reviewed.
In the UK, NICE has published clinical guidelines on the recognition and initial management of ovarian cancer with the aim of increasing awareness of the disease among general practitioners and decreasing the frequency of late diagnosis, which is thought to account for the lower survival rates observed in the UK and Ireland compared with other European countries. The NICE guidelines state that general practitioners should measure serum CA125 concentrations in women presenting with persistent and continuous symptoms (> 12 times a month) suggestive of ovarian cancer (e.g. abdominal pain, early satiety and loss of appetite or pelvic pain). If CA125 concentration is ≥ 35 kU/L, an ultrasound scan of the abdomen and pelvis should be arranged to enable calculation of the RMI. Women with an RMI score ≥ 250 should be referred to a specialist multidisciplinary team. Women with CA125 < 35 kU/L should be reviewed at six weeks if symptoms persist.
Prognosis
During primary treatment, CA125 concentrations both before and after surgery may be of prognostic significance. Patients with a preoperative CA125 concentration > 65 kU/L have a poorer five-year survival than patients with lower CA125 concentrations. A concentration of < 250 kU/L before chemotherapy, a fall of CA125 of greater than seven-fold during the first month of chemotherapy, an apparent CA125 half-life of < 20 days during chemotherapy and a CA125 concentration of < 35 kU/L before the third course of chemotherapy are all thought to indicate a good prognosis.
Detection of residual disease
Measurement of serum CA125 following surgery is only relevant in the small number of patients with disease limited to the ovary (Stage I) who would not automatically receive further treatment. It is essential that these patients have CA125 measurements at least once a week initially so that the rate of fall can be checked. The apparent half-life of CA125 in patients with no residual disease is five days.
Monitoring
Serum CA125 concentrations correlate well with the response to treatment in ~ 90% of patients. A rising or stable CA125 at the beginning of the second or third course of chemotherapy, together with an apparently poor clinical response, may be used as an indication either to change the treatment or to institute appropriate palliative therapy. While an increased CA125 concentration (> 35 kU/L) at the end of treatment is always associated with disease, the reverse is not true, as a low CA125 concentration does not exclude active disease.
Long-term surveillance
A confirmed rise in CA125 to more than twice the upper reference limit accurately predicts relapse with a sensitivity of 86% and a positive predictive value of 95%. Whether early treatment for relapse in patients who are asymptomatic improves outcome, is still unclear. Results of a Medical Research Council and European Organisation for Research and Treatment of Cancer randomized control trial (MRC OV05 and EORTC 55955) addressing this question suggests that instituting early chemotherapy based on a rising CA125 does not improve either survival or quality of life, and that it is appropriate to delay treatment until signs and symptoms of recurrence develop. Whether this would also be the case with the improved treatment regimens now available is not clear. However, the recommendation from the trial investigators is that it is appropriate to offer women with ovarian cancer two informed choices for follow-up, i.e. no routine CA125 measurements but rapid access to CA125 testing if there are symptoms or signs of relapse, or, alternatively, regular CA125 measurements.
Pancreatic cancer
In pancreatic ductal adenocarcinomas (i.e. non-endocrine tumours of pancreas), CA19-9 is the only tumour marker for which there is sufficient evidence to support its clinical use. It can be used as an adjunct to diagnosis in association with appropriate imaging but it is not suitable for screening even in high-risk populations. Measurements can provide independent prognostic information with regard to resectability and survival but should only be used in conjunction with other clinical information. The American Society of Clinical Oncology recommends serial measurements of CA19-9 every 1–3 months for pancreatic cancer patients with locally advanced or metastatic disease who are receiving active therapy. Increases in serial CA19-9 measurements suggest progressive disease, but confirmation by other means should be sought. It is important to note that there are significant method-related differences in CA19-9 results and particular care should be taken if changing methods. Many non-malignant conditions, including any that cause cholestasis, can cause elevated plasma concentrations of CA19-9 (see Table 42.2).
Prostate cancer
Prostate cancer is one of the most common malignancies in men, autopsy data indicating the presence of histologically apparent cancer in the prostates of ~ 42% of men aged > 50 years who have died from other causes. Recent increases in the apparent incidence of prostate cancer almost certainly reflect widespread measurement of serum PSA, elevations of which can identify disease long before it is symptomatic. Since many men have indolent prostatic cancers that pose little threat to their life or health, there is increasing concern that the use of PSA measurements, which cannot differentiate slow-growing cancers from aggressive cancers that require treatment, is causing over-diagnosis and over-treatment of some men. A further difficulty is that, while PSA is essentially organ-specific, it is not cancer-specific, and men with non-malignant conditions, such as benign prostatic hypertrophy, may also have raised PSA concentrations. This lack of specificity complicates the interpretation of results, as well as having major implications for use of PSA in screening. Many uncertainties also remain concerning optimal treatment of early stage disease, even when clinically localized to the prostate.
Screening and diagnosis
Screening men for prostate cancer using PSA is controversial, as is evident from the disparate recommendations, both for and against screening, that have been made by different professional organizations. Consensus is unlikely to be achieved until publication of the final conclusions of a number of major national and international prospective screening trials using PSA (with or without digital rectal examination). Results from the European Randomized Study of Screening for Prostate Cancer, which involved 182 160 men age 50–74 years at entry, showed that, at 11 years of follow-up, PSA-based screening significantly reduced mortality from prostate cancer but did not affect all-cause mortality. To prevent one death from prostate cancer at 11 years of follow-up, 1055 men would need to be invited for screening and 37 cancers would need to be detected. In contrast, after 13 years of follow-up, results from the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial in the USA showed no evidence of a mortality benefit for organized annual screening as compared with opportunistic screening. Various explanations for these apparently contradictory results have been put forward.
However, whatever the final outcome of the ongoing trials, men will continue to request ad hoc screening for prostate cancer, whether or not an official programme is established. There is therefore general agreement that men should be informed of the benefits and limitations of PSA measurement before deciding whether to have the test performed, particularly if asymptomatic. In the UK, the NHS Cancer Screening Committee’s Prostate Cancer Risk Management Programme provides helpful information about the test (Informed Choice Programme, see Further reading).
Early detection is likely to be most effective in younger men (in their 50s and 60s) who are at high risk of developing prostate cancer, African American men and men with a family history of prostate cancer. It is unlikely to benefit men with a life expectancy of less than ten years. Men with a strong family history (e.g. a first-degree relative diagnosed before the age of 60) may have a 3–4-fold higher than average risk of developing prostate cancer and should be carefully investigated.
Considerable effort has been made to improve the specificity of PSA assays, for example by considering the ratio of free to total PSA (the proportion of circulating free PSA is higher in benign disease), by assessing the rate of doubling of PSA (doubling time is more rapid in malignant than benign disease) or by implementing age-related reference intervals (PSA may increase with age as the size of the prostate gland increases). Promising data are also emerging regarding proPSA, a proenzyme molecular form of free PSA, and a few truncated proPSA isoforms, but these tests are still under evaluation and are not widely used.
While each of these measures improves the differentiation of benign and malignant prostatic disease, whatever PSA decision limit is selected and however the marker is used, a significant number of men with benign prostatic disease will still be recommended for biopsy on the basis of a high PSA result. Conversely, a significant number of men with malignancy but with PSA below the decision point will be missed. It is estimated that up to 20% of men with a clinically significant prostate cancer will have a normal serum PSA concentration, and as many as two in every three men with an elevated PSA do not have prostate cancer detectable at biopsy. Nevertheless, PSA is currently the best routinely available serum tumour marker for screening or diagnosis of prostate cancer. It has been suggested that screening might be made more effective by starting screening earlier and referring patients with screen-detected cancers for treatment at specialist centres, and be made less harmful by avoiding screening in older men and promoting active surveillance with well-organized follow-up programmes for those with disease that is of low risk of progression.
Management
Once a diagnosis of prostatic cancer has been confirmed by finding malignant cells in a biopsy specimen, the treatment selected depends on whether disease is confined to the prostate gland or has spread to other organs. Using predictive tables combining the pre-treatment PSA concentration with information on clinical stage and the immunohistochemical Gleason score (which provides a measure of the degree of differentiation in biopsy tissue and ranges from 2 in well-differentiated tumours to 10 in completely anaplastic cancers), a reasonable prediction of the stage of localized prostate cancer can be made. For example, patients with a Gleason score of ≤ 6 and a PSA of < 10 μg/L are unlikely to have bone metastases.
Such information can then be used to select the most appropriate treatment option. If the disease has not spread beyond the prostatic capsule, possibilities include radical prostatectomy (complete removal of the prostate), brachytherapy (insertion of radioactive needles to ablate the prostate) and external beam irradiation. Active monitoring, i.e. regular clinical assessment together with measurement of PSA, is as appropriate as active intervention in some patients with localized tumours. The benefit of treatment is not yet proven, except in men with localized disease who are candidates for radical prostatectomy. In these men, the absolute reduction in the risk of death after ten years is small, but the reduction in the risks of metastasis and local tumour progression is substantial.
Following radical treatment, PSA should decrease to undetectable concentrations, with measurable concentrations providing evidence of residual disease. However, undetectable PSA concentrations do not necessarily indicate cure. Similarly, while rising PSA concentrations following radical treatment provide biochemical indication of recurrent disease, clinical symptoms may never occur or may appear many years later. The use of ultrasensitive PSA methods with low detection limits (usually < 0.005 μg/L), which permit very early identification of rising PSA concentrations, therefore remains controversial and is probably most appropriate within the context of clinical trials.
Treatment options for patients with prostatic cancer that has spread beyond the prostate (most frequently to local lymph nodes and bone) include endocrine therapy (usually by androgen blockade as prostate cancer is hormone-sensitive), radiotherapy and chemotherapy. Men with serum PSA > 100 μg/L and clinical, biochemical or radiological evidence of metastatic disease may, in some cases, be treated without histopathological confirmation of the diagnosis, while in other patients, active monitoring may be appropriate.
A major clinical application of PSA is in evaluating the efficacy of therapy, sustained increases providing evidence of disease progression, usually earlier than other diagnostic procedures. An increase in PSA should always be confirmed, and intraindividual variation in PSA of up to 20–30% taken into account before any increase is considered to be clinically significant. The doubling time of PSA can give some indication of the likely time to clinical progression. A stable PSA concentration does not necessarily exclude progression if clinically suspected. In patients with advanced, androgen-independent prostate cancer, measurement of alkaline phosphatase may be helpful to detect bone involvement. As knowledge of increasing PSA concentrations may have adverse psychological consequences, it may be desirable to stop measuring PSA in some patients, particularly if effective alternative therapy is not available.
Analytical and reporting requirements
Rigorous adherence to the quality control measures described at the beginning of this chapter is essential. The NHS Prostate Risk Management Programme formally requires that PSA assays used in the Informed Choice programme be accurately calibrated in terms of the International Standard for PSA (Table 42.4) and that they recognize free and complexed PSA equivalently (i.e. are equimolar). Screening programmes place particularly high demands on analytical performance, since relatively minor differences in performance have a major influence on outcome, i.e. whether or not a prostatic biopsy is advised. Even when PSA assays are accurately calibrated and equimolar, results for individual patients obtained using different methods are not necessarily interchangeable. It is therefore strongly recommended that laboratory reports for PSA state both the name of the assay used and the appropriate clinical decision limits.
Testicular cancer
More than 90% of testicular tumours in adults are germ cell tumours: these are discussed above. Leydig and Sertoli cell tumours, which are rare, develop in the supportive hormone-producing tissue of the testicles (the stroma). These tumours represent 4% of adult testicular tumours and 20% of childhood tumours. Tumours of the Leydig cells (which normally produce androgens) do not usually spread beyond the testicle and can be cured by surgical removal. Those that do spread are resistant to chemotherapy or radiation. Tumours of the Sertoli cells (which normally support sperm-producing cells) are similar to Leydig cell tumours and are also difficult to treat if they spread beyond the testes. Inhibin is the marker of choice for Sertoli and Leydig cell tumours and the same analytical issues apply as for granulosa cell tumours of the ovary.
Thyroid cancer
Thyroid cancer is a relatively rare tumour, accounting for approximately 2% of cancers in all age groups and 4% of cancers in those < 20 years old. There are four major types: papillary, follicular and anaplastic thyroid cancer and medullary carcinoma of the thyroid (MCT). Calcitonin is an excellent marker for MCT, which can be associated with multiple endocrine neoplasia (see Chapter 41). Papillary and follicular cancers are differentiated epithelial thyroid cancers and can be monitored using thyroglobulin, a large glycoprotein synthesized by the follicular cells and stored in the colloid space. Thyroglobulin is present in benign and malignant thyroid tissue.
Screening, diagnosis and prognosis
Serum thyroglobulin measurement has no role in the screening for, or diagnosis of, thyroid cancer and is of no value as a prognostic indicator. However, immunohistochemical detection of thyroglobulin can be useful in identifying the thyroid as the site of primary tumour in individuals presenting with metastatic disease from an unknown primary.
Monitoring
Thyroglobulin has an important role in monitoring patients with follicular or papillary thyroid carcinoma after treatment with surgery and/or radioiodine. Since thyroid stimulating hormone (TSH) stimulates thyroid follicular cells to produce thyroglobulin, serum thyroglobulin concentrations will be higher in patients with a raised TSH if there is remaining thyroid tissue or in the presence of recurrent tumour. TSH should therefore always be measured at the same time as thyroglobulin.
Detectable concentrations of thyroglobulin may be appropriate in patients treated by surgery alone, provided they are stable, but not in patients who have been treated with radioiodine to ensure total ablation of any remaining thyroid tissue. In these patients, serum thyroglobulin concentrations should decrease to < 2 μg/L following ablation. In an individual with suppressed TSH, a rising thyroglobulin suggests recurrent tumour.
Historically, the difference in thyroglobulin values on and off thyroxine replacement was used to assess the presence of residual or recurrent tumour. This can now be done more effectively by measuring plasma thyroglobulin concentration after administering recombinant TSH (rhTSH).
Analytical and reporting requirements
It is important to be aware of several potential pitfalls in thyroglobulin measurement. As for other tumour markers, it is highly desirable that specimens for thyroglobulin measurement should be taken prior to any invasive procedure. A common cause of very high serum thyroglobulin results is inappropriate specimen collection following fine needle aspiration biopsy of the thyroid.
Analytically, radioimmunoassay (RIA) and immunometric methods for thyroglobulin are particularly vulnerable to interference from anti-thyroglobulin autoantibodies (TgAb), which may be present in some patient sera. Results obtained may be falsely low or falsely high, with immunometric methods being particularly prone to giving falsely low thyroglobulin results. Thyroglobulin antibodies should be measured in the same sample as thyroglobulin, using a sensitive immunoassay method. The presence of antibodies usually invalidates the thyroglobulin result. Comparison of results with RIA results available from specialist laboratories is informative. When interference is present, RIA results may be more reliable. It is important to note that interference may be absent even in the presence of antibodies or conversely present in their absence. Appropriate cautions should be added to patient reports.
Cancers of unknown primary
Cancers of unknown primary origin account for ~ 3% of all new cancer diagnoses and generally have a poor outlook, with median survival of less than a year. Although it is not clear to what extent tumour marker measurements may influence the clinical management of cancers of unknown primary, for a few patients the benefit may be considerable, enabling identification of appropriate treatment. Diagnoses for which this is most likely to be the case, together with the tumour marker measurements of relevance, are gestational trophoblastic neoplasm (hCG), germ cell tumours of the testis or ovary (hCG and AFP) and prostate cancer (PSA). These are potentially curable malignancies, which, once diagnosed, can rapidly respond to appropriate treatment. The NICE guidelines for metastatic malignant disease of unknown primary origin recommend measurement of serum AFP and hCG (particularly in the presence of midline nodal disease), PSA in men, CA125 in women with peritoneal malignancy or ascites, and a myeloma screen when there are isolated or multiple lytic bone lesions.
SUMMARY
Measurements of tumour markers already make an important contribution to the management of cancer patients. Maximizing their clinical usefulness and cost-effectiveness provides an excellent opportunity for close liaison between laboratory and clinical staff. Tumour marker requests should be made only when the results are likely to be of benefit; pre-analytical and analytical requirements should be met and those using tumour markers clinically should be familiar with the key points summarized in Box 42.2.
It is likely that over the next decade, new analytical techniques such as proteomic profiling and novel genetic tests will lead to the development of more targeted tumour markers. Their introduction into routine practice will provide new challenges, which clinical biochemists are well positioned to meet.
Further reading
Cancer Research UK website. http://www.cancerresearchuk.org/cancer-help/; [Accessed October 2013].
Patient-oriented source of helpful information about most cancers.
National Academy of Clinical Biochemistry website. http://www.aacc.org/members/nacb/lmpg/pages/default.aspx# [Accessed October 2013].
The NACB’s Laboratory Medicine Practice Guidelines (LMPG) for Use of Tumour Markers in the Clinic, focus particularly on laboratory aspects of tumour marker use in 16 major malignancies, with separate sections on quality requirements and new technologies.
National Cancer Institute (United States National Institutes of Health) website. http://www.cancer.gov/ [Accessed October 2013].
Information about most cancers for patients and for health professionals.
National Comprehensive Cancer Network (NCCN) website. http://www.nccn.org/professionals/physician_gls/default.asp [Accessed October 2013].
The NCCN is an alliance of 20 of the world’s foremost cancer centres. Detailed Clinical Practice Guidelines for most major malignancies are available on the website.
National Health Service (NHS) Cancer Screening Programmes website. http://www.cancerscreening.nhs.uk [Accessed October 2013].
Source of current information about NHS screening policy with respect to established and pilot screening programmes, including those for breast cancer (mammography), cervical cancer, (smear testing) colorectal cancer (FOB testing) and prostate cancer (Informed Choice Programme).
National Institute of Health and Care Excellence (NICE) website: http://www.nice.org.uk/ [Accessed October 2013].
Evidence-based guidelines on the management of a number of cancers, including breast cancer, ovarian cancer, prostate cancer and metastatic malignant disease of unknown primary origin.
Royal College of Obstetrics and Gynaecologists. Green-top 38: the management of gestational trophoblastic disease 2010, http://www.rcog.org.uk [Accessed October 2013].
Evidence-based guidelines on the treatment of gestational trophoblastic neoplasia.
Scottish Intercollegiate Guidelines Network (SIGN) website. http:// www.sign.ac.uk/guidelines/published/numlist.html [Accessed October 2013].
Evidence-based guidelines for more than 12 cancer sites (including breast, colorectal, lung and testicular cancers), are available on the SIGN website, together with guidelines relating to other aspects of medicine.