CASE 40 Should a Nonculprit Artery Undergo PCI in the Setting of Acute STEMI?
Cardiac catheterization
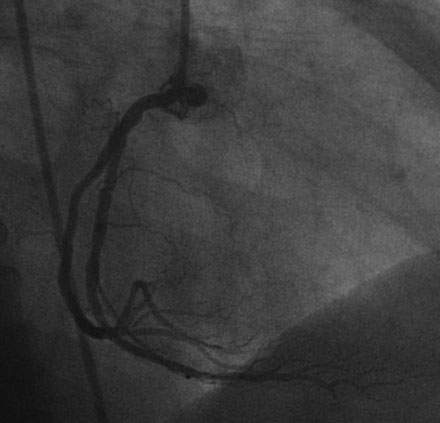
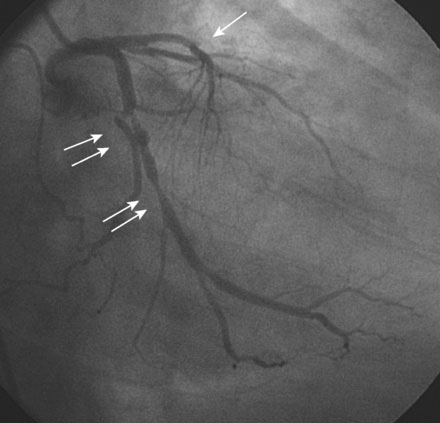
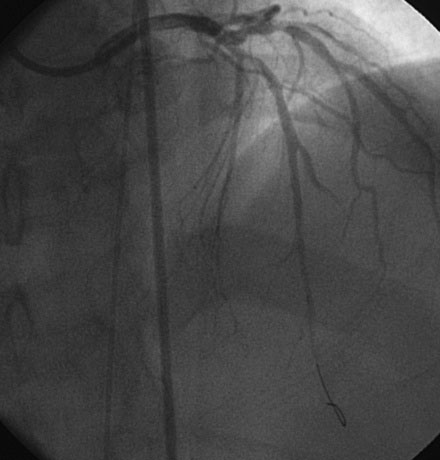
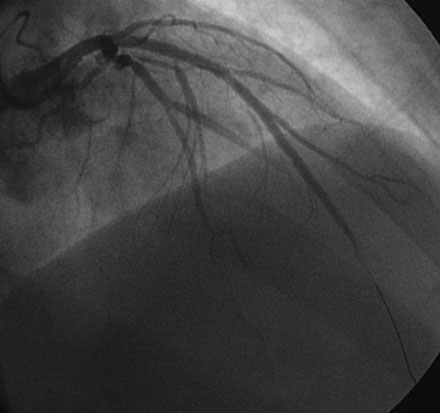
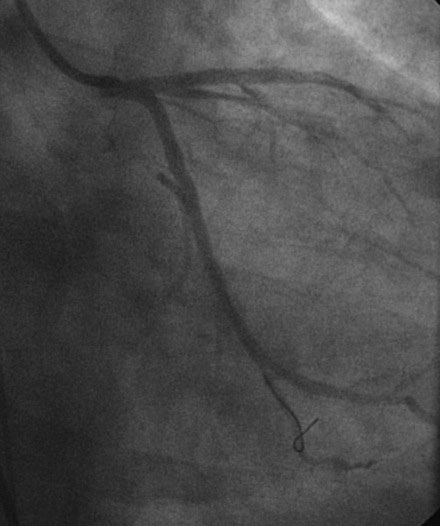
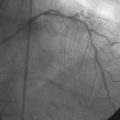
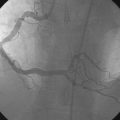
Hemodynamics obtained prior to coronary angiography revealed an elevated pulmonary capillary wedge pressure at 30 mmHg, elevated left ventricular end-diastolic pressure at 35 mmHg, a narrowed aortic pulse pressure, and tachycardia, consistent with the early phase of cardiogenic shock. Thus, the operator placed an intraaortic balloon pump for hemodynamic support. Angiography found a widely patent right coronary artery with mild luminal irregularities (Figure 40-1). Two consecutive severely narrowed tubular stenoses were present in a large, bifurcating, obtuse marginal branch of the circumflex artery. The LAD was occluded in its proximal portion just after the takeoff of a large septal branch (Figure 40-2 and Video 40-1). Anticoagulation was achieved with heparin and eptifibatide, and a 0.014 inch guidewire was easily advanced distally in the LAD. The lesion was predilated with a 2.5 mm diameter by 15 mm long compliant balloon with full balloon expansion and return of flow to the artery (Figure 40-3 and Video 40-2). Subsequently, a 3.0 mm diameter by 23 mm long sirolimus-eluting stent was placed successfully in the LAD with resultant TIMI-2 flow (Figure 40-4 and Video 40-3). His blood pressure remained low and he required a dopamine infusion (10 mcg/kg/min) for hemodynamic support. Given the patient’s deteriorating hemodynamic status, the operator was concerned about ongoing ischemia from the severe stenosis in the large circumflex artery and decided to perform percutaneous intervention on this vessel also. The 0.014 inch guidewire was removed from the LAD and advanced into the obtuse marginal. The sequential lesions were predilated with a 2.5 mm diameter by 15 mm long balloon with full balloon expansion. The distal lesion was treated with a 2.5 mm diameter by 13 mm long sirolimus-eluting stent, and a 3.0 mm diameter by 13 mm long sirolimus-eluting stent was deployed across the proximal lesion so there was overlap of the two stents. The final angiogram shows TIMI-3 flow in both the circumflex and LAD (Figure 40-5, and Video 40-4).
Discussion
In patients with ST-elevation myocardial infarction (STEMI), the initial goal of cardiac catheterization is to identify and open the infarct-related artery (IRA). A secondary goal is to image and evaluate the remaining coronary arteries for the presence of additional obstructive disease allowing for further risk stratification and management. In the current era, 40% to 50% of patients presenting with STEMI are found to have significant multivessel coronary disease.1,2,3 It is well-accepted that patients with multivessel disease in the setting of STEMI are at higher risk for both early and late cardiac events than patients with single-vessel disease.1,2,3 Several mechanisms may account for this. Disease in a non-IRA may prevent or impair collateral formation and function, creating more extensive ischemia and infarction during the acute occlusion. Additionally, previous infarctions may have lowered the ejection fraction, placing the patient at higher risk for heart failure or shock in the acute setting. And, finally, it has been shown that, in the setting of an acute myocardial infarction, plaques in non-IRAs have a higher frequency of rupture and vulnerable characteristics and non–infarct-related arteries have impairments in flow and flow reserve, suggesting a more global effect on the coronary circulation during an acute coronary syndrome.4–7
Given the increased risk in these patients, physicians are faced with the dilemma of how best to manage multivessel disease during catheterization for acute myocardial infarction. There is no definitive evidence to guide the physician in the proper management of the non-IRA, specifically whether the artery should be treated at the initial presentation, treated in a staged fashion, or if further risk stratification should be performed. Arguments can be made both for and against treatment of the non–infarct-related artery at initial presentation. The possibility of abrupt vessel closure or stent thrombosis in the non-IRA during intervention may lead to additional infarction and potentially devastating consequences. An equally compelling argument can be made that intervention on the non-IRA would decrease ischemic risk and improve collateral formation, thus resulting in decreased infarct size. Retrospective studies, prior to extensive use of drug-eluting stents and glycoprotein IIb/IIIa inhibitors, have suggested higher rates of reinfarction and target vessel revascularization if PCI of the non-IRA is performed during the initial presentation for STEMI.8,9 No large prospective trials address this dilemma. Accordingly, current practice guidelines do not recommend performance of an intervention on obstructive lesions in a non-IRA at the time of acute infarct angioplasty, with the exception of patients having hemodynamic compromise, where it might be considered reasonable.10
There are even fewer data addressing non-IRA PCI in patients with hemodynamic compromise. It is clear from prospective trials that PCI of the infarct-related artery is beneficial.11 However, retrospective data suggest higher in-hospital mortality in patients with cardiogenic shock undergoing multivessel PCI during primary PCI for STEMI.12 Definitive prospective studies need to be performed to better address this issue.
1 Muller D.W., Topol E.J., Ellis S.G., et al. Multivessel coronary artery disease: a key predictor of short-term prognosis after reperfusion therapy for acute myocardial infarction. Thrombolysis and Angioplasty in Myocardial Infarction (TAMI) Study Group. Am Heart J. 1991;121(4 Pt 1):1042-1049.
2 Jaski B.E., Cohen J.D., Trausch J., et al. Outcome of urgent percutaneous transluminal coronary angioplasty in acute myocardial infarction: comparison of single-vessel versus multivessel coronary artery disease. Am Heart J. 1992;124:1427-1433.
3 Kahn J.K., Rutherford B.D., McConahay D.R., et al. Results of primary angioplasty for acute myocardial infarction in patients with multivessel coronary artery disease. J Am Coll Cardiol. 1990;16:1089-1096.
4 Rioufol G., Finet G., Ginon I., et al. Multiple atherosclerotic plaque rupture in acute coronary syndromes: three-vessel intravascular ultrasound study. Circulation. 2002;106:804-808.
5 Kotani J., Mintz G.S., Castagna M.T., et al. Intravascular ultrasound analysis of infarct-related and non-infarct-related arteries in patients who presented with an acute myocardial infarction. Circulation. 2003;107:2889-2893.
6 Goldstein J.A., Demetriou D., Grines C.L., Pica M., Shoukfeh M., O’Neill W.W. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med. 2000;343:915-922.
7 Gibson C.M., Ryan K.A., Murphy S.A., et al. Impaired coronary blood flow in nonculprit arteries in the setting of acute myocardial infarction. The TIMI Study Group. Thrombolysis in myocardial infarction. J Am Coll Cardiol. 1999;34:974-982.
8 Roe M.T., Cura F.A., Joski P.S., et al. Initial experience with multivessel percutaneous coronary intervention during mechanical reperfusion for acute myocardial infarction. Am J Card. 2001;88:170-173.
9 Corpus R.A., House J.A., Marso S.P., et al. Multivessel percutaneous coronary intervention in patients with multivessel disease and acute myocardial infarction. Am Heart J. 2004;148:493-500.
10 2009 Focused updates. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction and ACC/AHA/SCAI guidelines on percutaneous coronary intervention. JACC. 2009;54:2205-2241.
11 Webb J.G., Lowe A.M., Sanborn T.A., et al. Percutaneous coronary intervention for cardiogenic shock in the SHOCK trial. JACC. 2003;42:1380-1386.
12 Cavender M.A., Milford-Beland S., Roe M.T., et al. Prevalence, predictors, and in-hospital outcomes of non-infarct artery intervention during primary percutaneous intervention for ST-segment elevation myocardial infarction. Am J Cardiol. 2009;104(4):507-513.