CASE 1
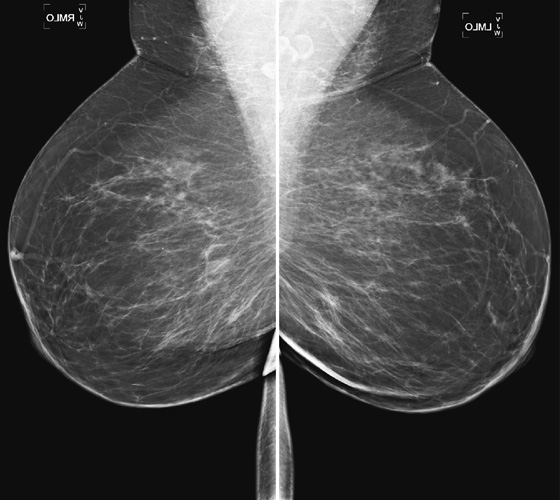
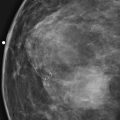
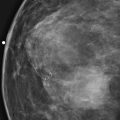
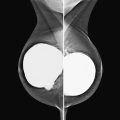
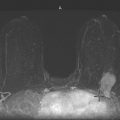
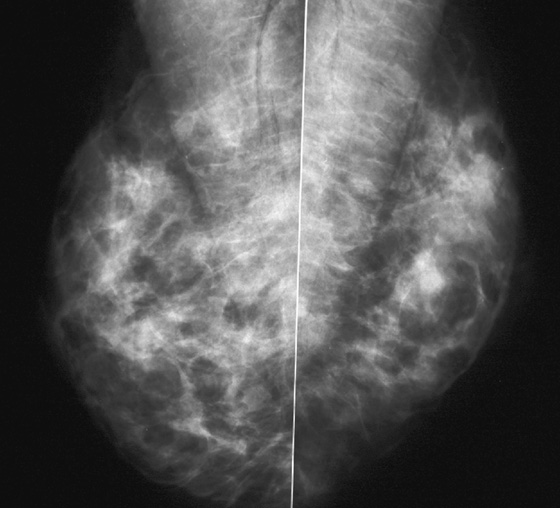
History: A 40-year-old woman presents for a baseline screening mammogram.
1. What is a screening population? (Choose all that apply.)
B. Patients with a lump that has been assessed as benign by the primary care provider
C. Patients with a nipple discharge on only one side
2. What is a diagnostic population?
A. Patients with a strong family history of breast cancer
B. Patients with a lump or nipple discharge
C. Patients with chronic cyclic breast pain
D. Patients who are extremely anxious about breast cancer
3. What is prevalence screening, and what is the approximate cancer detection rate in this population?
A. Patients who have ovarian cancer; 15/1000
B. Patients with a family history of breast cancer; 12/1000
C. First round of screening, no prior mammogram; 6-10/1000
D. Patients who have had many years of screening
4. What is incidence screening, and what is the approximate cancer detection rate in this population?
A. Screening in women who have had no prior mammogram; 10/1000
B. Screening in women undergoing annual mammography; 2-4/1000
D. Screening in high-risk women
ANSWERS
CASE 1
Incidence and Prevalence
1. A and D
2. B
3. C
4. B
References
Bassett LW, Jackson VP, Jahan R, et al. Diagnosis of Diseases of the Breast. Philadelphia: Saunders; 1997.
Cross-Reference
Ikeda D. Breast Imaging. THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010. p 39
Comment
The incidence of breast cancer is an estimate of the number of new cases of breast cancer over a specific period. It can also be stated as the incidence rate, which is the number of people with a diagnosis of breast cancer per 100,000 people. In the United States in 2008, the incidence rate of breast cancer in all women undergoing screening was 3/1000 women. This number reflects new cancers not previously detected.
The prevalence of breast cancer refers to the number of women living with breast cancer at any given time. Prevalence screening is the first mammogram performed in previously unscreened women, and the rate of cancer detected in average-risk women in this first screening event is higher than the incidence rate, at approximately 6 to 10 women with cancer detected per 1000 screens.
Mammography exams are divided into two broad types: screening and diagnostic exams. The screening exam consists of four standard imaging views (mediolateral oblique [MLO] (see the figure) and craniocaudal [CC] view of each breast), and the population is women who have no signs or symptoms of breast cancer. This group includes women who might have an increased risk of breast cancer because of family history. It also includes women with breast pain, which is not considered to be a symptom of breast cancer, particularly if the pain waxes and wanes. Exams of asymptomatic women with implants are also typically considered to be screening. Women with implants have an additional four views performed, with a special maneuver to displace the implants. This is to better visualize the breast tissue anterior to the implant.
Screening mammograms are often read in batches after the patient has left the department. If the exam is considered incomplete, she needs to be recalled for additional evaluation at a later time.
The diagnostic exam is tailored to an abnormality. If a patient presents with a clinical sign or symptom of breast cancer, such as a lump, nipple discharge, or red, swollen breast, the technologist typically marks the area of concern with a radiopaque marker and then performs the standard imaging mammographic views, as well as additional views to better image the area of concern. These views may include spot compression, magnification, tangential, 90-degree lateral, or rolled craniocaudal views. Ultrasound may also be performed for more complete evaluation. The diagnostic exam is directed by the radiologist, and results are given to the patient before she leaves.
CASE 2
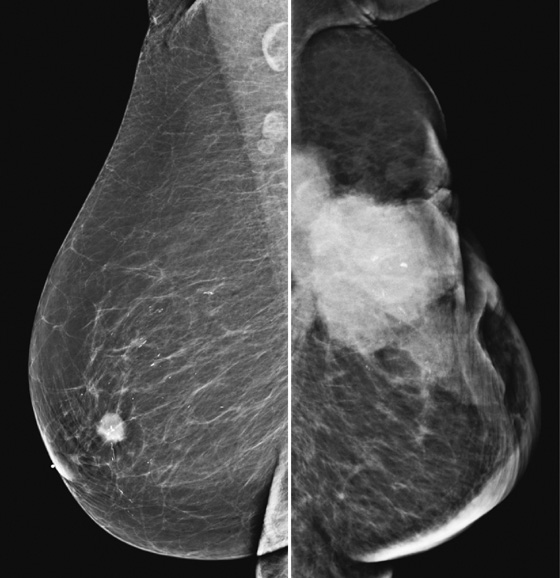
History: A 72-year-old woman presents for her first mammogram because of reduced mobility in her left arm and a draining wound in her left breast.
1. What should be included in the differential diagnosis? (Choose all that apply.)
B. Left breast infection, right breast cancer
D. Bilateral metastatic disease
2. What are the American Cancer Society and American College of Radiology guidelines for screening mammography in normal-risk women age 40 and older?
A. Baseline mammogram at age 35, then annual mammograms thereafter
B. Begin at age 40, then every other year until age 50, then annual
C. Begin at age 40, then every year until age 75
D. Begin annual screening at age 40
3. What is the reported reduction of breast cancer mortality associated with routine screening?
4. What is the incidence of breast cancer for women in the United States?
A. 1 in 15 women over a woman’s lifetime
B. 1 in 5 women over a woman’s lifetime
C. 1 in 8 women over a woman’s lifetime
D. 1 in 10 women over a woman’s lifetime
ANSWERS
CASE 2
Screening Guidelines
1. A and B
2. D
3. D
4. C
References
Cross-Reference
Ikeda D. Breast Imaging. THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010. p 1
Comment
The goal of screening mammography is to detect breast cancer as occult disease—before the patient and clinician know it is there. In this patient, her baseline mammogram, performed at age 72, was prompted by the presence of a large, ulcerating mass in her left breast, with bulky left axillary adenopathy. A small cancer is incidentally noted in the contralateral breast. This case illustrates the benefit of screening: The small right breast mass is detected on mammography before it is detected clinically; the locally advanced left breast cancer was detected late (see the figure). Had this patient started routine annual screening mammograms at an earlier age, it is likely that the left mass would have been seen earlier before it was found clinically. This patient had local spread of cancer to the skin and axillary nodes and widespread distant metastases at diagnosis.
Early detection is the attempt to find breast cancer before it has spread beyond the breast, improving morbidity and mortality from breast cancer. Mammography has been shown to decrease mortality from breast cancer by 30%, based on more recent data from the Swedish Two-County trial, including 130,000 women followed for 25 years.
Women in the United States have a slightly less than 1 in 8 lifetime risk for developing invasive breast cancer. The chance of dying from breast cancer is decreasing and is now approximately 1 in 35. The American Cancer Society estimated that there were 230,480 new cases of invasive breast cancer in 2011. This number is increasing and does not include carcinoma in situ. The American Cancer Society estimated that 57,650 new cases of in situ carcinoma were found in 2011. In 2011, 39,520 deaths from breast cancer were estimated to occur. This number is decreasing, likely owing to earlier detection and more effective treatments.
The American Cancer Society more recently updated their recommendations for screening for breast cancer: annual mammograms beginning at age 40 and continuing as long as the woman is in good health. They recommend clinical breast examination about every 3 years for women in their twenties and thirties and annually for women 40 and older. The American Cancer Society added that women should know how their breasts normally look and feel and that they should report any change to their health care provider; this might be termed breast awareness rather than breast self-examination. Guidelines have been also established for the screening of high-risk women.
CASE 3
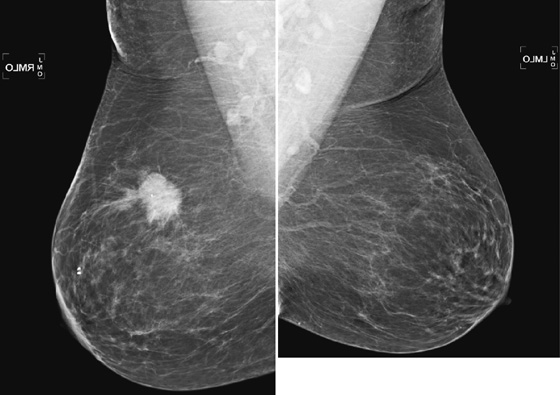
History: A 66-year-old asymptomatic woman presents for routine screening.
1. What are the three most important risk factors for developing breast cancer?
C. Family and personal history of breast cancer
E. Known inherited gene mutation
2. How does estrogen play a role in breast cancer risk?
A. Estrogen stimulates the development of breast ducts and affects cell division.
B. Women who have late menarche and early menopause are at greater risk.
D. Taking exogenous estrogen does not increase the risk of breast cancer.
3. What percentage of breast cancers is due to known genetic mutations?
4. How do benign breast biopsies affect breast cancer risk?
A. There is no relation between benign breast biopsy and cancer.
B. Some lesions that are biopsied contain atypical cells that might be a precursor to cancer.
C. There is about a 10-fold increase in breast cancer risk with certain benign biopsy results.
ANSWERS
CASE 3
Risk Factors
1. A, B, and C
2. A
3. D
4. B
References
Bassett LW, Jackson VP, Jahan R, et al. Diagnosis of Diseases of the Breast. Philadelphia: Saunders; 1997. p 308
Evans DG, Howell A. Breast cancer risk-assessment models. Breast Cancer Res. 2007;9(5):213.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:24.
Comment
The most important risk factor for developing breast cancer is being female. Males have a low incidence and have approximately 1% of the total of breast cancers diagnosed. The second most important risk factor is increasing age. Risk steadily increases as the woman ages. Exposure to estrogen is an important risk factor, because estrogen influences cell division and breast duct development. The figure shows a suspicious mass in the right breast seen on a routine screening mammogram.
The longer the exposure to estrogen, the greater the chance of breast cancer, so women who begin menses early and who have a late menopause have higher risk. Women who have no pregnancies are at higher risk. The late timing of the first pregnancy also is thought to increase risk, because the developing breast of the adolescent and young adult has a longer exposure to estrogen. Estrogen supports the growth of estrogen-sensitive tumors.
Family history of breast cancer is an important risk factor, although the majority of women with breast cancer have no family members affected. Family history is present in approximately 25% of cancers diagnosed. The more important family members to assess in risk determination are the first-degree relatives: mother, sister, daughter, father, brother, and son. Second-degree relatives—grandmother, grandfather, aunt, and uncle—also play a role in risk, but to a lesser degree. The age of the relative is also important: The younger the age of the relative at diagnosis, the more significant the risk. Multiple premenopausal women with breast cancer in the family raises the concern for a possible gene mutation. Other factors include the presence of male breast cancer in the family and Ashkenazi Jewish heritage.
The two most common known genetic mutations are BRCA1 and BRCA2. Having one of these two mutations significantly increases the likelihood of developing breast cancer; breast cancer is 50% to 85% more likely to be diagnosed in a woman with either mutation in her lifetime. Recent data suggest that women who have a 20% or greater lifetime risk of breast cancer by a risk-assessment model should be screened with MRI as well as mammography annually. Risk-assessment models are available to practitioners and patients. The Gail, Claus, and Cuzick-Tyrer models are commonly used.
Breast cancer is thought to develop stepwise, through abnormalities in cell proliferation, so that normal cells develop into atypical hyperplasia, then into in situ cancer, then into infiltrative cancer. An abnormality that is detected in screening or by palpation and that is shown to be proliferative on histology confers an increased risk of developing cancer in that patient of 1.5 to 2 times, even though the lesion itself is benign. These lesions include sclerosing adenosis, papillomatosis, complex fibroadenoma, and hyperplasia without atypia. If the lesion is atypical hyperplasia, either lobular or ductal type, the risk increases to 4 to 5 times her normal risk.
CASE 4
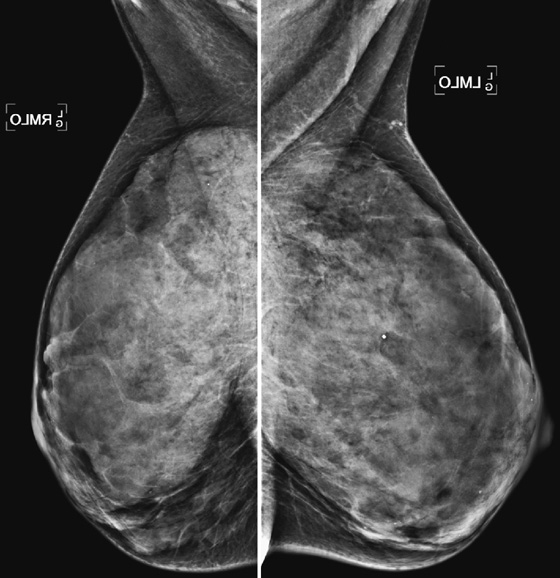
History: An asymptomatic 42-year-old woman presents for a routine screening mammogram.
1. What is in the differential for diagnosis, tissue density, and Breast Imaging Reporting and Data System (BI-RADS) code of the mammogram views presented? (Choose all that apply.)
A. Normal fatty breast, BI-RADS 1
B. Indeterminate calcification in left breast, dense, BI-RADS 0
C. Dense breasts with punctate calcification in both breasts, BI-RADS 2
D. Normal dense breasts, BI-RADS 1
2. The descriptor of tissue density “scattered fibroglandular densities” corresponds to what percentage of gland tissue on the mammogram?
3. What is the MQSA?
A. Mammography Quality Standards Act
B. A state-by-state law, not federally mandated
C. A voluntary system for accreditation of mammography centers.
4. Why is the BI-RADS code important?
B. Although it is not mandated by law, it is good medical practice.
C. It helps speed the interpretation of the mammogram.
ANSWERS
CASE 4
The Mammogram Report
1. C and D
2. C
3. A
4. A
References
American College of Radiology: Breast Imaging Reporting and Data System. Reston, VA, American College of Radiology, 2003
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:39.
Comment
The mammogram report should follow a standard format, which includes the following elements:
1. Reason for exam—screening (routine) versus diagnostic (problem solving)
2. Tissue density (breast composition)
A. Almost entirely fat (<25% glandular)
B. Scattered fibroglandular densities (25% to 50% glandular)
C. Heterogeneous dense, which could obscure detection of small masses (50% to 75% glandular)
3. Description of any significant findings
B. Calcifications (which may be scattered and punctate, as in the figure)
4. Comparison to previous exam, if available
5. Impression; include BI-RADS categories
B. BI-RADS 2—benign finding(s) (as in the mammogram in the figure)
C. BI-RADS 3—probably benign (<2% chance of malignancy)
D. BI-RADS 4—suspicious abnormality, biopsy should be considered
E. BI-RADS 5—highly suspicious, appropriate action should be taken (>95% chance of malignancy)
The inclusion of a recommendation at the end of the report is good medical practice. This should state if the patient is to have her next mammogram at the standard interval, needs a follow-up at a short interval, needs additional views, or needs a biopsy.
Screening mammography results should be limited to normal (BI-RADS 1 or 2) or incomplete (BI-RADS 0), needs more evaluation. BI-RADS 4 or 5 is not given at screening. Suspicious findings should be further evaluated with additional views before rendering a final impression. BI-RADS 3 is used after the full evaluation has been performed and the finding is a small chance of malignancy (<2%), and a short-interval follow-up is recommended.
CASE 5
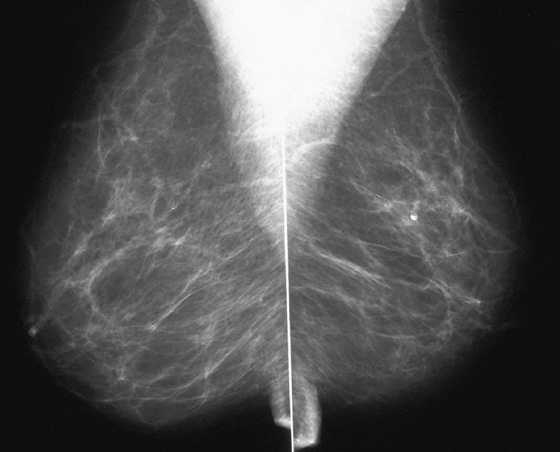
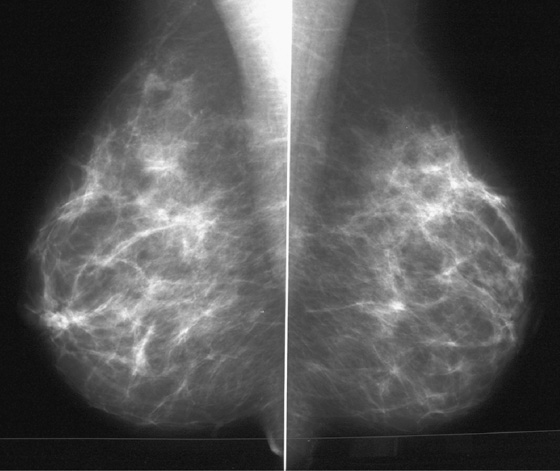
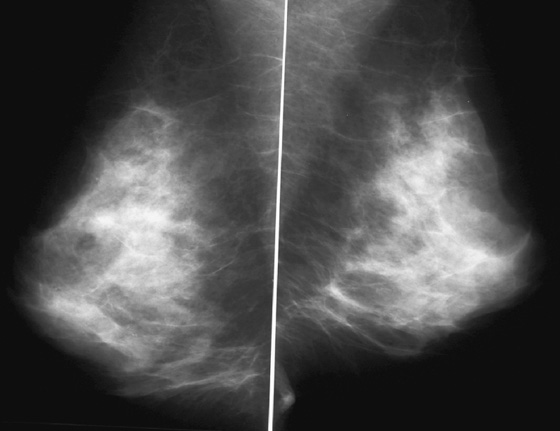
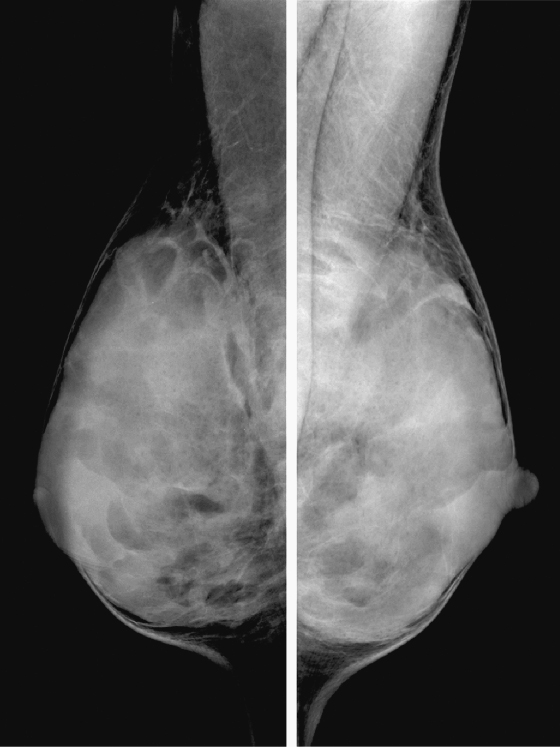
History: Screening mammograms.
1. What is your differential diagnosis for the four different patients? (Choose all that apply.)
B. Normal mammograms with four different tissue densities
2. Why is it important to report the tissue density in the mammogram report?
A. The breast density reflects your confidence in excluding cancer.
B. The mammogram is not very sensitive in detecting cancer in the fatty-replaced breast.
C. It is required by the Mammography Quality Standards Act (MQSA).
D. It is a very precise way of describing the character of the breast tissue.
3. How does the breast density affect the sensitivity for detecting breast cancer on the mammogram?
A. Breast cancer sensitivity is highest in the fatty breast.
B. Sensitivity is highest in the dense breast.
C. Sensitivity is lower when the density is lower.
D. Breast density is not related to mammographic sensitivity.
4. Does breast density change over the woman’s life?
A. No, breast tissue density is inherent, and it is stable over time.
B. Yes, the breast becomes denser as the woman ages.
ANSWERS
CASE 5
Breast Tissue Density Examples
1. A and B
2. A
3. A
4. C
References
American College of Radiology. Breast Imaging Reporting and Data System (BI-RADS). Reston, VA: American College of Radiology; 2003.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:29.
Comment
Mammographic density is reported because it tells the referring physician the sensitivity of the mammogram in detecting breast cancer. In the fatty breast, the contrast between the background dark fat and the white tumor is the greatest; therefore, sensitivity for detecting cancer is the highest in this type of breast. In the dense breast, the background density is white, similar to the density of tumor, so a tumor can be missed.
The four-category system of breast density is as follows:
1. Almost entirely fat, <25% glandular
2. Scattered fibroglandular densities, 25% to 50% glandular
Dense breasts are seen commonly in young women, and the density can decrease with age. However, many young women do not have dense breasts, and postmenopausal women with no exogenous hormone use can have dense breasts. The density is also affected by lactation. During lactation the breast is commonly very dense. The patient’s weight can affect breast density. Typically, thin women have dense breasts, and obese women have fatty-replaced breasts. The sensitivity of the mammogram varies with the patient’s age and with breast density.
CASE 6
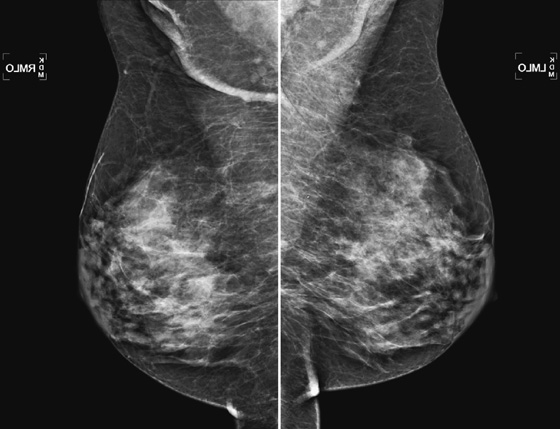
History: Routine mammogram in an 82-year-old asymptomatic woman with no history of breast surgery. She is not taking hormones.
1. What is your differential diagnosis for this patient, including BI-RADS diagnostic code? (Choose all that apply.)
A. Benign mammogram, BI-RADS 2
B. Focal asymmetric density in right upper posterior breast, BI-RADS 0
C. Heterogeneously dense breasts, BI-RADS 2
D. Dense breasts, advise screening MRI, BI-RADS 0
E. Suspicious calcifications in left breast, BI-RADS 0
2. Does this breast density confer a higher risk of malignancy?
A. No, risk is not related to breast density.
B. No, her only risk factor is her age.
C. Yes, there is an increased risk of malignancy in women with dense breasts.
D. No, fatty breasts have the highest risk of malignancy.
3. Is this tissue density seen more often in premenopausal or postmenopausal women?
A. Dense breasts are more common in postmenopausal women.
B. There is no relation of breast density to menopausal status.
C. Breast density is related only to the degree of fat in the breast, not to menopausal status.
D. Dense breasts are more common in premenopausal women.
4. In the postmenopausal woman, is this density related to hormone therapy?
B. Yes, hormones can increase breast density.
C. Yes, but hormones cause increased density only in the upper outer quadrant of the breasts.
D. Yes, but it also always causes pain.
ANSWERS
CASE 6
Dense Breast in an 82-Year-Old Woman
1. A and C
2. C
3. D
4. B
References
Harvey JA, Bovbjerg VE. Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology. 2004;230:29–41.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:29.
Comment
Denser breasts are commonly seen in mammography. This type of mammogram is more difficult to interpret because the radiographic density of the glandular tissue and a mass or cyst is similar, meaning that masses may be obscured in a dense breast. The dense tissue on the mammogram represents the ducts and lobules and also fibrous connective tissue. In a dense breast, there is relatively little fat interspersed between the glandular elements.
Increased density imparted an increased risk of breast cancer in several studies using a quantitative measurement of breast density, with an odds ratio of 4.0 or greater, meaning that women with dense breasts had a fourfold increase in risk of breast cancer compared to those with the least dense breasts. Due to the breast density and advanced age, older women with dense breasts have an even higher risk.
Breast tissue is responsive to hormone changes. Estrogen levels are higher in younger women, and after menopause, estrogen levels diminish and cause the breast lobules to regress. The mammogram then becomes less dense. About 65% of women in their twenties have at least 50% breast density. This decreases to 50% of women in their forties and to 30% of women in their seventies. Therefore, this relatively dense breast is uncommon in older women.
Hormone replacement therapy increases the glandular density of the breast in up to 73% of women, and the greatest increase in density occurs in the first year of use. This increase in density is due to stimulation of the cells of the ducts, lobules, and stroma to proliferate and increase mitotic activity.
The 82-year-old patient reported here is not on hormone replacement therapy, and the mammogram is unchanged compared to previous exams.
CASE 7
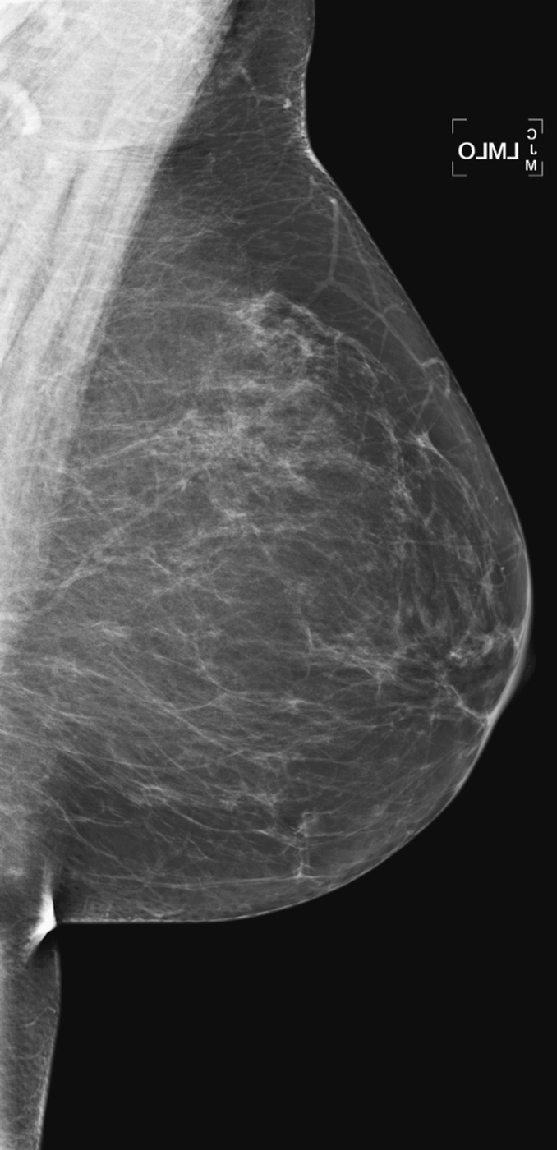
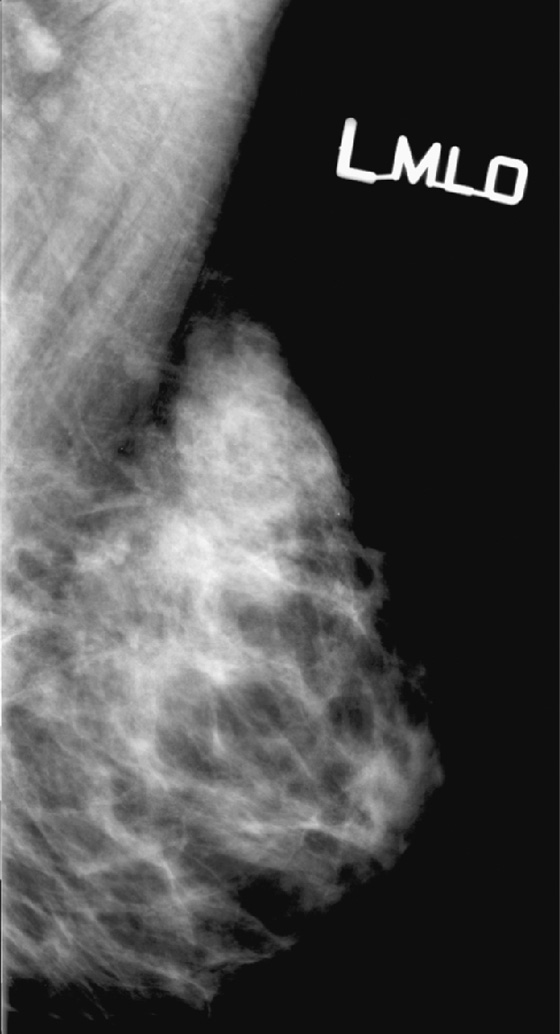
History: Left mediolateral oblique (MLO) mammograms of a 55-year-old woman taken 1 year apart. On the day of the later exam, she complains of bilateral breast tenderness and swelling.
1. What is your differential diagnosis for the change in the mammogram of this patient? (Choose all that apply.)
C. Hormone replacement therapy
D. Typical changes of menopause
2. What personal history question is typically asked of the patient who comes in for a mammogram?
B. History of clotting function
C. Family history of renal and liver disease
3. What would you do next to work up the patient’s symptoms and the change in the mammogram?
A. MRI is needed for more complete evaluation.
B. Take a clinical history regarding exogenous hormone use and any focal findings.
C. Nothing; this is normal for this age.
4. What is your recommendation for management of this patient?
A. Exogenous hormone therapy should be stopped because of the change on the mammogram.
C. Recommend routine mammography.
D. The patient should have needle biopsy of random sites in both breasts.
ANSWERS
CASE 7
Hormones
1. A, B, C, and E
2. A
3. B
4. C
References
Berkowitz JE, Gatewood OM, Goldblum LE, Gayler BW. Hormonal replacement therapy: mammographic manifestations. Radiology. 1990;174:199–201.
National Cancer Institute: Menopausal hormone replacement therapy use and cancer (factsheet)
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:392.
Comment
The breasts bilaterally show an increase in density, although only one view is shown here (see the figures). The density is now “heterogeneously dense,” whereas before the density was “scattered fibroglandular densities.” The most common cause of bilaterally symmetric increasing density that involves the glandular tissue and not the skin (no edema or skin thickening) is exogenous hormone therapy (HRT), as in this case. The patient had begun taking a combined estrogen and progesterone supplement.
Normally, as a woman enters menopause, involutional changes occur in the breast parenchyma (see the figures). The volume of the mammographically dense areas tends to decrease as the glandular elements involute and are replaced by fat. HRT reverses the normal involution, and histologically the breast epithelium and stromal elements proliferate. This is due to the effects of the estrogen component of the HRT. The progesterone effects include an increase in epithelial mytotic activity and lobular hyperplasia.
These effects have been shown to increase the incidence of breast cancer in the postmenopausal population taking exogenous hormones, particularly combined therapy. In 2002, the Women’s Health Initiative (WHI), a study of exogenous hormone use, found an additional eight cases of breast cancer per 10,000 women in women on combined hormonal therapy for 1 year, compared to the placebo group. The choice to remain on hormone therapy is up to the patient and her clinician, and mammography is not typically performed at any different schedule because of this increased risk.
The imaging evaluation is based on the clinical findings. Ultrasound is not indicated when there is a diffuse bilateral increase in density when it can be explained by exogenous hormonal therapy. In our practice, we do not generally perform ultrasound on women who have bilateral diffuse breast pain. If there is a focal area of tenderness or an area of palpable concern, a directed ultrasound is performed. Occasionally, the mammographic and clinical findings are more unilateral and focal, in which case physical exam, directed ultrasound, and possibly MRI may be needed to exclude a developing malignancy.
CASE 8
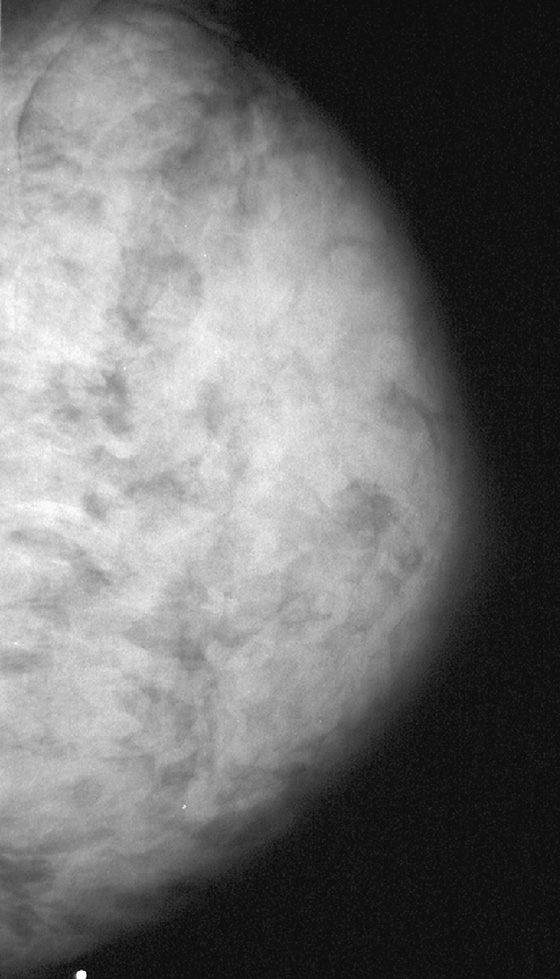
History: A 40-year-old woman presents for routine screening mammogram. The first figure is the right mediolateral oblique (MLO) view 4 years earlier, when she had presented with a palpable lump. The second figure is the current exam.
1. What is your differential diagnosis for the difference in the appearance of the mammogram between the two exams? (Choose all that apply.)
A. The patient gained weight, causing more fat to develop in the breast.
B. The patient began taking birth control pills.
C. The patient was lactating on the earlier exam.
D. The patient had inflammatory breast cancer on the initial image, which was successfully treated.
2. Can you be sure this is the same patient? What can you do to try to verify the patient’s identity when reading mammograms?
A. Check the patient’s name on the image because this will always be correct.
B. Check for unique patterns of blood vessels and lymph nodes on the image.
C. Ask the patient if this is her previous mammogram.
D. When the appearance of the glandular tissue is very different, assume it is not the same patient.
3. Why was only one view performed when the patient presented with a palpable lump?
A. Mammography is limited during lactation, owing to breast density.
B. Mammography is dangerous to the infant being nursed, owing to radiation exposure.
C. Ultrasound alone can be used to evaluate the finding in all cases of palpable masses.
4. Why is mammography used at all for a lactating patient?
A. It should not be used in this situation.
B. Even though a woman is lactating, she should still have routine screening mammograms.
D. If the ultrasound exam demonstrates a simple cyst, mammography must also be used.
ANSWERS
CASE 8
Lactational Change
1. A, B, and C
2. B
3. A
4. C
Reference
Ahn BY, Kim HH, Moon WK, et al. Pregnancy- and lactation-associated breast cancer: mammographic and sonographic findings. J Ultrasound Med. 2003;22:491–497.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:378.
Comment
This 40-year-old woman presented for a routine mammogram. Her prior exam was made available for comparison, yet it is not at all similar to the current exam. It is important to verify the identity of the patient when interpreting studies, because human error can result in the wrong patient’s name on the exam. In this case, this is her prior exam, and the breast appearance is different because of lactation. This case illustrates the difficulty that can occur in interpreting the mammogram during lactation. During lactation, milk is produced by the lobules and carried in the ducts, which can increase the size and density of the breast, as in this case (see the figures). There is no danger to the nursing infant in performing mammography during lactation.
Ultrasound is often used as the initial exam for evaluating a palpable mass during lactation. If ultrasound demonstrates a simple cyst or a benign-appearing lactating adenoma or other benign mass that corresponds to the palpable finding, no mammogram is indicated. However, if the ultrasound is negative, mammography should be performed for more complete evaluation, essentially to check for a suspicious mass or microcalcifications (which are not present in this patient). If ultrasound demonstrates a suspicious mass, then mammography should be performed to check for extent of disease, such as microcalcifications or additional masses.
CASE 9
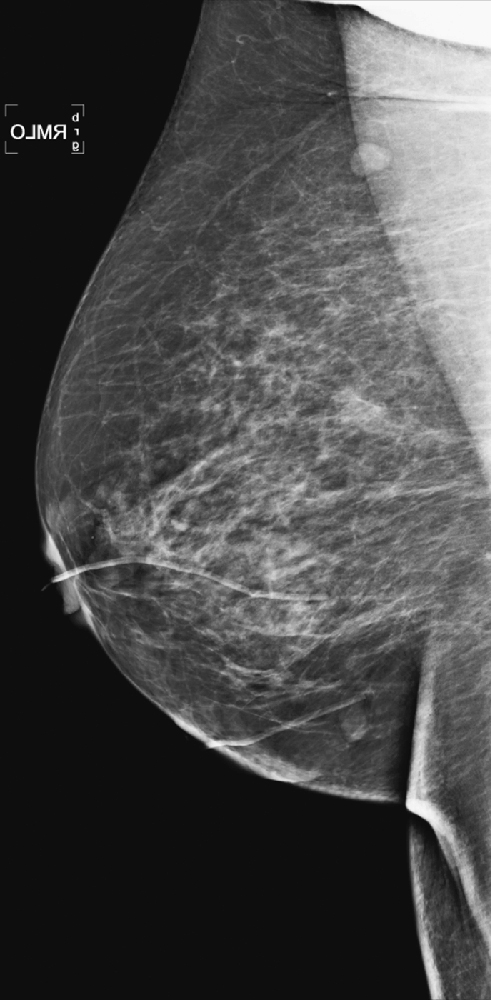
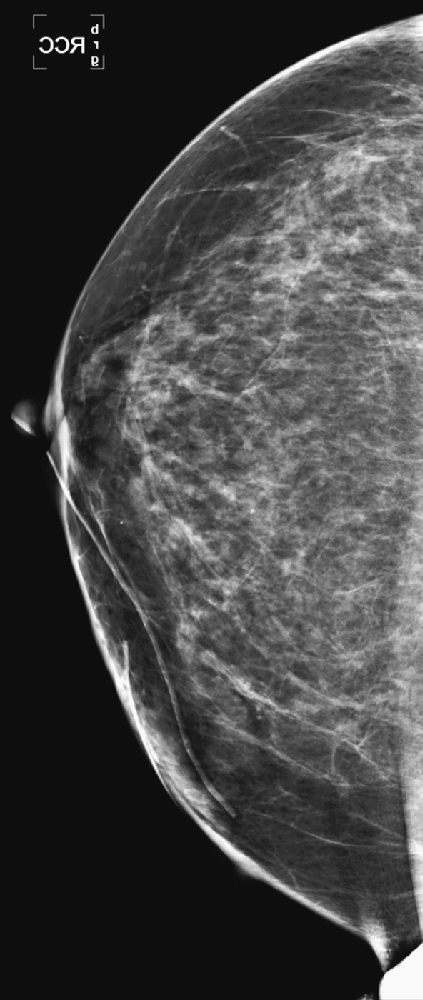
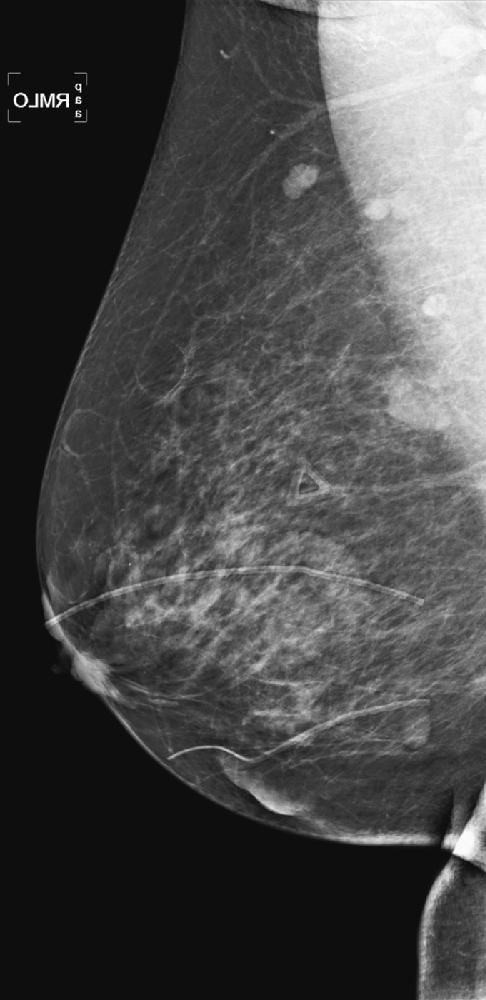
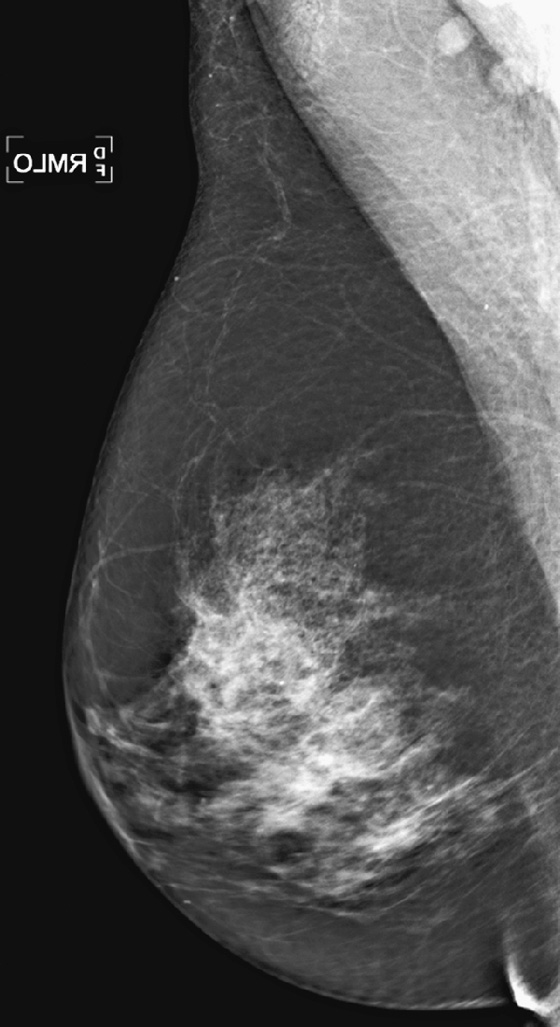
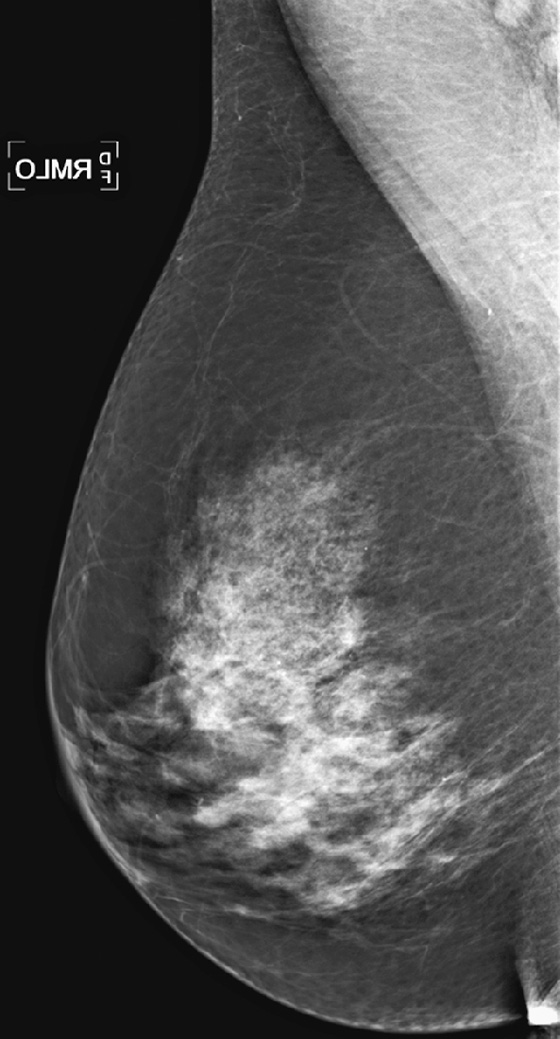
History: A 64-year-old woman with keloids from prior benign surgery presents for a routine screening mammogram. Right mediolateral oblique (MLO) and craniocaudal (CC) views are shown (see the first two figures). The patient returns for a right mammogram when she palpates a lump. Right MLO and CC views are shown from the second mammogram (see the second two figures).
1. What should be included in the differential diagnosis of the two sets of mammograms of the right breast? (Choose all that apply.)
A. Suspicious mass at 9 o’clock position, not present on first mammogram
B. Benign-appearing mass in the 4’clock position, new since previous mammogram
C. Suspicious mass at 1 o’clock position
D. Benign mass at 1 o’clock position
2. What is the posterior nipple line?
A. A line connecting the nipples on three views of the same breast
B. An imaginary line connecting the nipple to the posterior chest wall
C. A line drawn parallel to the chest wall, at the level of the nipple
D. A line drawn to measure the distance from the nipple to a lesion
3. If you think that screening mammographic views are not correctly positioned, what is the next step?
A. The patient should be recalled for proper positioning.
C. The radiologist should make a note in the report that positioning is suboptimal.
4. What is the next step in management of this patient?
ANSWERS
CASE 9
Poor Positioning, Missed Cancer
1. C and D
2. B
3. A
4. C
References
Eklund GW, Cardenosa G. The art of mammographic positioning. Radiol Clin North Am. 1992;30(1):21–53.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:6.
Comment
In this patient, a mass was seen only after she presented with a palpable lump. It was not recognized on the routine mammogram performed earlier because of inadequate positioning.
Proper positioning is an important part of breast imaging. It requires constant diligence on the part of the radiologic technologist and cooperation by the patient. Positioning must be evaluated by the radiologist on every examination. If positioning is inadequate, the patient needs to be recalled to have a well-performed mammogram, and the technologist needs to be informed that the positioning was not done to satisfaction.
The posterior nipple line is a way to assess the proper positioning of the mammogram. An imaginary line is drawn from the nipple to the chest wall or edge of the MLO image, perpendicular to the pectoral muscle (see the figures). This line is then drawn on the CC view, from the nipple to the edge of the then image. The length of the line on the CC view should be within 1 cm of the length of the line on the MLO view. In this case, it is not; the CC view is short. The mass present in the medial aspect of the breast was not included on the examination.
CASE 10
History: An asymptomatic woman presents for a routine screening mammogram.
1. A single right mediolateral oblique (MLO) view is shown in the first figure; repeat right MLO view is shown in the second figure. What is your one best reason for recalling this patient for an additional right MLO view?
A. The right breast MLO view is poorly positioned.
B. Microcalcifications in the breast need to be evaluated.
C. The first view has motion blur.
D. A possible mass in the central right breast needs to be evaluated.
2. Where is motion blur most likely to occur?
A. On the craniocaudal (CC) view
B. In the upper aspect of the breast
3. How can motion blur best be avoided?
A. Minimize the length of exposure.
D. Perform a “true lateral” view instead of an MLO view.
4. What is a technical recall?
B. A patient is recalled for magnifications or spot compression views.
C. The patient cannot tolerate compression.
D. The patient has never had a mammogram before.
ANSWERS
CASE 10
Motion Unsharpness
1. C
2. D
3. A
4. A
References
Bassett LW. Clinical image evaluation. Radiol Clin North Am. 1995;33(6):1027–1039.
Eklund GW, Cardenosa G. The art of mammographic positioning. Radiol Clin North Am. 1992;30(1):21–53.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:6.
Comment
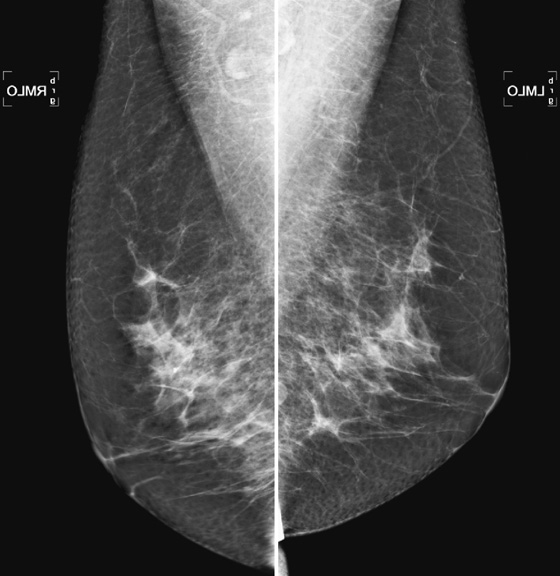
Technical aspects of a good-quality image of the breast include positioning, compression, exposure, sharpness, noise, artifacts, and contrast. This case demonstrates motion unsharpness in the right MLO view (see the figures). The repeat image (see the figures) reveals sharper detail. Motion unsharpness can result when the patient moves during the exposure, and inadequate compression can contribute to it. This type of motion unsharpness often occurs in the inferior aspect of the breast on the MLO view, and it can be recognized by poor separation and blurriness of the edges of linear structures and tissue borders. This can be difficult to recognize, but it is important, because malignancy can be missed owing to unsharpness of the image. This is particularly true for microcalcifications and small masses. Compression thickness is greater on the MLO view than on the craniocaudal (CC) view, and it is more common to see motion unsharpness on the MLO view. Adequate compression immobilizes the breast and decreases the likelihood of motion unsharpness, reduces breast thickness, and reduces the dose needed for a proper exposure. If blur is seen, mammographic detail is compromised, and the image is not adequate for interpretation. The patient should be recalled for a repeat image (“technical recall”).
CASE 11
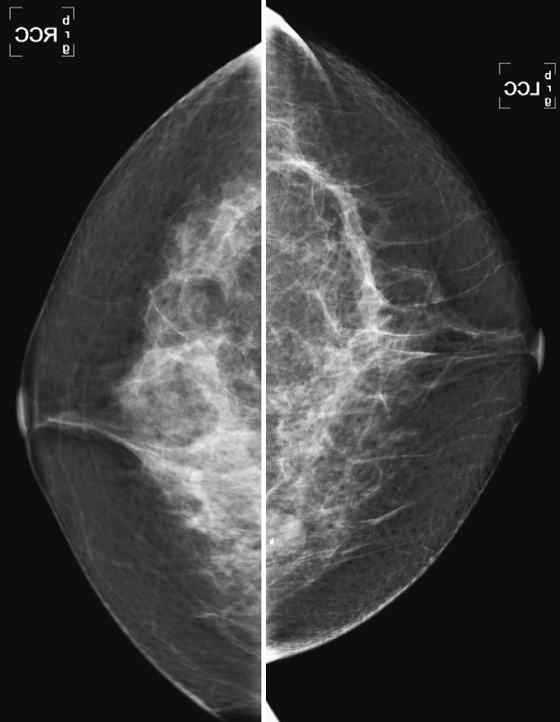
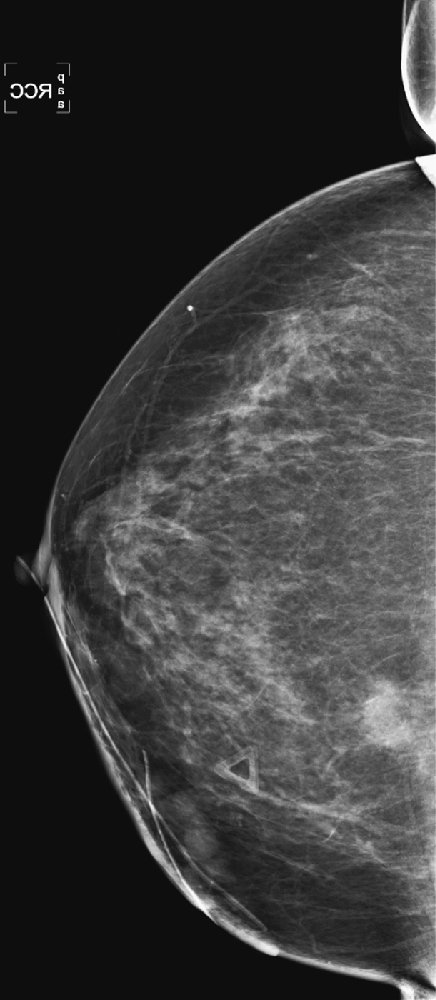
History: A 40-year-old woman presents for a baseline screening mammogram.
1. What should be included in the differential diagnosis, based on the four-view screening mammogram? (Choose all that apply.)
B. Malignant mass in the left medial breast
C. Benign mass in the left medial breast
D. Cyst in the left medial breast
2. What is the next step in the evaluation?
A. Recommend routine screening mammogram in 1 year.
B. Recall for additional spot compression views and ultrasound.
C. Perform MRI of the left breast.
D. Refer patient to a surgeon.
3. Why is the mass seen better on the craniocaudal (CC) view?
A. The finding is a superimposition of densities, not a true mass.
C. Typically, the medial breast is not included well on the MLO view.
D. The CC view is better positioned.
4. What are Tabar’s “danger zones”?
A. Areas of the world in which it is dangerous to practice breast imaging
B. Areas of the mammogram that are most likely to contain malignancy
C. Areas of the breast that are more likely to have malignant spread to the lymph nodes
D. Areas of the breast that are usually normal fat and to which special attention should be paid
ANSWERS
CASE 11
Medial Mass
1. B, C, and D
2. B
3. C
4. D
References
Eklund GW, Cardenosa G. The art of mammographic positioning. Radiol Clin North Am. 1992;30(1):21–53.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:38.
Comment
Masses or densities in the medial breast are in what is termed a “danger zone.” Typically, only fat and minimal gland tissue is seen in this area. The other “danger zones” include the retroglandular fat and the edge of the image. This case illustrates two of these “danger zone” findings: a mass in the medial breast (see the figures) and a mass that is just barely seen at the edge of the image on the MLO view (see the figures).
Most of the gland tissue of the breast is in the upper outer quadrant. For this reason, the MLO view was designated a standard view, rather than the orthogonal mediolateral view. The MLO view includes the upper outer quadrant more completely. However, the medial breast is not as well seen on the MLO view. For this reason, the medial breast must be included on the CC view as completely as possible. Medial masses seen on the CC view may not be seen on the MLO view because of the relative limitation of the MLO view in the medial breast. Focal densities seen only on one view, in the medial breast on the CC view, should be viewed with suspicion. Additional imaging should be performed.
This patient was recalled for spot compression views and ultrasound. The mass was seen on both spot compression views, and ultrasound showed a 17-mm solid mass in the 8 o’clock position of the lower inner left breast. The patient underwent a core needle biopsy, and histology showed a fibroadenoma.
CASE 12
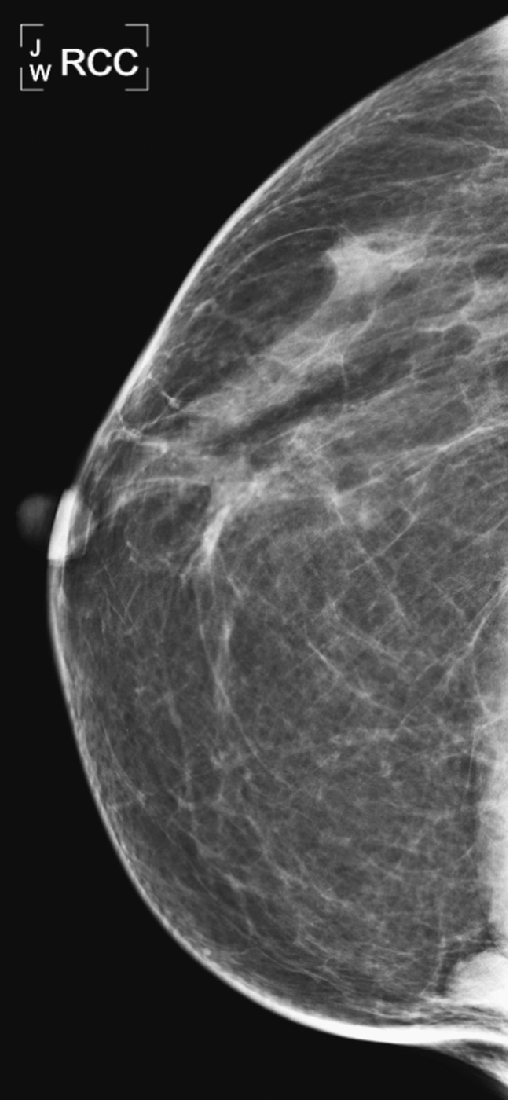
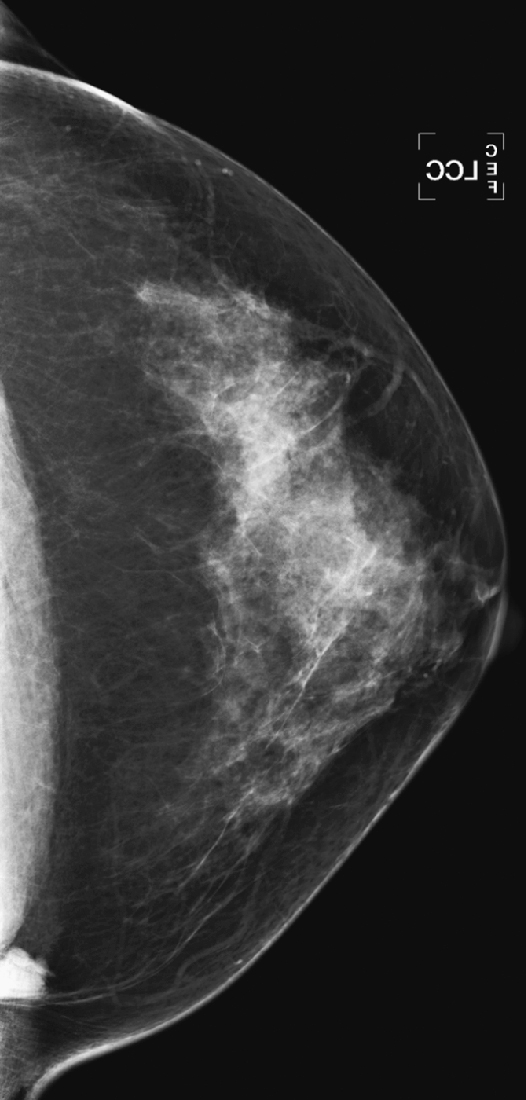
History: Craniocaudal (CC) view from routine mammogram in two different women.
1. What is your differential diagnosis for the finding in the medial aspect of the CC view in these two different patients? (Choose all that apply.)
A. Abnormality in the pectoralis muscle
B. Sternalis muscle, a normal variant in some patients
2. What further work-up is performed next?
3. If there is a 5-cm mass in the medial breast on the CC view, does the differential diagnosis still include a sternalis muscle?
A. No, the sternalis muscle is typically less than 2 cm in cross section.
B. No, because the sternalis muscle is not located medially.
C. Yes, the finding could still represent the sternalis muscle.
4. Where is the sternalis muscle?
A. It is posterior to the pectoralis muscle and runs parallel to the pectoralis.
B. It is lateral to the sternum, running vertically, perpendicular to the pectoralis.
C. It is lateral to the pectoralis, running vertically in the mid-axillary line.
D. It runs parallel to the clavicle, adjacent to the sternum.
ANSWERS
CASE 12
Sternalis Muscle
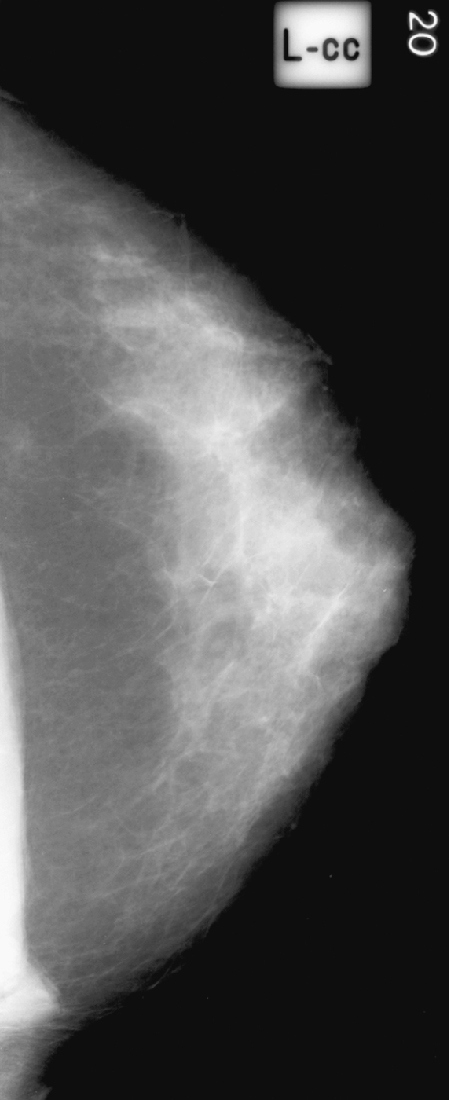
Prior left CC view from patient 2.
1. B and D
2. A
3. A
4. B
References
Bradley FM, Hoover HC Jr. Hulka CA, et al: The sternalis muscle: an unusual normal finding seen on mammography. AJR Am J Roentgenol. 1996;166(1):33–36.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:28.
Comment
The sternalis muscle is a variation of the chest wall musculature that is seen in approximately 8% of the population according to cadaveric studies. This muscle is long and narrow and runs vertically along the sternum at 90 degrees to the pectoralis muscle. It may be unilateral or bilateral; more often it is unilateral. It is seen in the far medial aspect of the breast on the CC view only, and it can be mistaken for a medial breast mass. It can have a rounded, triangular, or flame-shaped configuration, and it is usually surrounded by fat. It is typically less than 2 cm in diameter.
It is important to recognize this normal variant muscle (see the figures) and to avoid any additional work-up. Spot compression views and ultrasound are unrevealing, as is the physical exam. If the additional work-up is done and no mass is seen on ultrasound or felt on physical exam, and there is still diagnostic concern, cross-sectional imaging with CT or MR will demonstrate the sternalis muscle running perpendicular to the pectoralis, along the sternum. If prior mammograms are available, comparison can be helpful to observe the stability of the finding (see the figures).
The sternalis should be differentiated from the pectoralis muscle, which is present in nearly all patients, and the technologist should attempt to include the pectoralis on the CC as well as the mediolateral oblique (MLO) views, to demonstrate that the entire breast has been included in the image.
CASE 13
History: Craniocaudal (CC) views from screening mammograms in the same patient are presented.
1. What should be included in the differential diagnosis? (Choose all that apply.)
2. What is the next step in the work-up?
A. MRI to evaluate the chest wall
B. Breast-specific gamma imaging (BSGI) to evaluate for enhancing lesions
C. Spot compression views of the denser areas at the chest wall
D. Referring the patient to a surgeon for a physical examination
3. Is it desirable to position the breast so that the pectoralis muscle is included on the CC view?
A. No, there is no need to see the pectoralis muscle.
B. No, it is important only to see the glandular tissue and retroglandular fat.
C. No, the pectoralis muscle is seen only on the mediolateral oblique (MLO) view.
D. Yes, it is ideal to see the pectoralis muscle on the CC view.
4. How is the sternalis muscle different from the pectoralis muscle on the CC view?
A. It is ideal to include the sternalis muscle on every patient.
B. The sternalis and pectoralis muscles overlap and can be difficult to differentiate.
C. The sternalis muscle is seen in less than 10% of patients on a mammogram.
ANSWERS
CASE 13
Pectoralis Muscle on Craniocaudal View
1. A, B, and D
2. C
3. D
4. C
Reference
Eklund GW, Cardenosa G. The art of mammographic positioning. Radiol Clin North Am. 1992;30(1):21–53.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:28.
Comment
The pectoralis muscle lies posterior to the breast, the only part of the chest wall that is seen on the mammogram. Positioning the breast to include as much of the breast tissue as possible on the image is one of the most important roles of the mammography technologist. Recognizing that the breast is completely or incompletely imaged is an important responsibility of the radiologist interpreting the mammogram.
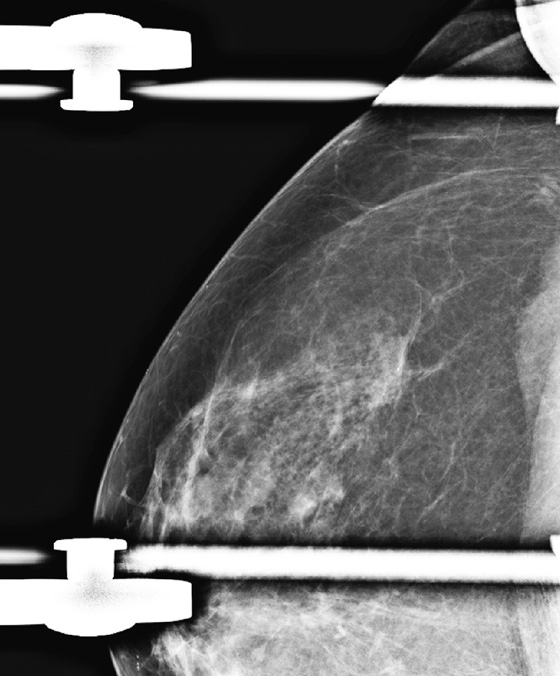
The pectoralis muscle is valuable in recognizing the adequacy of breast positioning. It should be seen on the MLO view, extending to the nipple and having a convex anterior margin. On the CC view, it is seen as a convex density at the posterior edge of the image in approximately 25% of patients (see the figures). When the pectoralis muscle is seen, the radiologist can be confident that posterior breast tissue has been adequately included on the image.
The pectoralis muscle may have an undulating contour (see the figures), and this may be confused as a mass. Spot compression views and ultrasound can be used as needed (see the figures) to help evaluate a lumpy contour, but this appearance of the pectoralis muscle is not unusual on the CC view.
CASE 14
History: A 60-year-old woman presents for a routine screening mammogram and is recalled for additional views.
1. What should be included in the differential diagnosis for the images shown? (Choose all that apply.)
D. Infiltrating ductal carcinoma
2. Why is the mass not fully seen on the routine views?
A. It is obscured by surrounding tissue.
B. It is in the posterior breast.
C. It is inferior to the central cone of the breast.
D. It is too far lateral to be included.
3. Which portion of the breast is not well seen on the mediolateral oblique (MLO) view?
4. Where in the right breast is this mass located?
ANSWERS
CASE 14
Hidden Mass
1. B, C, and D
2. B
3. C
4. D
References
Harvey JA, Nicholson BT, Cohen MA. Finding early invasive breast cancers: a practical approach. Radiology. 2008;248(1):61–76.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:29. 408
Comment
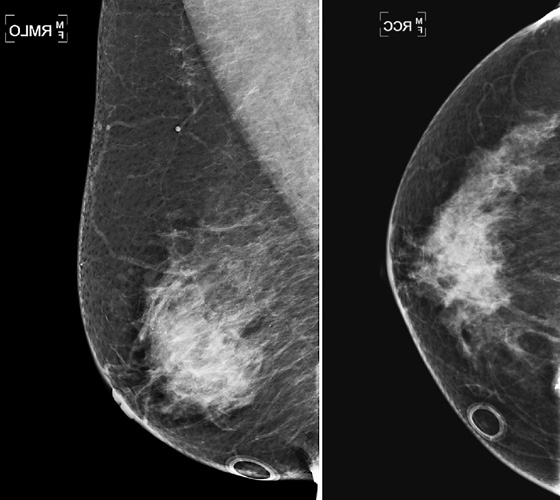
Breast lesions may be missed on mammography for various reasons. This case illustrates a mass that is in the posterior breast, adjacent to the chest wall (see the figures). The posterior breast may be difficult to include on the mammogram. The medial aspect of the breast is another area that may not be well included on both the MLO and craniocaudal (CC) views, so medial lesions can also be missed.
In positioning the breast for the mammogram, the technologist should use the natural features of breast mobility to help include as much breast tissue as possible into the image. The lower portion of the breast is mobile, and the lateral aspect of the breast is mobile. The technologist can mobilize the lateral aspect of the breast medially in positioning the patient for the MLO view. The technologist can mobilize the inferior breast upward for the CC view. In both views, the breast must be pulled gently but firmly away from the chest wall to include as much posterior tissue as possible. Despite these maneuvers, lesions present in the breast may not be included in standard views (see the figures).
The radiologist must pay special attention to the posterior edge of the image to check for the anterior margin of a posterior mass (see the figures). Additional views are needed to try to include this area on the image, such as spot compression (see the figures). If the anterior margin is still not well seen, ultrasound is a useful imaging tool because it is not hampered by the same limitations as mammography.
This patient in this case was aware of the mass in her right breast and knew it was stable by palpation. She reported that a biopsy was performed more than 10 years previously at another institution, and the biopsy finding was a fibroadenoma.
CASE 15
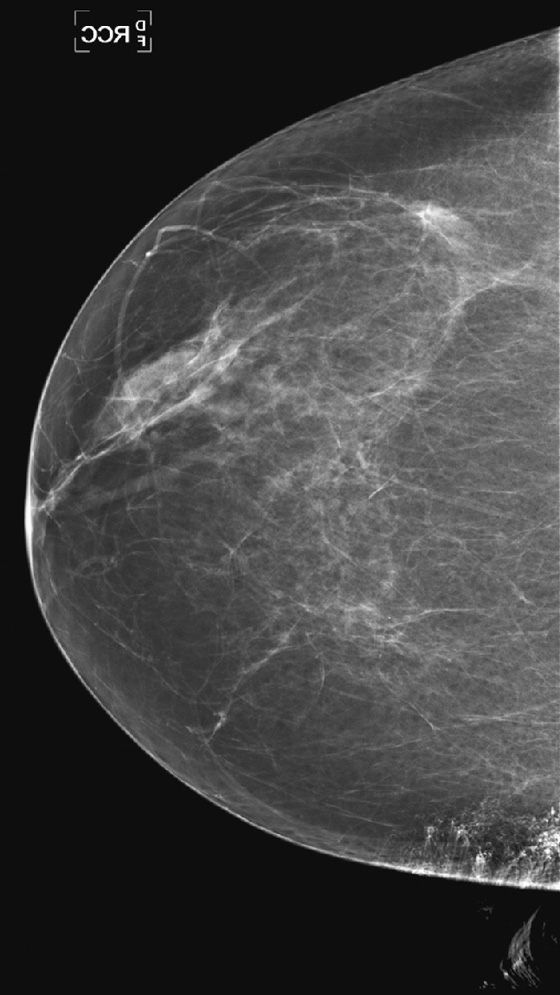
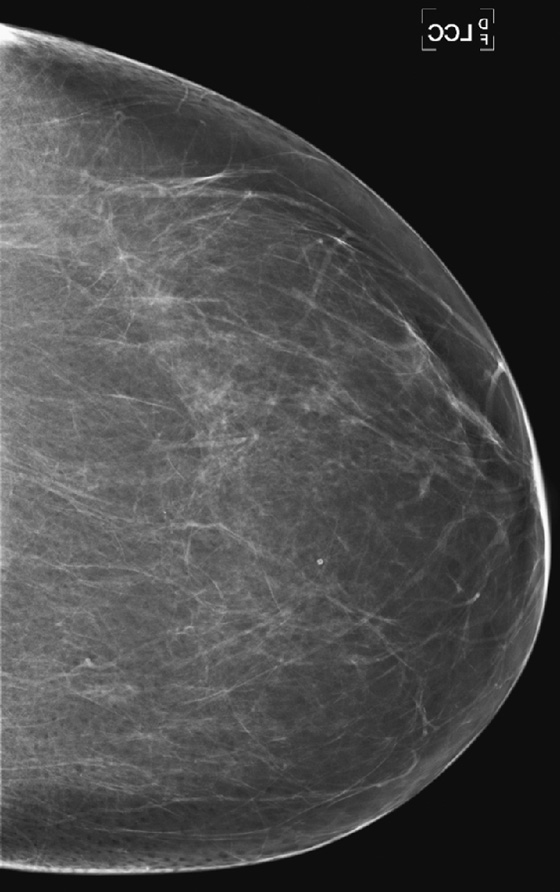
History: A 72-year-old woman presents for a routine screening mammogram. The craniocaudal (CC) views are shown.
1. What should be included in the differential diagnosis of the minus-density finding in the medial right breast? (Choose all that apply.)
A. Artifact from scratches on the film
B. Artifact from patient’s hair on the image
C. Linear branching calcifications in the medial right breast
D. Artifact stuck to the medial aspect of the patient’s breast
2. What is the next step in the work-up?
A. The patient should be recalled for a repeat right CC view.
B. The patient should be contacted and informed that she should tie back her hair.
C. Nothing should be done because this is an obvious artifact; there is no breast abnormality.
D. Spot magnification views of the medial right breast should be obtained.
3. Why is it important to be aware of artifacts?
A. They are not important because they are obvious when seen.
B. They can interfere with image interpretation.
C. They improve image quality.
D. They can be easily controlled, and there is no excuse for having artifacts.
4. What is an artifact?
A. An object that is always outside the patient
B. An object that interferes with interpretation of the image
C. An object that may mimic a true finding
ANSWERS
CASE 15
Artifact: Hair
1. B and D
2. A
3. B
4. E
Reference
Hogge JP, Palmer CH, Muller CC, et al. Quality assurance in mammography: artifact analysis. Radiographics. 1999;19(2):503–522.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:74.
Comment
Artifacts must be recognized when they are present on an image. There are certain pathognomonic appearances. Once these artifacts are seen, they should be easily recognized when they reappear. Hair artifact is one such finding. The long white curvilinear markings seen at the chest wall aspect of the image should not be confused with breast pathology (see the figures). A hair artifact is typically present on the CC view, as the patient leans in over the image receptor as the exposure is being made. Hair overlying the breast can obscure detail of the tissue underneath, so the image must be repeated.
The radiologist needs to be aware of artifact appearance because the technologist may not recognize the artifact at the time of the examination. If the image is not repeated at the time of the visit, the patient must be recalled for a repeat view that is technically adequate for interpretation.
CASE 16
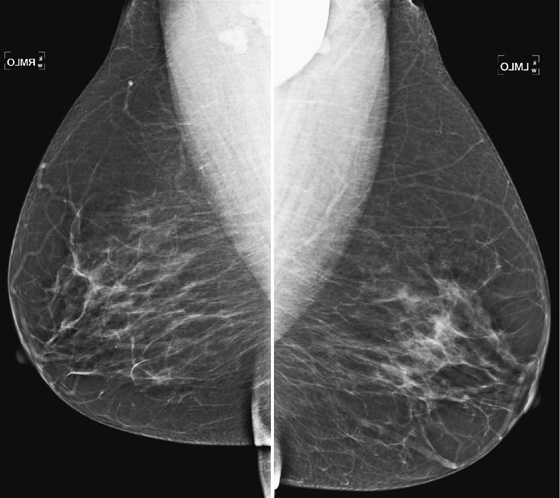
History: Bilateral mediolateral oblique (MLO) views of asymptomatic 85-year-old woman.
1. What is the differential diagnosis for the dense object in the left axilla? (Choose all that apply.)
B. Pacemaker battery overlying the left upper chest
2. What Breast Imaging Reporting and Data System (BI-RADS) code should be given to this exam?
3. What should be done next?
A. The patient should be recalled for a repeat left view.
B. The patient should be recalled for an ultrasound of the left axilla.
C. The patient should be scheduled for an MRI.
D. A 90-degree lateral view should be performed of the left breast.
4. Why does this common artifact occur?
B. The patient’s breast is not positioned deeply enough.
C. The patient moved during the exposure.
D. The image receptor was positioned too high into the patient’s axilla.
ANSWERS
CASE 16
Artifact: Chin
1. B and C
2. D
3. A
4. A
References
Bassett LW, Hirbawi IA, DeBruhl N, Hayes MK. Mammographic positioning: evaluation from the view box. Radiology. 1993;188:803–806.
Eklund GW, Cardenosa G. The art of mammographic positioning. Radiol Clin North Am. 1992;30(1):21–53.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:6.
Comment
This artifact of the chin obscuring the upper portion of the axilla is commonly seen in daily practice, and it is more often seen in elderly patients, like this one. Ideally, this case should not be presented to the radiologist, because the technologist performing the exam should recognize the artifact at the time of the exam and repeat the image. This is particularly true in digital mammography, because the technologist previews the image before accepting it, as she is performing the mammogram at the acquisition work station.
Although the area obscured by the patient’s overlying body part is relatively small (see the figures), this film should not be accepted. It should be given a Breast Imaging Reporting and Data System (BI-RADS) 0, and the patient recalled for a repeat left view. It is possible that the chin is obscuring important pathology that could be present in the axilla, and the radiologist is responsible for that area of the anatomy on the mammogram. This additional imaging is a “technical callback” and is performed free of charge.
If the technologist feels that the patient cannot cooperate for improved positioning, as is sometimes the case in extreme kyphosis or in wheelchair-bound patients, for example, then the technologist needs to make that situation clear to the radiologist, and it should be mentioned in the radiology report. If the patient is quite limited, you may instruct your technologists to show the images to the radiologist before the patient leaves, and the radiologist may meet the patient and observe attempts at positioning, to see if improvements can be made.
In this case, the view was repeated, and no abnormality was observed in the axilla.
CASE 17
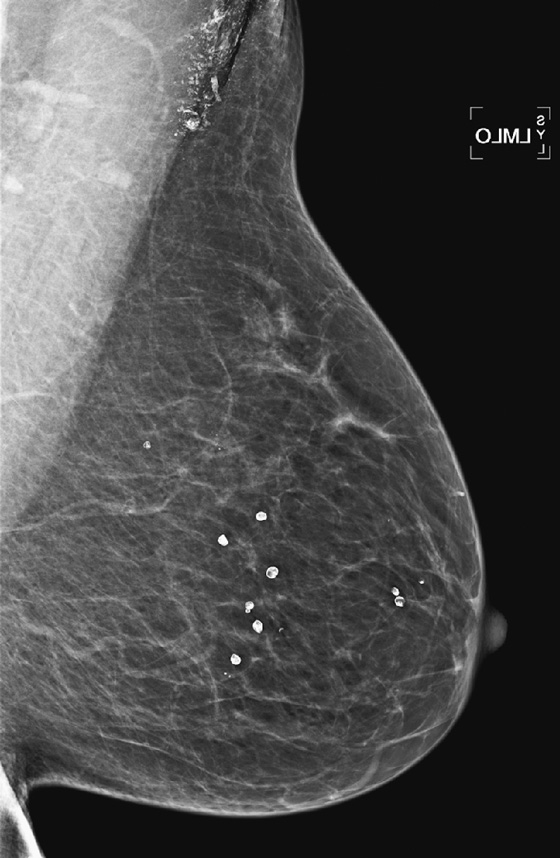
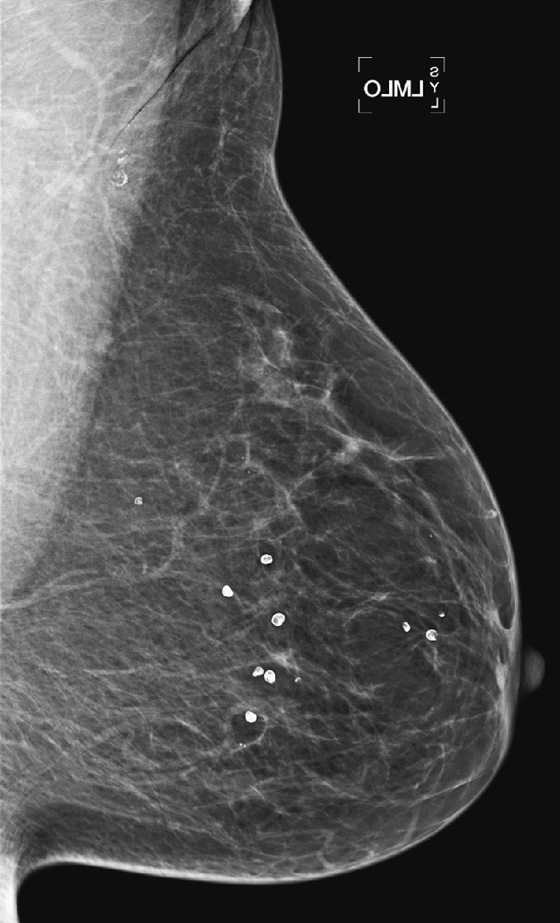
History: A 52-year-old woman presents for routine screening.
1. What is the differential diagnosis for the density in the left axilla in the first figure? (Choose all that apply.)
B. Artifact from lotion containing zinc oxide
C. Artifact from gunshot wound
D. Calcifications in the breast
2. What is your recommendation and Breast Imaging Reporting and Data System (BI-RADS) based on these images?
A. Because the density is definitely on the skin, it can be reported as such, BI-RADS 2.
C. The patient should return for magnification views of the left axilla, BI-RADS 0.
D. The patient should return for an ultrasound of the left axilla, BI-RADS 0.
3. What radiographic density is this?
4. What is the one most important reason for recognizing this artifact?
A. Patients should always follow instructions not to use deodorant.
B. Technologists should always see this artifact when performing mammography.
C. It is necessary to differentiate this from microcalcifications in axillary breast tissue.
ANSWERS
CASE 17
Artifact: Deodorant
1. A and B
2. B
3. A
4. C
References
Bassett LW, Jackson VP, Jahan R, et al. Diagnosis of Diseases of the Breast. Philadelphia: Saunders; 1997. pp 363–364
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:74.
Comment
Most patients use deodorant, and most facilities instruct their patients not to use deodorant, lotions, powders, and talc on their breasts or underarms on the day of the exam. However, many patients forget this instruction, or they expect to clean the skin before the mammogram and then neglect to do so or do not do so thoroughly enough. Technologists should ask each patient if she has applied any skin product, and if so, should arrange for the patient to remove the product completely.
There may still be residue of the product clinging to skin pores (see the figures) or trapped in the irregular surface of moles or skin keratoses even after cleaning. In that case, the patient needs to return for additional views or to repeat the view after the skin is recleaned (see the figures). If there is a mole or keratosis, this should be marked to help recognize skin product trapped on the lesion’s surface. Deodorant or antiperspirant can contain aluminum, and this metal, trapped in skin pores, gives the characteristic appearance seen in the second figure.
The important aspect of this artifact is that such tiny metallic densities can mimic microcalcifications, which need to be evaluated for the possible presence of malignancy.
If the artifact is clearly on the skin, seen tangentially or along skin folds in the axilla, or in a marked mole, the patient might not need to return for technical callback views.
CASE 18
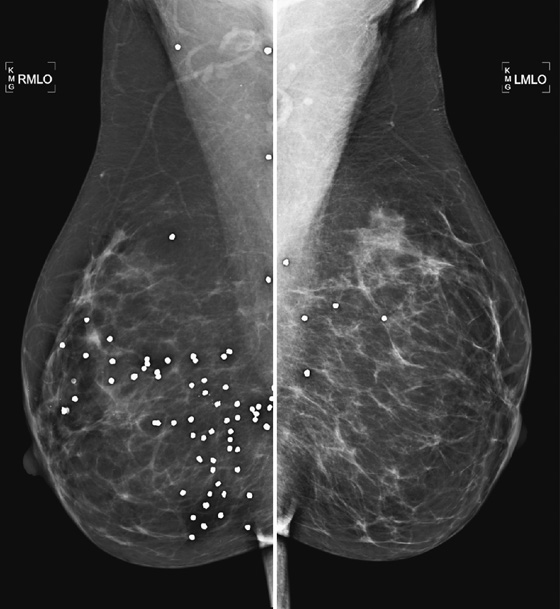
History: A 42-year-old patient presents for routine screening mammogram.
1. What is the differential diagnosis for this mammogram? (Choose all that apply.)
A. Scattered punctate calcifications
B. Multiple calcified fibroadenomas
C. Multiple round metal pellets in the breast, consistent with shotgun injury
2. What is the Breast Imaging Reporting and Data System (BI-RADS) diagnostic code for this mammogram?
A. BI-RADS 5—highly suspicious
3. Is there a reason this patient should not have an MRI?
A. No, the metal in shotgun pellets is nonferromagnetic and does not pose a problem.
B. No, the motion of the metal pellets within the breast during the scan would not pose a problem.
D. The patient may choose to have an MRI once she understands the potential problems.
4. Should the metal pellets be removed or biopsied?
A. No, the metal pellets typically do not cause a health risk.
B. Yes, as much of the metal should be removed as possible.
C. If any of the pellets are palpable, they must be removed.
D. Needle biopsy would be difficult, so excision is recommended.
ANSWERS
CASE 18
Artifact: Shotgun Pellets in Breast
1. C and D
2. D
3. C
4. A
References
Frenna TH, Meyer JE, DiPiro PJ, Denison CM. Gunshot residua simulating microcalcifications on mammography. Breast Dis. 1994;7:175–178.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:403–407.
Comment
Metal artifacts in the breast are relatively common. Needle core biopsies of benign lesions are often performed, and the physician may leave behind a localizing metal clip. Other iatrogenic metal material, including portions of catheters or needle localization devices, can be seen. Surgical clips are common after excision biopsy. Sewing needles and pencil lead fragments can be seen. Shotgun pellets or bullets may be seen.
The important factor in interpreting mammograms with artifacts is to realize that the finding is metal density, not calcification, and thus not native to the breast. Metal artifacts in the breasts are generally well tolerated and do not need to be removed. They do not typically cause symptoms, although superficially located pellets or other artifacts may be palpable.
MR imaging of the breast is best avoided in this patient, because the material in the pellets is unlikely to be known and might contain ferromagnetic material. This material causes two concerns: The pellets could move during the scan and could heat up, and there is an artifact from the metal that would lead to multiple signal-void artifacts on the image in this patient. The signal voids are usually larger than the size of the pellet, and no diagnostic information can be gained in these areas.
CASE 19
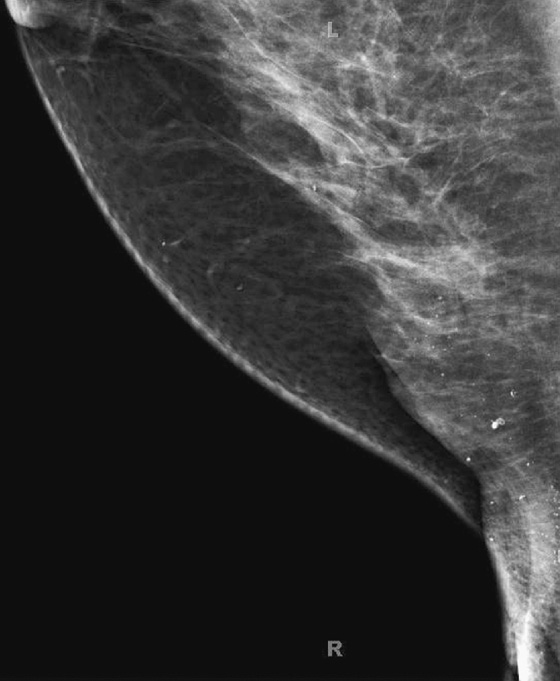
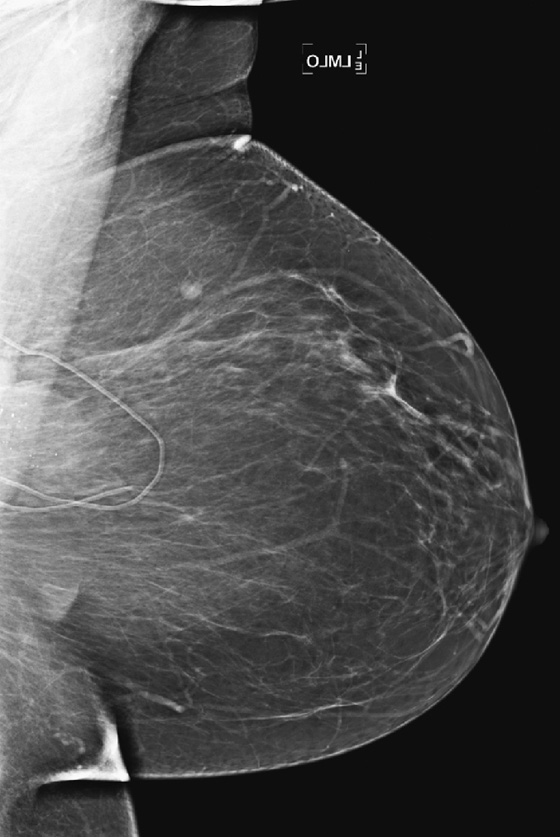
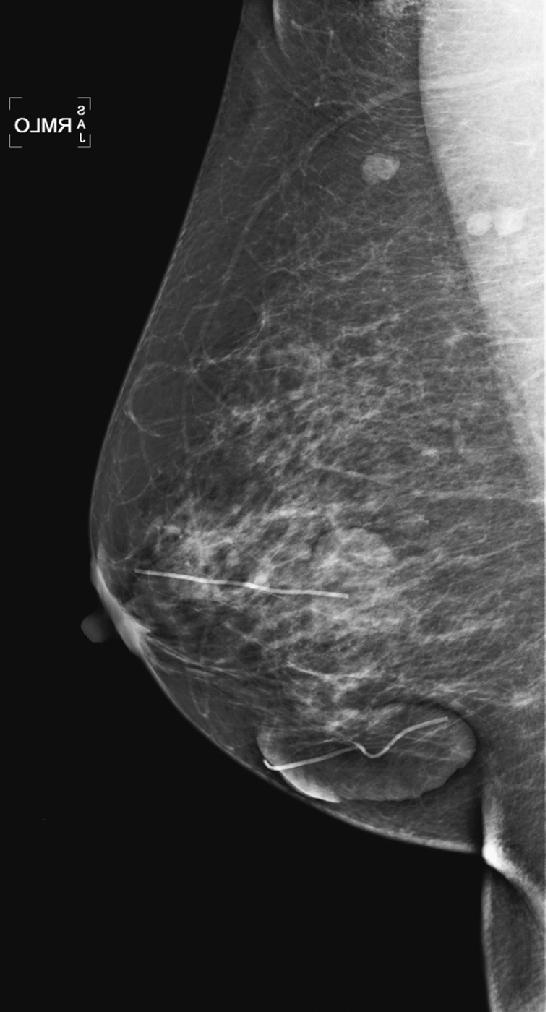
History: Three different women, of different ages, all with history of previous benign breast surgery, present for routine mammograms.
1. What is the differential diagnosis for the three different patients presented here? (Choose all that apply.)
2. Are the calcifications seen in the first image suspicious?
A. No, calcifications are often seen in keloids.
B. Yes, they are pleomorphic and should be biopsied.
C. Yes, magnification views should be performed.
D. Keloids are scar tissue and should not calcify.
3. Is it important to mark the skin in this condition?
A. Yes, keloids can mimic a breast mass.
B. No, it is not important; this condition is obvious on the mammogram.
C. Yes, as the skin thickening of keloids is indistinguishable from malignant skin thickening.
D. Yes, keloids are premalignant and must be followed closely.
4. What is the etiology of keloids?
A. They are always the result of surgical incision.
B. They are overexuberant scar tissue.
C. They are more common after burns than after surgical incision.
D. They are caused by scar tissue that has stretched and thinned.
ANSWERS
CASE 19
Keloid
1. B, C, and D
2. A
3. A
4. B
References
Kilkenny TE, Swenson GW. Keloids of the breast: mammographic findings. AJR Am J Roentgenol. 1995;164(4):1022.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:407.
Comment
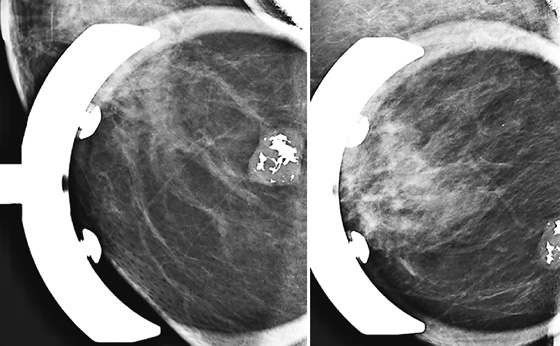
These three women all have the same condition: keloids formed on the skin after benign breast surgery. Keloids are composed of overexuberant collagenous scar tissue that forms in the skin in some patients after trauma from surgery, needle biopsy, insect bite, infection, or burn. The new tissue is elevated, rounded, and firm. Young women and African-Americans are particularly susceptible to keloid formation. On the mammogram, keloids are recognized by a smooth area of increased density in the skin, which typically follows the contour of the scar on the skin and so is usually tubular. The portion of the keloid outlined by air has a sharp contour (see the figures). Calcifications can develop within the keloid and should be superimposed over the area of skin thickening on both views. If in doubt, the technologist can obtain a tangential view to show that the calcifications are in the dermis. The air outline helps in the differentiation of a skin lesion versus a lesion inside the breast, such as a cyst or fibroadenoma. Ultrasound is usually not needed for evaluation, because the keloid should be obvious on clinical evaluation of the skin. The technologist may mark the location of the keloid on the skin so that this dermal lesion is not confused with breast pathology.
CASE 20
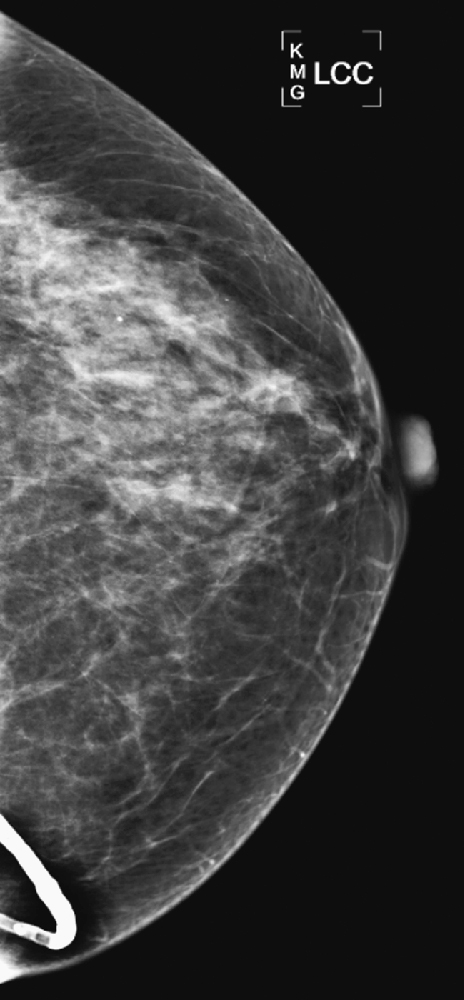
History: A 78-year-old woman presents for routine screening mammogram.
1. What is the differential diagnosis for this left CC mammographic view? (Choose all that apply.)
A. Normal mammogram view with a catheter superimposed
C. Normal mammogram view with an artifact from film handling
D. Vascular calcifications in the breast
2. Nothing is seen on the patient’s skin, and the gown is not in the path of the beam. What is the next step?
A. Ask the patient if there is an indwelling catheter.
C. Call the referring doctor’s office for information, if the patient is unaware.
D. Take an additional view, with spot compression of the area of concern.
3. What is the significance of this finding to the mammogram? What is the Breast Imaging Reporting and Data System (BI-RADS) code?
A. No significance, BI-RADS 2.
B. The finding obscures breast tissue, and the patient should be recalled, BI-RADS 0.
D. The finding is suspicious for malignant calcifications, BI-RADS 4.
4. Is any additional work-up needed?
A. Yes, spot magnification views of the calcified finding
B. Yes, ultrasound of the medial right breast
C. No, no additional work-up needed
D. Yes, clinical exam to try to palpate the tubular structure
ANSWERS
CASE 20
Artifact: Ventriculoperitoneal Shunt
1. A and B
2. F
3. A
4. C
Reference
Hogge JP, Palmer CH, Muller CC, et al. Quality assurance in mammography: artifact analysis. Radiographics. 1999;19:503–522.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:74.
Comment
Artifacts are abnormalities in mammographic density that are not native to the breast. Artifacts can occur in any component of the imaging process. Artifacts related to the patient can be due to motion or to superimposed objects, such as deodorant, lotion, hair, jewelry, and implanted medical devices. In this case, the patient has a ventriculoperitoneal shunt for hydrocephalus, and the tubing is seen on the mammogram.
In this patient, the shunt tubing is calcified, but this shunt may be seen without calcification. The figure above shows a different patient with the same history.
CASE 21
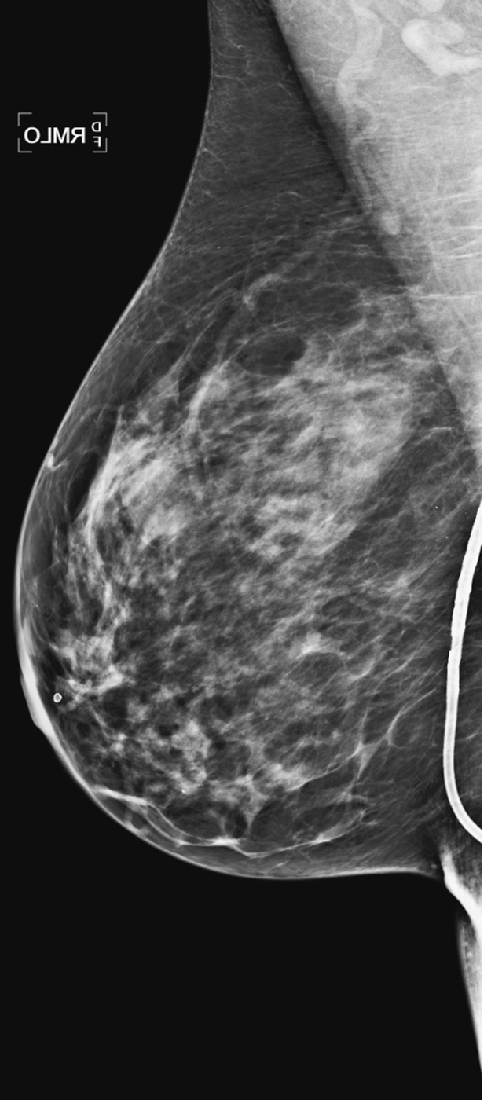
History: A 43-year-old asymptomatic patient has a mass seen in the left breast during routine screening.
1. What should be included in the differential diagnosis? (Choose all that apply.)
2. What is the next diagnostic step to differentiate these possibilities?
A. Examination of the patient’s skin
D. Additional mammographic views
3. What is an accessory nipple?
A. A molelike raised skin lesion that looks like a nipple but is dermatologic, not breast related
B. An additional nipple that develops only after pregnancy and lactation
C. A rudimentary, additional nipple along the primitive milk line
D. A mass of concern for malignancy, owing to abnormal duct formation
4. How common is this entity?
A. It is very common, seen in at least 50% of women, although it may be tiny.
B. It is extremely common; nearly all women have one.
C. It is rare, seen in 2% to 6% of women.
D. It is common, seen in approximately 20% of women.
ANSWERS
CASE 21
Accessory Nipple
1. B, C, and D
2. A
3. C
4. C
References
Bassett LW, Jackson VP, Jahan R, et al. In: Diagnosis of Diseases of the Breast. 6th ed Philadelphia: Saunders; 1997:399.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:38.
Comment
Supernumerary nipples (accessory nipples) are located on the embryonic milk line, which extends bilaterally from the upper axilla into the inner thigh. The most common presentation is the nipple alone. The accessory nipple represents the failure of complete regression of embryonal tissue and is more common in men than in women (about 1.7:1).
More than 75% of accessory nipples are smaller than one-third the size of the normal nipple. Most are below the breast (see the figure), and most are single. Most are found to be unrelated to other disease syndromes and have no clinical significance. The finding may include the nipple alone or be associated with an areola or underlying subareolar gland tissue or both. This gland tissue may produce milk in a lactating woman. The incidence of accessory nipples is estimated to be 2% to 6% of women, but this figure may be low because small accessory nipples may be mistaken for moles.
CASE 22
History: A 60-year-old woman presents for a routine mammogram. She has a history of bilateral surgical biopsies.
1. What should be included in the differential diagnosis for this mammogram? (Choose all that apply.)
B. Multiple benign solid masses, such as fibroadenomas
D. Bilateral multifocal cancer
2. How can you differentiate if the lesions are on the skin, rather than in the breast?
A. There is no way to differentiate on the mammogram.
B. You must be told by the technologist if there are skin lesions.
C. Ultrasound must be used to determine whether these are cystic versus solid.
3. Can neurofibromas be found in the breast as well as on the skin?
A. No, they are only in the cutaneous layer of the breast.
B. Yes, they are more often found in the breast as well as on the skin.
C. Yes, but they are very rarely found in the breast compared with skin manifestations.
4. Is there an increased risk of breast cancer in patients with NF?
A. No, NF is not related to malignancy.
B. No, the neurofibromas in the breast are never malignant.
C. No, but there is an increased risk of benign tumors.
D. Yes, there is an increased risk of breast cancer in these patients.
ANSWERS
CASE 22
Neurofibromatosis
1. A, B, and C
2. D
3. C
4. D
References
el-Zawahry MD, Farid M, Abd el-Latif A, et al. Breast lesions in generalized neurofibromatosis: breast cancer and cystosarcoma phylloides. Neurofibromatosis. 1989;2(2):121–124.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:408.
Comment
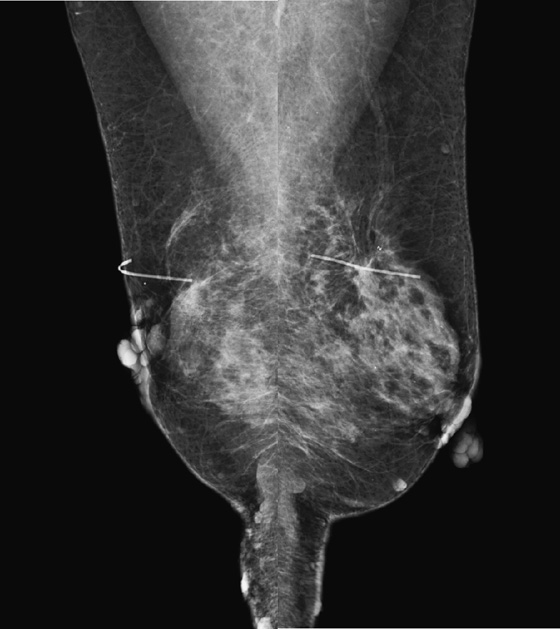
Neurofibromatosis (NF), or von Recklinghausen’s disease, is a disorder of the neurocutaneous system. It is relatively common, occurring in 1 in 4000 persons. The disease is characterized by neurofibromas in the skin and nervous system, café-au-lait spots, bone defects, and visual disorders. It is inherited in autosomal dominant fashion.
The mammogram shown is from a woman who has multiple skin neurofibromas (see the figures). She has a history of a malignant cystosarcoma phyllodes removed from the right breast and ductal carcinoma in situ removed from the left breast. This case illustrates the increased risk of breast cancer in patients with NF and the difficulty in finding a breast mass with the overlying multiple skin masses superimposed over the breast. Some of the masses have a rim of air around them, showing that they project outward from the skin rather than being inside the breast.
In patients with NF, not only are breast masses harder to find, but also NF is associated with an increased risk of developing benign and malignant tumors, including breast cancer. A five-times elevated risk of breast cancer has been found in neurofibromatosis patients. If a patient has a palpable mass in the breast, evaluation should proceed with additional mammographic views and ultrasound as needed, the same as in patients who do not carry the NF mutation.
Because no suspicious masses were seen in this mammogram, it was read as benign findings, Breast Imaging Reporting and Data System (BI-RADS) category 2. It was recommended that the patient return for bilateral mammography in 1 year.
CASE 23
History: A 48-year-old woman with factor V Leiden mutation undergoes routine baseline screening mammogram.
1. What should be included in the differential diagnosis for the images presented? (Choose all that apply.)
A. Dilated ducts in the left breast
B. Mondor’s disease, left greater than right
C. Varicose veins secondary to hemodialysis access complication
D. Chronic superior vena cava obstruction
2. What is the BI-RADS (Breast Imaging Reporting and Data System) code for this mammogram?
3. What is the next step?
A. Report the findings to the patient’s physician because the venous obstruction may not be known.
B. The patient should be referred for vascular work-up.
C. The patient should undergo MRI to evaluate for occult breast malignancy.
D. Do not include comments about enlarged veins in the mammogram report.
4. What is an etiology of central venous obstruction or stenosis?
C. Catheter insertion into a central vein
D. Phlebitis of a lower extremity superficial vein
ANSWERS
CASE 23
Superior Vena Cava Syndrome
1. C and D
2. C
3. A
4. C
References
Krishnan P, Uragoda L, Rao H, et al. Venous dilatation seen on routine mammography: a clue to superior vena cava obstruction. Chest. 2002;121(4):1361–1363.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:389.
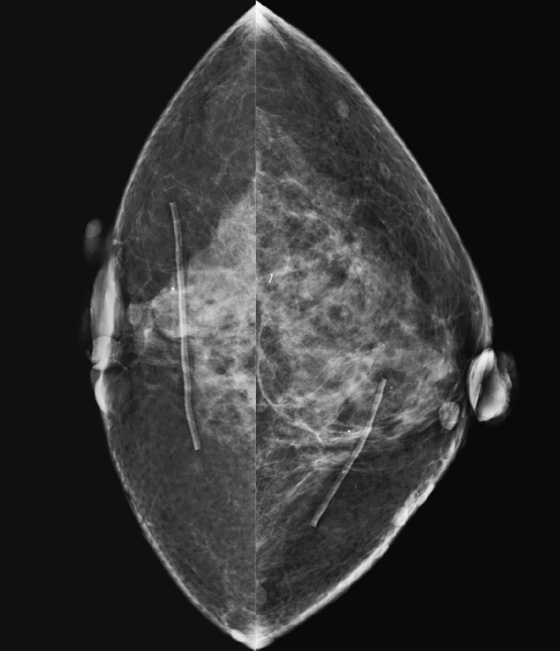
Comment
Dilated veins may be seen in the breast and are consistent with collaterals that develop to bypass an obstruction or stenosis in a central vein, typically the superior vena cava. This condition may be called superior vena cava syndrome or central venous occlusion. In patients with chronic central vein obstruction, the presence of collaterals can mean there are no other symptoms of the obstruction. In the patient in this case, who has a hypercoagulable state, involvement of the superior vena cava was unknown. The patient had no clinical symptoms relative to the upper extremities, chest, or neck. No edema was present in the skin or in the breast. The presence of dilated veins in the breast (see the figures) on her baseline screening mammogram led to the discovery of superior vena cava syndrome. In this patient, the obstruction was not due to external compression by a lung or mediastinum mass or from a catheter but rather thrombosis secondary to factor V deficiency.
The collaterals in the breast are not related to breast pathology but are a sign of a systemic process. If the collaterals had not been present, allowing the venous blood to bypass the obstruction and return to the right atrium, one would expect breast edema to be present on the mammogram. Because the presence of collaterals means that no clinical symptoms may be present, it is important to report this finding to the referring physician.
CASE 24
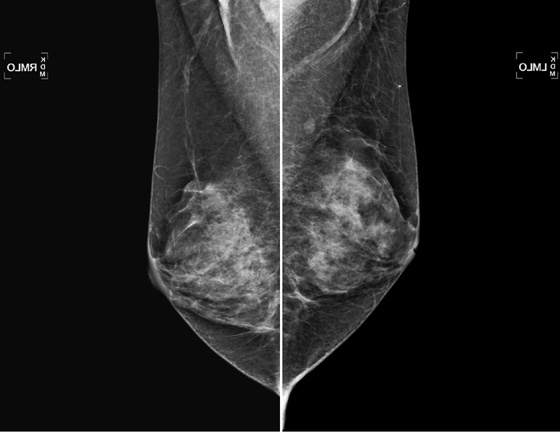
History: A 52-year-old woman, who has a family history of breast cancer in her mother at age 49, presents for routine screening mammogram. The focal density in the upper posterior left breast was not seen on any previous mammogram.
1. What should be included in the differential diagnosis for this mammogram? (Choose all that apply.)
A. Highly suspicious mass in the left upper breast
B. Lymph node in the upper left breast
C. Newly developing fibroadenoma in the left upper breast
D. Developing cyst in the left upper breast
2. What is the next step in management?
A. No work-up needed for benign lymph node—recommend annual screening
B. Scheduling the patient for stereotactic biopsy
C. MRI of the left breast to check for brisk enhancement
D. Ultrasound of the left upper breast to evaluate the density further
3. If the ultrasound examination shows a lymph node with thin symmetric cortex, what is the next step?
A. The patient can return to routine screening.
B. Short-interval follow-up is needed, to assess for change.
C. Biopsy is needed, using ultrasound guidance.
D. Refer the patient for whole-body imaging, preferably with positron emission tomography (PET).
4. What is the anatomic location of this lymph node?
ANSWERS
CASE 24
Normal Lymph Nodes
1. B, C, and D
2. D
3. A
4. B
Reference
Leibman AJ, Wong R. Findings on mammography in the axilla. AJR Am J Roentgenol. 1997;169(5):1385–1390.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:135. 152
Comment
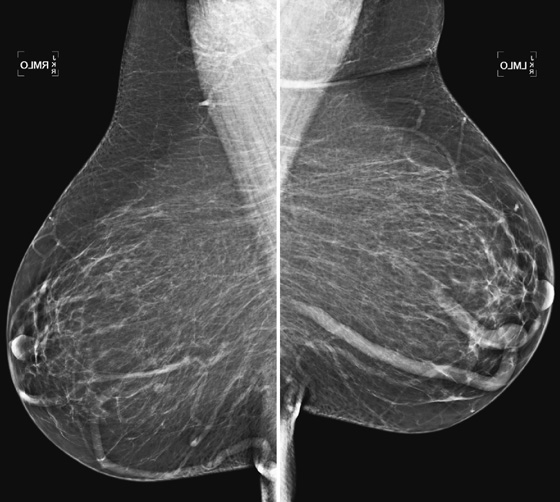
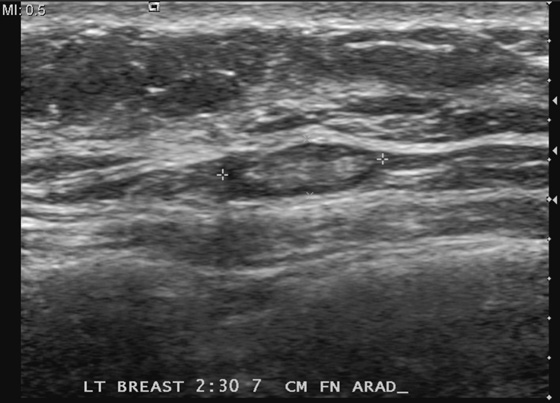
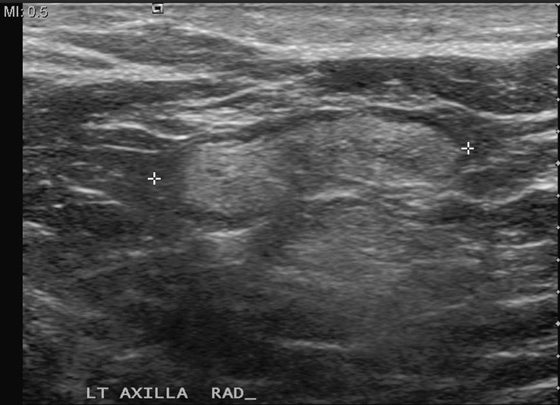
Lymph nodes are commonly seen on mammogram. They are often seen in the axilla and throughout the breast, most often in the upper outer quadrant. Normal nodes have a typical appearance on mammography, and when they are stable and have a characteristic appearance, no further evaluation is necessary. They are characteristically oval with sharp contours and contain a fatty hilum. A notch may be seen where the vessels and lymphatics enter the node. They can be any size, and large nodes with large central fatty hilum are commonly seen, particularly in the axilla. Abnormal nodes may herald otherwise occult breast pathology or systemic illness and require work-up. They become larger and denser and lose the fatty hilum.
If a new finding is seen on a mammogram, a work-up is needed, unless the new finding fits the description of a normal node. Ultrasound is useful in the evaluation of an unknown mass. The ultrasound appearance of the normal node is an oval or reniform mass, parallel to the chest wall, with a thin, concentric cortex and an echogenic central fatty hilum. The abnormal node has an eccentric thickened cortex and loss of the central hilar fat.
In this patient, the oval, circumscribed focal density in the left upper posterior breast had not been previously seen, and she was recalled for additional evaluation. Ultrasound revealed a normal node (see the figures above) with a thin cortex and oval shape, parallel to the chest wall. Also shown is a typical axillary node in the same patient (see the figures), which is larger, owing to an increased size of the fatty hilum. Because of the thin cortex, this node is also classified as benign. Overall size of the nodes in the axilla is irrelevant to the presence of pathology. This patient’s mammogram is now BI-RADS (Breast Imaging Reporting and Data System) 1, and she can return to routine screening.
CASE 25
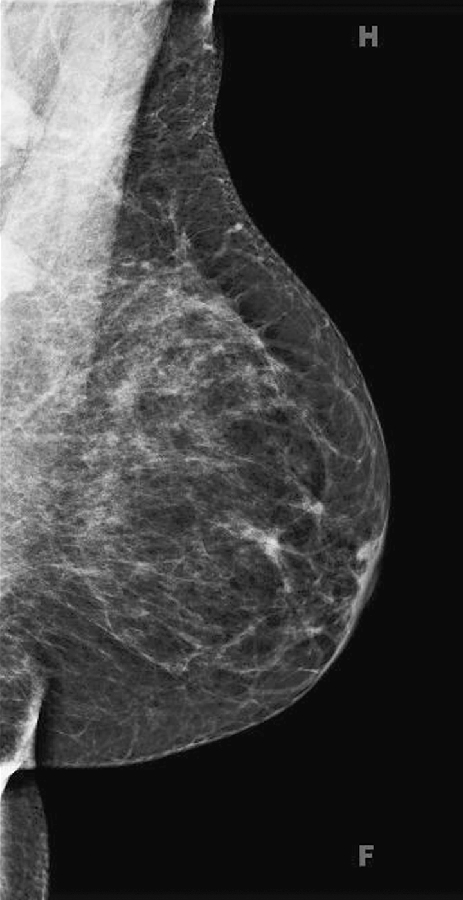
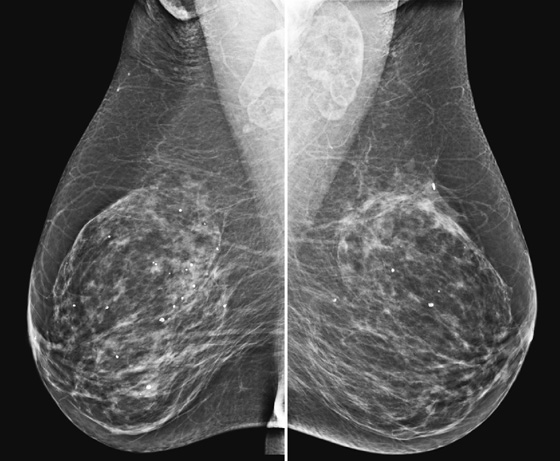
History: An asymptomatic 45-year-old woman presents for baseline screening mammogram; multiple masses are seen in both axillae on the mediolateral oblique (MLO) view. The image at right represents a companion case.
1. What should be included in the differential diagnosis? (Choose all that apply.)
A. Malignant mass in the lower medial aspect of the right breast
B. Normal axillary lymph nodes
C. Enlarged axillary lymph nodes
D. Lipomas in the axillary tail of Spence
2. Is ultrasound useful in the work-up?
A. No, because the patient did not note a palpable concern.
B. No, ultrasound is useful only for distinguishing a cyst from a solid mass.
C. No, a physical examination can reveal the correct diagnosis.
D. Yes, ultrasound is useful in the work-up of bilateral axillary adenopathy.
3. What is the next step in management of this patient?
A. Careful history to elicit evidence of infectious, inflammatory, or lymphoproliferative disorders
B. Full-body PET scan to check for extent of the disorder
C. Surgical consultation to excise a node
D. Image-guided core biopsy of a node
4. What Breast Imaging Reporting and Data System (BI-RADS) category and clinical recommendation should be given for bilateral enlarged axillary nodes seen on a routine baseline screening mammogram?
A. BI-RADS 4—suspicious, recommend biopsy
B. BI-RADS 5—highly suspicious for malignancy, biopsy strongly recommended
C. BI-RADS 1—normal, recommend annual mammography
D. BI-RADS 0—incomplete, recommend additional evaluation
ANSWERS
CASE 25
Enlarged Axillary Lymph Nodes
1. C
2. D
3. A
4. D
References
Alvarez E, Anorbe P, Alcorta F. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am J Roentgenol. 2006;186(5):1342–1348.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:395. 397
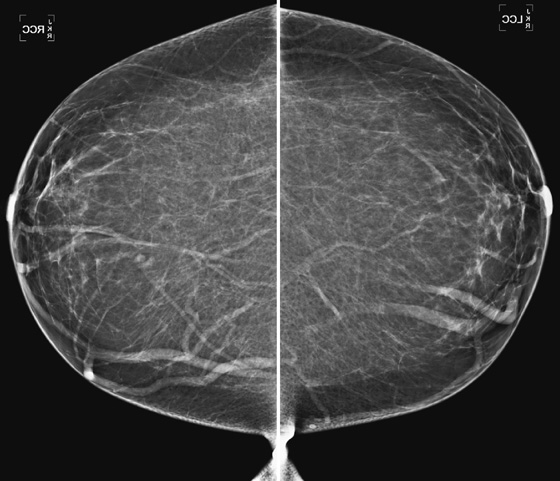
Comment
Bilateral axillary adenopathy is defined as multiple enlarged axillary nodes that do not contain fat (see the figures). The nodes are commonly larger than 2 cm and are typically rounded in contour and denser than normal. The margins may be irregular. Normal axillary nodes can be quite large if they are composed predominantly of fat (see the figures). Abnormal nodes are seen in approximately 0.3% of routine mammograms. The differential diagnosis includes HIV, lymphoma, leukemia, rheumatoid arthitis, scleroderma, lupus, sarcoidosis, and tuberculosis.
In the patient in this case, bilateral axillary adenopathy was due to HIV, and her enlarged nodes are not a new finding. The nodes are symmetric, although more are included in the right mammogram view compared with the left, likely secondary to positioning (see the figures).
CASE 26
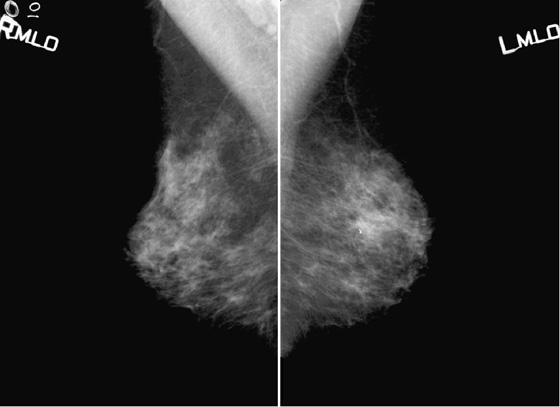
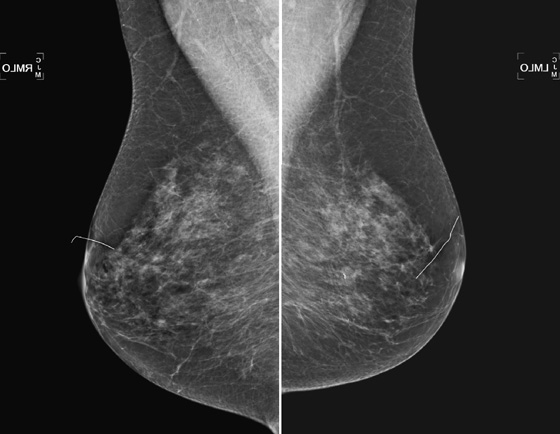
History: A 49-year-old woman presents for routine screening mammogram; two separate screening examinations taken 3 years apart are shown.
1. What advantages does digital mammography have over film-screen mammography? (Choose all that apply.)
2. What did the Digital Mammographic Imaging Screening Trial (DMIST) conclude?
A. There is no improvement in detection of breast cancer with digital mammography.
D. Digital mammography detected more cancers in women with fatty breasts.
3. What is the reason for improvement in sensitivity with digital mammography?
A. Spatial resolution is improved.
B. Contrast resolution is improved.
C. Digital display allows for magnified image.
D. Soft-copy display is better than hard-copy films.
4. If DMIST showed benefit only in selected groups of women, why are breast centers changing from film-screen to digital mammography?
A. Equipment is less expensive.
B. Digital process couples the image acquisition to image display.
C. Digital acquisition, display, and storage are separated.
D. Patient satisfaction is much higher.
ANSWERS
CASE 26
Digital Mammography
1. A, C, and D
2. C
3. B
4. C
References
Hendrick RE, Pisano ED, Averbukh A, et al. Comparison of acquisition parameters and breast dose in digital mammography and screen-film mammography in the American College of Radiology Imaging Network digital mammographic screening trial. AJR Am J Roentgenol. 2010;194(2):362–369.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:7–15.
Comment
Digital mammography offers advantages over film-screen mammography (see the figures). The elimination of film processing and storage, as was already accomplished with all other radiology applications, is a major advantage. Other advantages include improved contrast resolution and the visualization of the entire dynamic range across the image, out to and including the skin (see the figures). Allowing for the use of computer-aided detection, tomosynthesis, and telemammography is an additional advantage.
Several large studies have been performed, using film-screen mammography as the gold standard, to ensure that digital mammography was at least as good as film in detecting breast cancer. DMIST in the United States, which concluded its recruitment in 2003 and was published in 2005, showed that digital mammography was as sensitive as film across all surveyed groups and had improved sensitivity in the detection of breast cancer in several subgroups, including women younger than 50, premenopausal and perimenopausal women, and women with dense or heterogeneously dense breasts.
In digital mammography, spatial resolution is not as good as in film, but contrast resolution is higher. Spatial resolution is most important in the detection of tiny objects, such as microcalcifications. Studies have shown that digital mammography more accurately characterizes microcalcifications compared with film mammography despite the poorer spatial resolution; this may be due to the higher contrast resolution.
In this patient, the initial mammogram was film-screen (see the figures), and the subsequent mammogram was digital (see the figures). Compare the visualization of the dynamic range of tissue density across the image, which can be attained with digital and not with film technology. This range allows better detection of abnormalities adjacent to the skin compared with film. This case is an example of a woman who was shown in DMIST to benefit from digital mammography because she is younger than 50 with heterogeneously dense breasts.
CASE 27
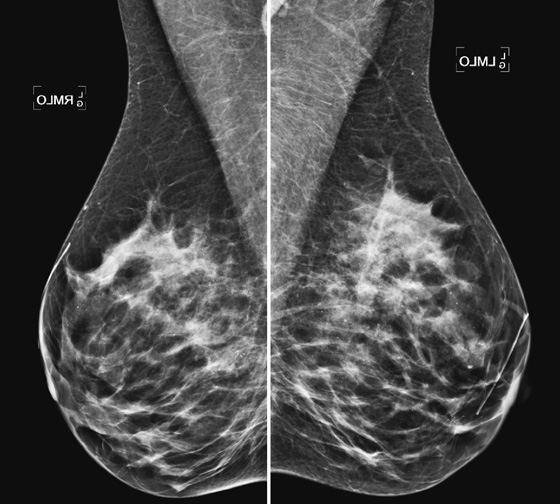
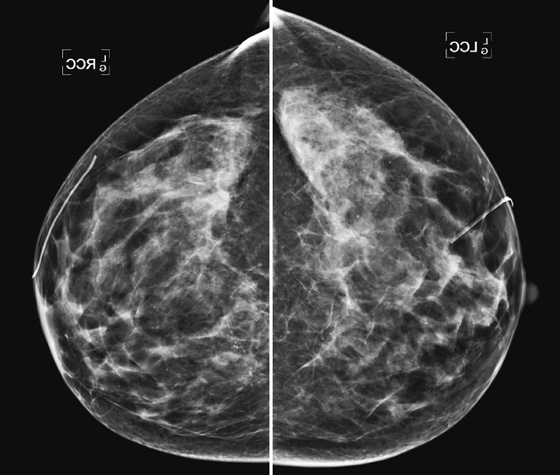
History: A 54-year-old asymptomatic woman who underwent a surgical biopsy previously for papillomas associated with atypical ductal hyperplasia presents for routine screening mammogram.
1. What should be included in the differential diagnosis for the images shown? (Choose all that apply.)
A. Bilateral suspicious calcifications, possible ductal carcinoma in situ
B. Bilateral benign fibrocystic calcifications
C. Bilateral dermal calcifications
D. Calcifications in multiple tiny bilateral masses such as fibroadenomas or papillomas
2. What is the distribution of these calcifications?
B. Bilateral segmental distribution
3. What is the risk of malignancy associated with these calcifications?
4. What is the Breast Imaging Reporting and Data System (BI-RADS) code for this mammogram?
ANSWERS
CASE 27
Punctate Calcifications
1. B, C, and D
2. C
3. A
4. A
References
Burnside ES, Ochsner J, Fowler K, et al. Use of microcalcification descriptors in BI-RADS 4th edition to stratify risk of malignancy. Radiology. 2007;242(2):388–395.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:74.
Comment
Many mammograms contain calcifications. It is important to analyze the calcifications to determine if they can be placed into a benign category and dismissed from consideration for possible malignancy. Digital mammography has been shown to identify more calcifications compared with film-screen mammography; in one study, calcifications were seen in 45% of digital mammograms versus 36% of film mammograms.
It is important to determine both the morphology and the distribution of calcifications. The benign morphology category includes round and punctate calcifications. Round calcifications differ from punctate only by size; round calcifications are larger than 0.5 mm. This morphology is typically benign, but it can be seen in malignant calcifications if they are in a suspicious distribution. Benign calcifications should be in a benign distribution, including stable clusters, regional, multiple clusters, and diffuse distribution (see the figures).
It has been shown in multiple studies that round or punctate calcifications in a benign distribution do not represent malignancy. They are typically due to fibrocystic change or sclerosing adenosis. The calcifications should be noted, and routine follow-up is recommended. If more suspicious calcifications develop in these patients, they should be evaluated further with magnification views.
CASE 28
History: A 53-year-old woman who was diagnosed with left breast cancer 13 years ago, in a different state, now presents for routine mammogram after a 3-year delay.
1. What should be included in the differential diagnosis of the right mammogram? (Choose all that apply.)
A. Distortion in the right breast from prior surgery
C. Radial scar in the right upper breast
D. Fibroadenoma in the right upper breast
2. Why is it important to know surgical history?
A. The surgical scar can cause distortion.
B. The patient’s risk of cancer increases markedly if there is a history of previous benign surgery.
C. Fat necrosis developing at the surgical site increases risk of breast cancer at that site.
D. Pain at a prior surgery site may affect the decision on imaging needed.
3. What is the best way to work up suspected distortion?
4. What is the next best step in management of this finding?
B. Needle biopsy with imaging guidance
C. MRI to evaluate for abnormal enhancement
D. PET/CT to evaluate for distant metastases
ANSWERS
CASE 28
Tent Sign
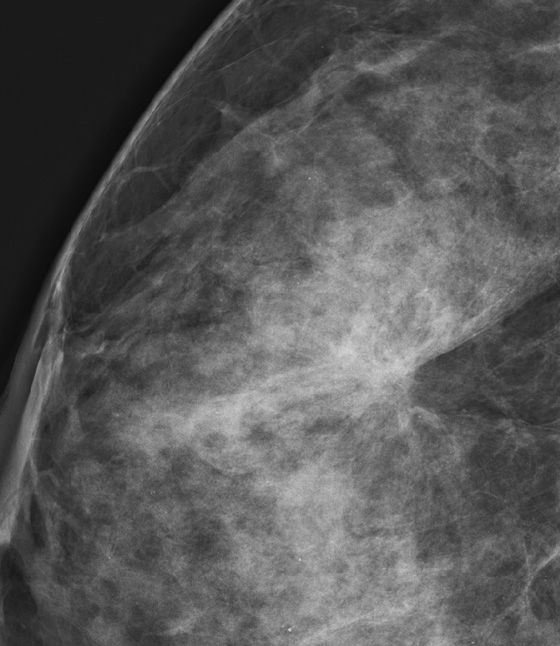
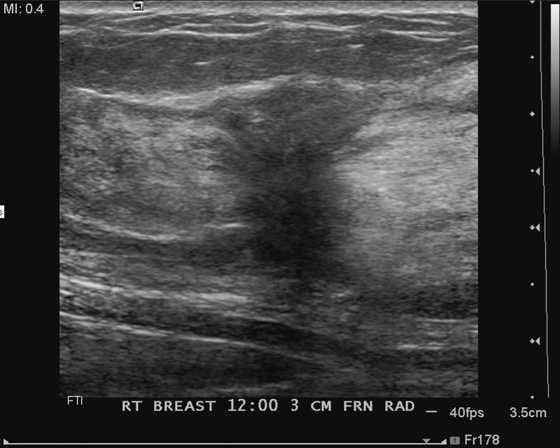
1. A, B, and C
2. A
3. B
4. B
Reference
Bird RE, Wallace TE, Yankaskas BC. Analysis of cancers missed at screening mammography. Radiology. 1992;184(3):613–617.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:34.
Comment
The tent sign is a description of the effect of a spiculated mass on the surrounding tissue. Masses, particularly in the dense breast, can be obscured by surrounding gland tissue. One way to identify a mass is by examining the contour of the glandular tissue. The margins of the anterior and posterior breast should be smooth, scalloped, and adjacent to the subcutaneous and deep posterior fat lobules. Any interruption in this pattern should be examined. The “tenting” of tissue, or the triangular shape of the fat adjacent to the gland tissue, indicates that there is a distortion in the gland tissue. This distortion can be caused by a spiculated mass, scar tissue, radial scar, or fat necrosis. Spot compression or spot magnification views should be obtained to analyze the tenting, when it is seen.
This patient was diagnosed with a mass in the right breast after undergoing a routine mammogram (see the figures on p. 57); the distortion was identified on the craniocaudal (CC) view (see the figures on p. 57). A spot magnification view (see the first figure above) accentuated the distortion and tenting of the adjacent fat. Ultrasound was performed and showed a spiculated mass (see the second figure above).
CASE 29
History: A 42-year-old woman undergoes routine baseline mammogram.
1. What should be included in the differential diagnosis of the mammogram shown? (Choose all that apply.)
2. What is the BI-RADS (Breast Imaging Reporting and Data System) code for this mammogram?
3. This patient was unaware of this mass. How does this finding usually manifest?
A. A tender mass, fixed to the skin
C. A soft mass that is nontender
D. Skin dimpling and peau d’orange
4. Is biopsy indicated if the mass is palpable?
A. Yes, biopsy should be performed of any palpable mass.
B. Yes, the mixed elements in this mass require biopsy to exclude cancer.
C. No, this mass does not require biopsy, even if palpable.
D. No, but the mass should be carefully followed because malignant transformation is common.
ANSWERS
CASE 29
Hamartoma
1. A and B
2. B
3. C
4. C
References
Georgian-Smith D, Kricun B, McKee G, et al. The mammary hamartoma: appreciation of additional imaging characteristics. J Ultrasound Med. 2004;23(10):1267–1273.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:135.
Comment
A hamartoma is a benign overgrowth of fibrous tissue, gland elements, and fat that occurs in women during the reproductive years. A hamartoma can contain different amounts of these three components; fat may predominate, as in this patient (see the figures), or the mass may have relatively little fat and manifest as a dense mass on mammography. The mass is typically well circumscribed and encapsulated. Cancer can arise within a hamartoma but has no special propensity to do so. Hamartoma is coined “breast within a breast” because it contains the histologic elements of normal breast tissue.
If there are no suspicious calcifications within the mass, as in this patient, biopsy may be unnecessary. If biopsy is performed, pathologists may have difficulty distinguishing the mass from normal breast tissue, especially on core biopsy. Ultrasound evaluation is unnecessary in a patient, such as this one, who has a classic presentation. If ultrasound is performed, the mass is oval with heterogeneous internal echoes and circumscribed margins.
CASE 30
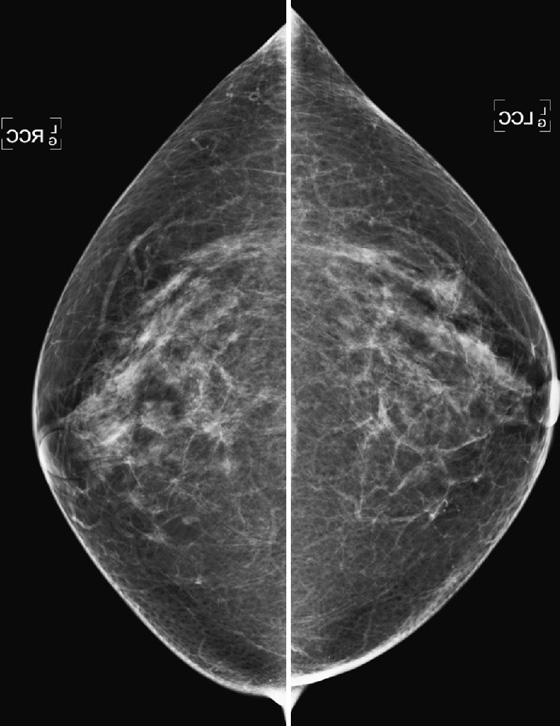
History: A 49-year-old woman underwent a baseline screening mammogram; craniocaudal (CC) views are shown.
1. What should be included in the differential diagnosis for these two views? (Choose all that apply.)
B. Cluster of calcifications in the medial left breast
C. Dermal calcifications seen on the left CC view
D. Suspicious mass with associated calcifications in the left breast
2. What is the next step in management?
A. Recall the patient for magnification views.
B. Use the digital magnification capability of the workstation to evaluate the calcifications fully.
C. Recommend routine screening mammogram in 1 year.
D. Perform ultrasound of the left breast to check for possible mass.
3. Why is it important to know if the calcifications are dermal or in the breast?
A. Dermal calcifications are suspicious for spread of breast cancer to the skin.
C. Dermal calcifications can be related to skin cancer.
4. How can you show calcifications to be in the skin?
A. Perform ultrasound of the calcification area and look for calcifications in the skin.
D. Perform stereotactic biopsy.
ANSWERS
CASE 30
Dermal Calcifications Localization
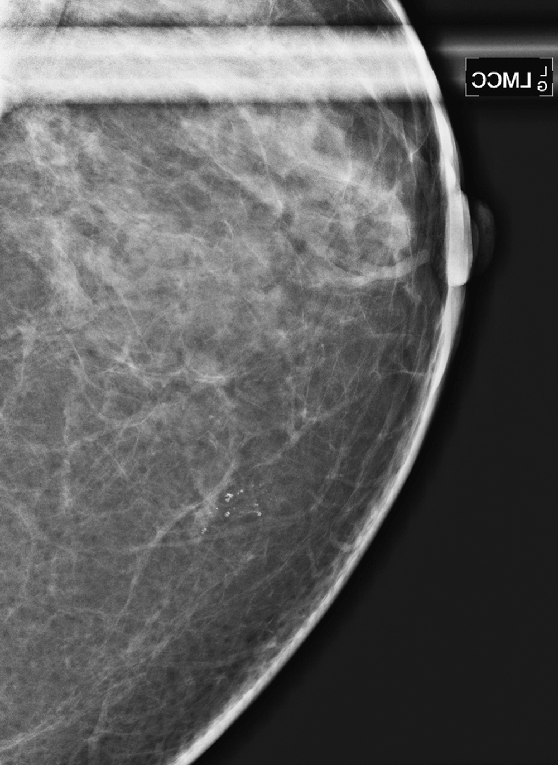
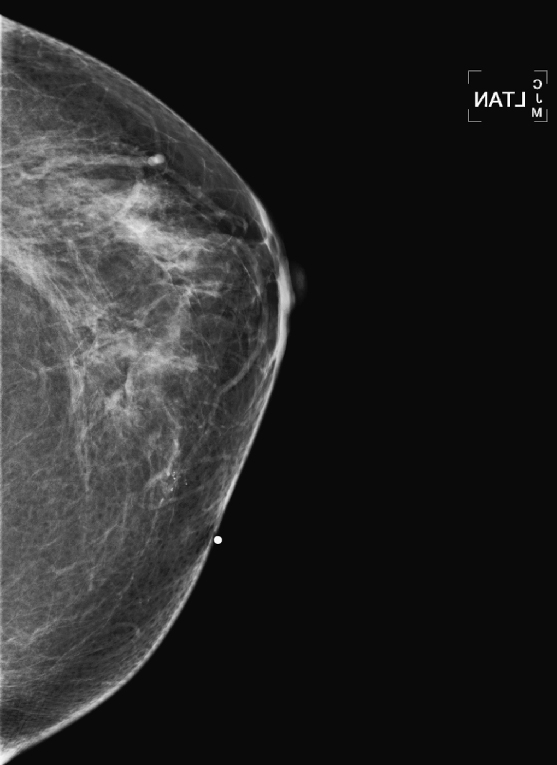
1. B and C
2. A
3. B
4. C
References
Berkowitz JE, Gatewood OM, Donovan GB, et al. Dermal breast calcifications: localization with template-guided placement of skin marker. Radiology. 1987;163(1):282.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:76.
Comment
Dermal calcification is very common, with individual calcifications typically 1 to 2 mm in size and having a smooth, round contour. Calcifications that are in the skin never represent primary breast malignancy. They are of no clinical significance and do not need follow-up. They arise from sebaceous glands and often have lucent centers. This appearance may be characteristic, and the calcifications may be dismissed easily on screening mammogram, without the need for further characterization. Skin calcifications may be grouped and may have a fixed relationship within the group, a finding termed the tattoo sign, which may also help in differentiation from breast calcifications. When calcifications are seen for the first time and are near the skin surface, as in this patient (see the figures), further evaluation can be done to determine if the calcifications are in the skin and can be dismissed, rather than performing a biopsy.
Magnification views are performed first (see the figures). If there is a possibility the calcifications are dermal, skin localization views can be performed. The technologist places the breast in compression using an alphanumeric fenestrated paddle, such as is used for a needle localization procedure (see the figures). The image is viewed, and the location of the calcification within the fenestration is marked on the skin with a BB, and this is confirmed with another image. The breast is removed from that compression paddle, and the BB marker is imaged in tangent (see the figures). If the calcifications are in the skin, this view confirms them in the dermal layer. In this patient, the calcifications are superficial in the breast but are not in the dermis, and biopsy is recommended.
Skin calcifications are seen much more easily with digital mammography than with film-screen mammography because the tissue density is equalized out to the skin.
CASE 31
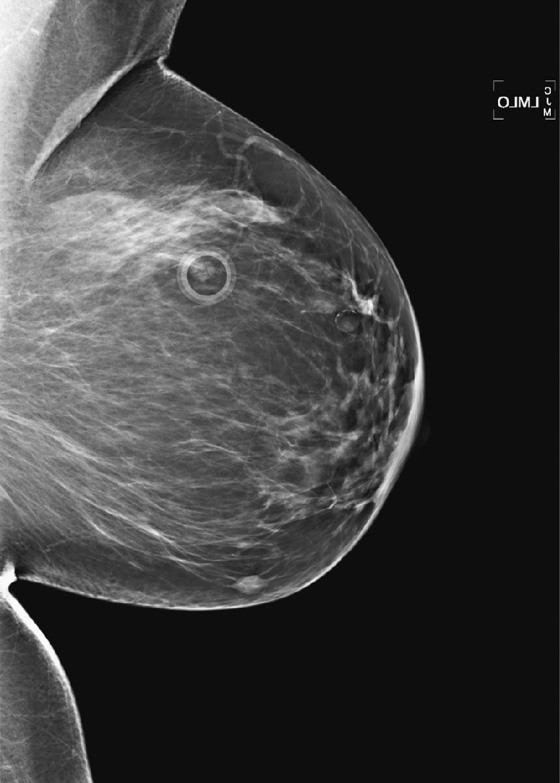
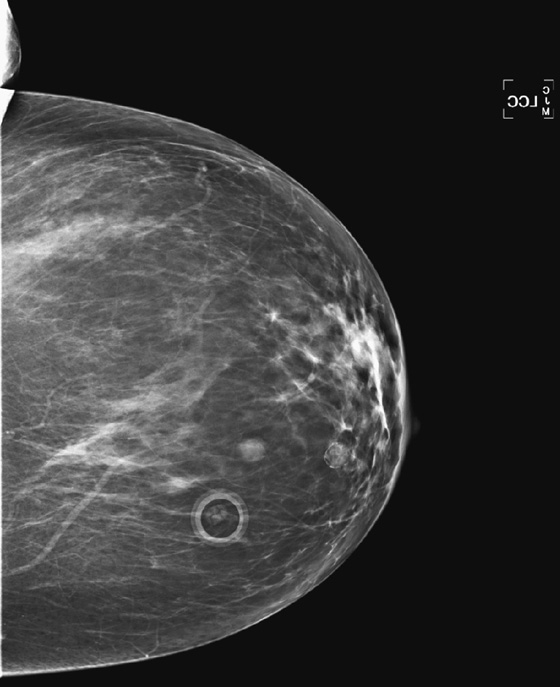
History: A 55-year-old woman undergoes routine screening mammogram. She had a left breast fibroadenoma diagnosed by core needle biopsy 14 years ago.
1. What should be included in the differential diagnosis of the two mammogram views shown? (Choose all that apply.)
A. Three benign masses in the left breast
B. A fibroadenoma, an oil cyst, and a mole on the skin
C. A cyst, an oil cyst, and a mole on the skin
D. A suspicious solid mass, an oil cyst, and a mole on the skin
2. In what quadrant is the structure marked with a ring marker?
3. In what quadrant are the two lesions in the left breast?
A. The oil cyst is in the upper inner quadrant; the fibroadenoma is in the lower inner quadrant.
B. The oil cyst is in the upper inner quadrant; the fibroadenoma is in the lower outer quadrant.
C. The oil cyst is in the upper outer quadrant; the fibroadenoma is in the lower outer quadrant.
D. You cannot tell which quadrant the lesions are in without a true mediolateral view.
4. Why are moles marked before mammogram views are performed?
A. Moles can become malignant.
B. Moles can be confused for masses in the breast.
D. The patient may palpate the mole as a suspicious breast lump.
ANSWERS
CASE 31
Triangulation
1. B and C
2. C
3. A
4. B
References
Park JM, Franken EA Jr. Triangulation of breast lesions: review and clinical applications. Curr Probl Diagn Radiol. 2008;37(1):1–14.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:49.
Comment
Triangulation is the determination of the location of a mass in the breast by assessing its appearance on the standard views. In this patient, both lesions are seen in both views, but this may not always be the case. Triangulation can also refer to locating a lesion that is seen only on one view.
In this patient, there is a known fibroadenoma in her left breast, a calcified oil cyst in the left breast, and a mole on the skin (see the figures). When a lesion is seen in two projections of the mammogram, the location can be known, even though the mediolateral oblique (MLO) view is not orthogonal to the craniocaudal (CC) view. Not all lesions that project above the nipple on the MLO view are truly above the nipple. A mass in the upper inner breast may project below the nipple, and a mass in the lower outer breast may project above the nipple. It is important to understand triangulation, based on the MLO and CC views, so that you can be certain you are locating the same lesion on ultrasound.
First, assess the location of the lesion on the CC view: medial or lateral to the nipple? How far medial or lateral? Then look at the MLO view, and try to judge where the lesion would be on the mediolateral (ML) view, which is the true location in the breast. If the lesion is lateral on the CC view, it appears higher on the MLO view than it truly is. The further lateral on the CC view, the more discrepant the location on the MLO view. Conversely, if the lesion is in the medial breast, it appears lower on the MLO view than it truly is. If the lesions are close to the nipple on the CC view, as these two lesions are (see the figures), the location in the breast is similar to the location on the MLO view.
The calcified oil cyst is in the upper inner quadrant and about 1 to 2 cm medial to the nipple. This means that the location would not be very different on the ML view compared with the MLO view, and you would expect to find this lesion at the 11 o’clock position. The solid oval mass is also in the medial breast, about 1 to 2 cm from the nipple. However, on the MLO view, it is at the inferior aspect of the breast, so its location is at the 7 o’clock position in the left breast. The mole can be found easily by inspecting the skin, but it can be confused for a mass inside the breast, so it is good practice to mark moles before performing mammography.
CASE 32
History: A 75-year-old woman presents for routine mammogram.
1. What is the differential diagnosis for the finding in the right breast? (Choose all that apply.)
A. Mass suspicious for malignancy
2. What is the likely cause of this finding?
3. Is any further work-up needed? What Breast Imaging Reporting and Data System (BI-RADS) score would you give?
A. No additional work up is needed: BI-RADS 2.
B. Yes, spot magnification views are needed for the calcifications: BI-RADS 0.
C. Yes, ultrasound is needed to confirm the suspected benign diagnosis: BI-RADS 0.
D. Yes, ultrasound is needed to guide needle core biopsy: BI-RADS 4.
4. What are the characteristic mammographic findings of this condition?
C. Thick, irregular radiodense rim
ANSWERS
CASE 32
Oil Cyst
1. C
2. A
3. A
4. D
References
American College of Radiology. In: Illustrated BI-RADS. 4th ed Reston, VA: American College of Radiology; 2004:76–81.
Sickles EA. Breast calcifications: mammographic evaluation. Radiology. 1986;160:289–293.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:137.
Comment
The calcified oil cyst is one of the common benign calcifications seen on the mammogram. It is caused by trauma, which results in focal tissue death, with necrosis of the fat, which is then surrounded by a thin wall that can calcify.
The oil cyst is recognized by the central fat lucency and the thin, often calcified wall. This thin calcification is referred to as eggshell calcification and is diagnostic of an oil cyst. The trauma is typically caused by surgery to the breast, from either excisional biopsy or reduction mammoplasty, and it can occur in a motor vehicle accident. In the case of trauma from surgery, the oil cysts are typically in the area of scar. In a motor vehicle accident, the seat belt tightening against the breast can cause bruising and subsequent fat necrosis. This may be seen in a typical linear pattern across the chest, tracing the path of the shoulder harness of the seat belt. In this patient (see the figures), the oil cyst was likely caused by a benign surgical biopsy many years before. It is not palpable. This was seen on routine screening mammogram, and no further work-up is needed.
Patients may present with a palpable mass that is an oil cyst. You may be able to elicit a history of trauma or surgery. The mass is usually superficial and in the area of the scar or the seat belt. If the typical findings of an oil cyst are seen on mammogram, no further work-up is needed. If ultrasound is performed, the appearance can be variable and can cause some doubt about the diagnosis. The mass will be round on ultrasound, and the central fat portion can have varying degrees of echogenicity. If the rim is calcified, it can shadow on ultrasound, giving it a more ominous appearance. If the typical findings of fat lucency and thin rim are seen on mammography, ultrasound is not needed.
CASE 33
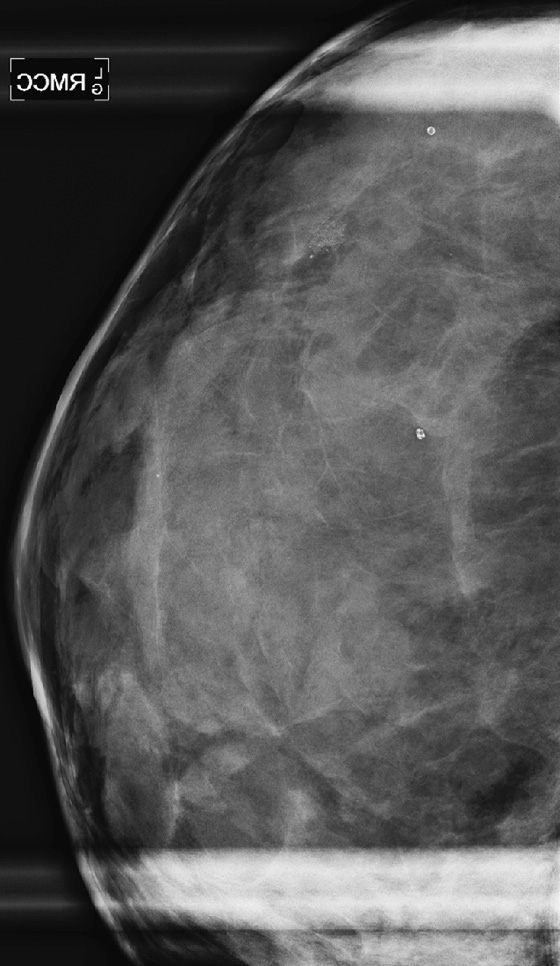
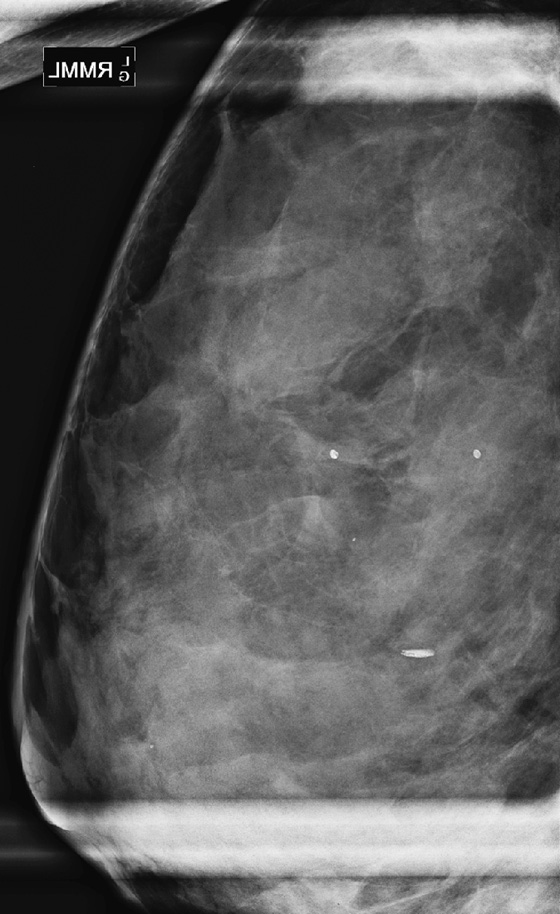
History: An asymptomatic woman having a routine mammogram had calcifications in the lateral right breast on standard images. She was recalled for magnification views, which are shown.
1. What is the differential diagnosis based on the magnification views? (Choose all that apply.)
A. Ductal carcinoma in situ (DCIS)
B. Amorphous, indeterminate calcifications
D. Rod-shaped calcifications typical of secretory type
2. How do the magnification views give you the diagnosis?
A. The calcifications remain linear in two projections.
B. The calcifications are pleomorphic on the craniocaudal (CC) view.
C. The calcifications are indistinct on both views.
3. What is the next step in follow-up?
A. The calcifications should be biopsied.
B. The patient should return in 6 months for follow-up magnification views.
C. Routine annual mammogram should be scheduled.
D. Diagnostic mammogram should be done in 1 year, with magnification views repeated.
4. What is milk of calcium?
A. It is calcium in ducts, related to lactation.
B. It is calcium sediment in a cyst, which layers to the bottom.
C. It is often seen adjacent to DCIS.
D. Also called “secretory type,” it is calcium in milk ducts.
ANSWERS
CASE 33
Milk of Calcium
1. C
2. D
3. C
4. B
Reference
Linden SS, Sickles EA. Sedimented calcium in benign breast cysts: the full spectrum of mammographic presentations. AJR Am J Roentgenol. 1989;152(5):967–971.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010.
Comment
This patient demonstrates the common condition of milk of calcium calcifications in the breast. In this case (see the figures) the calcifications are clustered on the CC view and layer in a meniscus shape on the ML view. This is characteristic of sedimented calcifications that are present in a macrocyst. The ML view is of utmost importance, with the x-ray beam perpendicular to the breast cyst, to display the calcifications dependent in the cyst. If this view is not performed, the dependent layering can be missed, and the calcifications are seen only as clustered and may be suspected to be malignant. The calcifications have a very different appearance on the ML magnification view (layering or linear) and the CC magnification view (smudgy or punctate). If the typical appearance is seen on the magnification views, the calcifications can be correctly assigned to Breast Imaging Reporting and Data System (BI-RADS) category 2, benign, and no biopsy or follow-up is needed.
Milk of calcium can also manifest as a single calcification in each of many tiny imperceptible cysts. In such cases, many layering separate calcifications are seen on the ML magnification view.
CASE 34
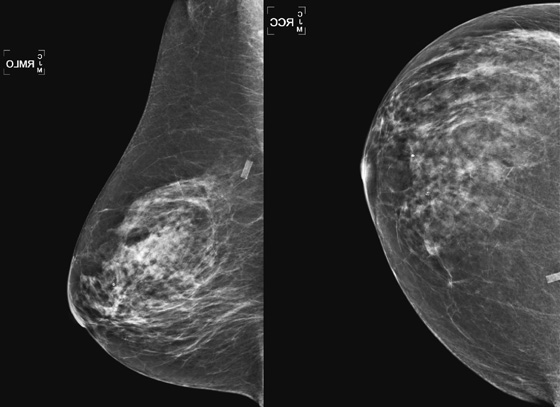
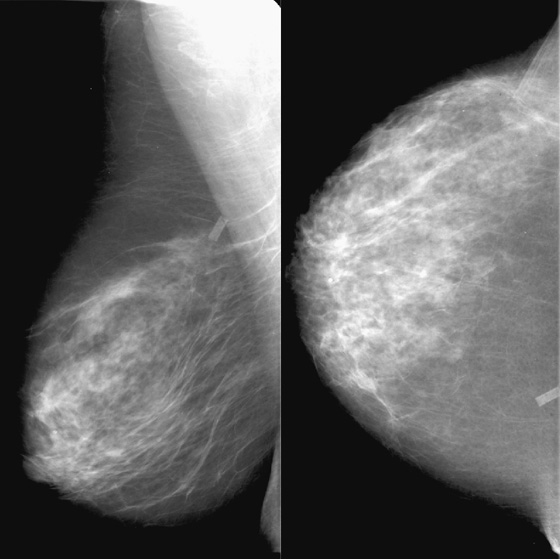
History: A 64-year-old woman undergoes routine screening mammogram.
1. What should be included in the differential diagnosis of the right mammogram shown? (Choose all that apply.)
A. Normal mammogram with artifact from retained catheter cuff in the right upper inner chest
B. Suspicious mass in the right upper inner breast
C. Evidence of infection around a retained sheath from a removed catheter
2. What BI-RADS (Breast Imaging Reporting and Data System) would you give this mammogram?
3. Which statement is not related to the origin of this artifact?
A. The patient had a central line placed.
B. The central line catheter was removed by traction.
C. The central line was removed by cutting down on the catheter surgically.
D. The sheath is in a subcutaneous tunnel.
4. What side effect of the retained sheath is most commonly seen on the mammogram?
A. Breakage of the sheath into smaller pieces
D. Hematoma formation around the catheter
ANSWERS
CASE 34
Retained Catheter Cuff
1. A and D
2. B
3. C
4. C
References
Beyer GA, Thorsen MK, Shaffer KA, et al. Mammographic appearance of the retained Dacron cuff of a Hickman catheter. AJR Am J Roentgenol. 1990;155(6):1203–1204.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:404.
Comment
Central venous catheters are placed for many reasons—chiefly to supply a patient with long-term delivery of parenteral nutrition, chemotherapy, antibiotic therapy, or hemodialysis. The catheters are tunneled under the skin and have a Dacron cuff. The purpose of the cuff is to help maintain the catheter in position, as fibrous tissue is stimulated to grow around it. The cuff is positioned in the subcutaneous tunnel.
Catheters are removed most commonly by pulling, or “traction.” According to one report, in about 10% of cases, the cuff remains inside the subcutaneous tunnel when the catheter is pulled out. This retention does not occur if the catheter is removed by cutting down on the catheter surgically. Most catheters are placed into the central venous system on the right side, so the retained cuffs are most commonly seen in the right upper inner breast on the mammogram, as in our patient (see the figures).
Catheters are removed either because the function is no longer needed or because the catheter becomes infected. If the cuff is retained after the catheter is removed, it does not need to be removed. Infection of the retained cuff is unusual. If this occurs, there is usually a palpable, erythematous mass in the area of the subcutaneous tunnel. The infection may be recognized on the mammogram with an area of increased density and spiculation around the cuff. However, these findings are not exclusive to infection; many noninfected cuffs have an area of increased density around them owing to fibrosis from scarring. When density is seen around a retained cuff, correlation with patient symptoms should be made.
CASE 35
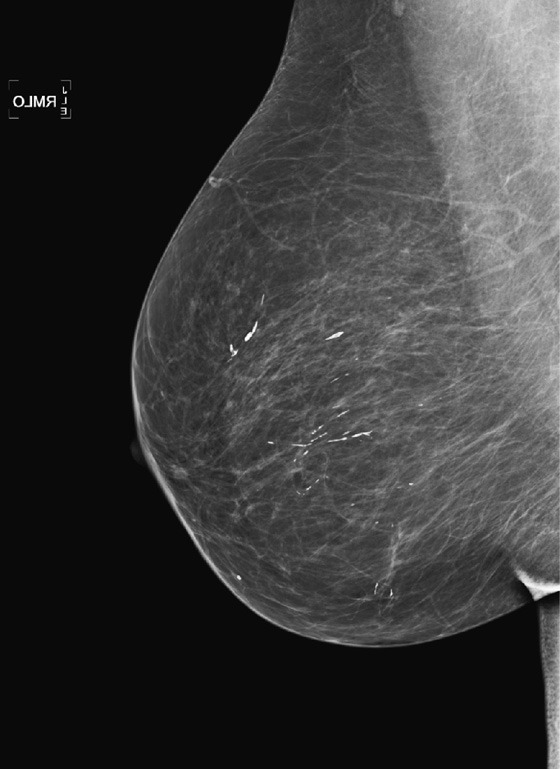
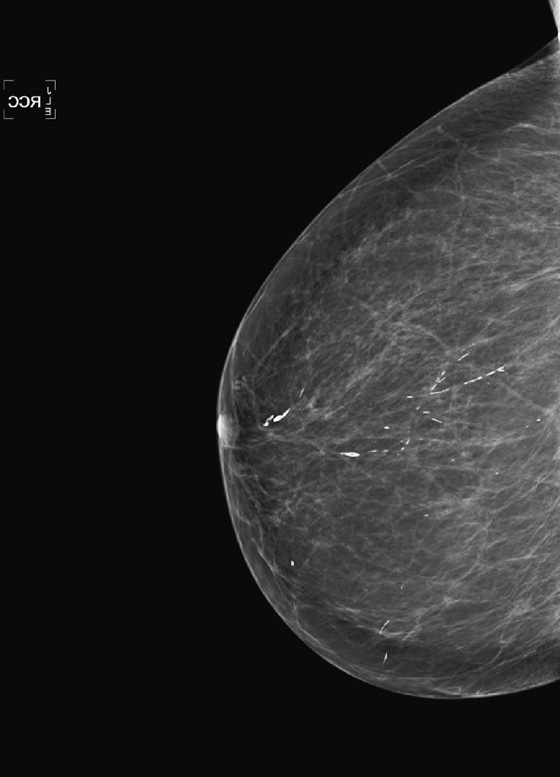
History: A 68-year-old woman presents for routine mammogram.
1. What is the differential diagnosis for the right breast calcifications? (Choose all that apply.)
A. Ductal carcinoma in situ (DCIS)
C. Secretory-type calcifications
2. Are any additional mammographic images needed?
A. Yes, magnification views are needed to assess these calcifications.
B. Yes, spot compression views are needed.
C. No additional views are needed for this finding.
D. Yes, the patient should have true lateral (ML) projections to check for layering.
3. What is your recommendation and Breast Imaging Reporting and Data System (BI-RADS) category?
A. Routine annual mammogram: BI-RADS 2
B. Recall for additional views: BI-RADS 0
C. Short-interval follow-up: BI-RADS 3
D. Recommend biopsy: BI-RADS 4
4. Is this condition in a spectrum of ductal carcinoma or in a spectrum of benign disease?
A. Because this condition involves the duct, it is related to ductal carcinoma.
B. Calcifications in a ductal distribution are always of concern for ductal carcinoma.
C. This is a benign condition of the ducts.
D. There is overlap between this condition and ductal carcinoma in situ.
ANSWERS
CASE 35
Secretory Calcifications
1. C
2. C
3. A
4. C
References
Bassett LW, Jackson VP, Jahan R, et al. Diagnosis of Diseases of the Breast. Philadelphia: Saunders; 1997.
Cardenosa G. The Core Curriculum. Breast Imaging. Baltimore: Lippincott Williams & Wilkins; 2004.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:81.–83
Comment
Secretory-type calcifications are one of the benign types of calcification commonly seen on the mammogram. The calcifications are located histologically in the periductal cells and are associated with chronic inflammation. The ducts are dilated and may be filled with secretions. This process develops over time, and the calcifications are more commonly seen in the older population. A condition termed plasma cell mastitis, described in the literature of the early 20th century, described this condition when it manifests as a palpable mass due to the inflammatory response.
If the calcifications have the typical smooth, rodlike forms, usually greater than 1 mm in diameter, such as seen here (see the figures), then no further work-up is needed. These calcifications can have lucent centers, can taper (cigar-shaped), and can branch. This is a benign condition, the condition does not typically produce symptoms, and it is given a BI-RADS score of 2, with routine annual mammography recommended.
Occasionally, there can be some doubt about the etiology of linear calcifications, with concern for possible DCIS. The calcifications of DCIS are typically thinner and more irregular, with a crushed-stone or snakeskin appearance. The calcifications of DCIS can also follow a ductal or segmental distribution. If the etiology is in doubt, magnification views can be performed.
CASE 36
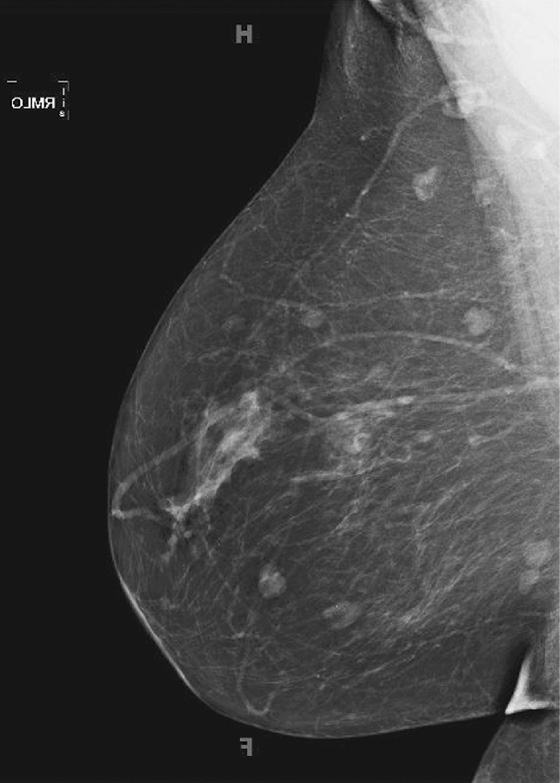
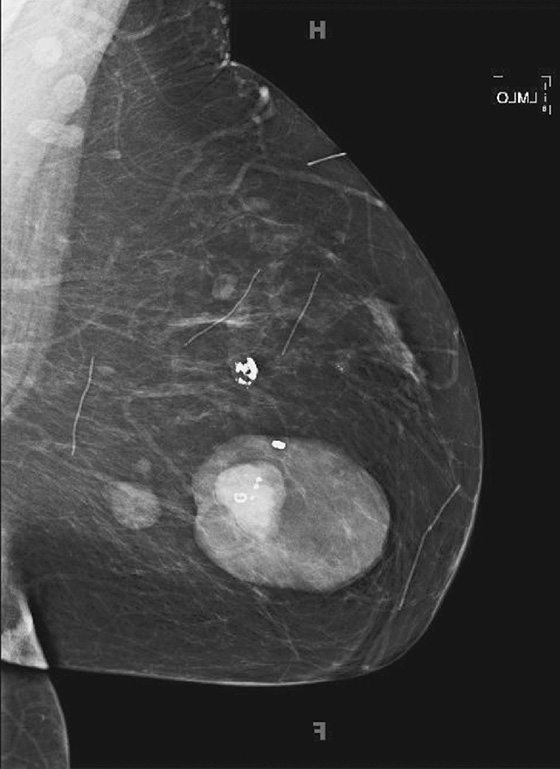
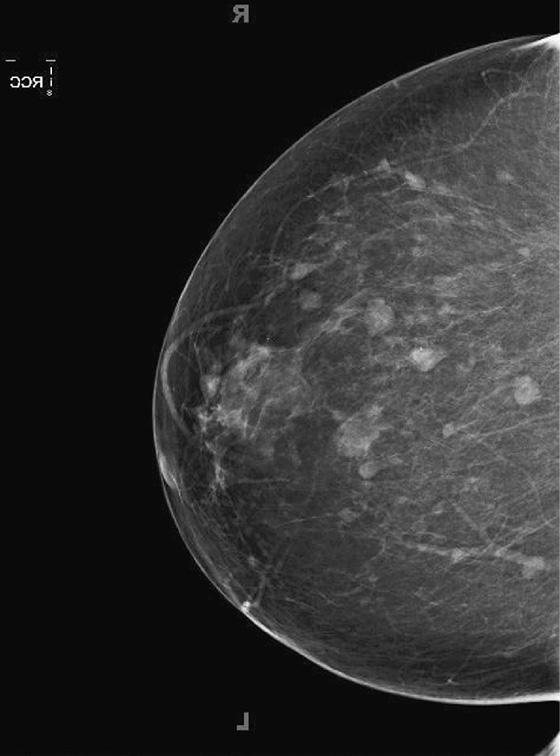
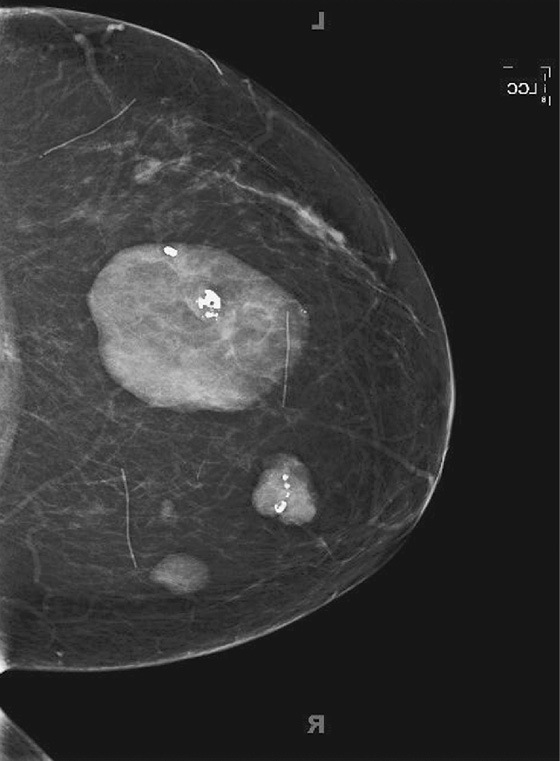
History: A 56-year-old asymptomatic patient presents for a routine baseline screening mammogram.
1. What is the differential diagnosis in this patient? (Choose all that apply.)
A. Multiple bilateral fibroadenomas
2. What work-up is indicated?
B. MRI is needed to evaluate for the typical enhancement pattern of fibroadenomas.
C. Biopsy of the largest mass should be performed.
3. What type of calcifications is seen on this mammogram?
A. Suspicious pleomorphic calcifications
B. Indeterminate, coarse heterogeneous calcifications
C. Benign popcorn-type calcifications
D. Clustered calcifications typical of fat necrosis
4. How often is this finding multiple or bilateral?
ANSWERS
CASE 36
Multiple Fibroadenomas
1. A and B
2. D
3. C
4. C
References
Campbell RE. Image interpretation session: 1993. Fibroadenoma of the breast. Radiographics. 1994;14(1):209–210.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:117–120.
Comment
This asymptomatic patient has multiple bilateral benign masses (see the figures). The fibroadenoma is the most common solid mass of the breast and is benign. Characteristically, fibroadenomas are round or oval, with sharply circumscribed, smooth borders. The contour can be macrolobulated. The density is equal to that of breast tissue on the mammogram. They are composed of stromal (fibrous) and epithelial (adenoma) elements. Some of the masses contain the classic calcifications seen in fibroadenomas, termed popcorn calcifications. The calcifications are thought to look like popped kernels of corn. The calcifications can reflect sclerosis and hyalinization of the mass.
Calcifications commonly form in fibroadenomas and help in characterizing the mass as benign, Breast Imaging Reporting and Data System (BI-RADS) 2. They can form initially in the periphery of the mass, and then become large and dense. In the early stage of the calcifications’ development, they may be faint and irregular and difficult to differentiate from malignant calcifications. In that case, they should be biopsied. Malignancy in a fibroadenoma is rare, occurring in about 1 in 1000 cases.
Fibroadenomas can be multiple and bilateral. In the dense breast, they may be difficult to distinguish from the background fibroglandular tissue, because the density is the same. Ultrasound can be used for further evaluation. On ultrasound, the mass is typically hypoechoic or isoechoic to the surrounding fat and is oval, with the long axis of the mass parallel to the skin (wider than tall) and a thin capsule.
CASE 37
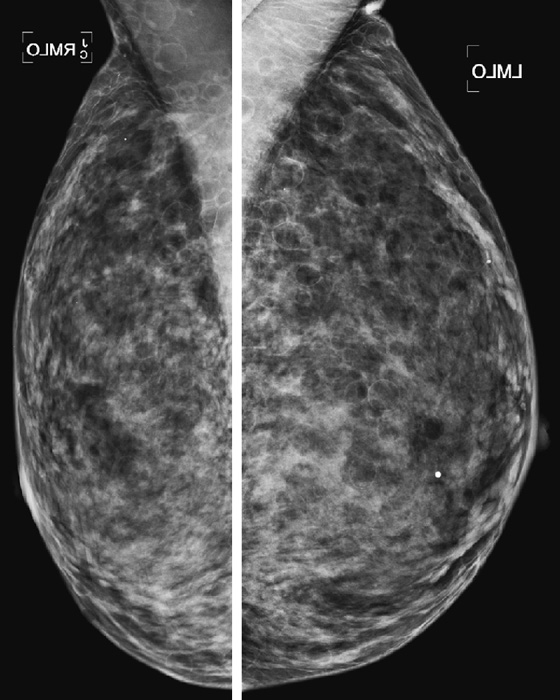
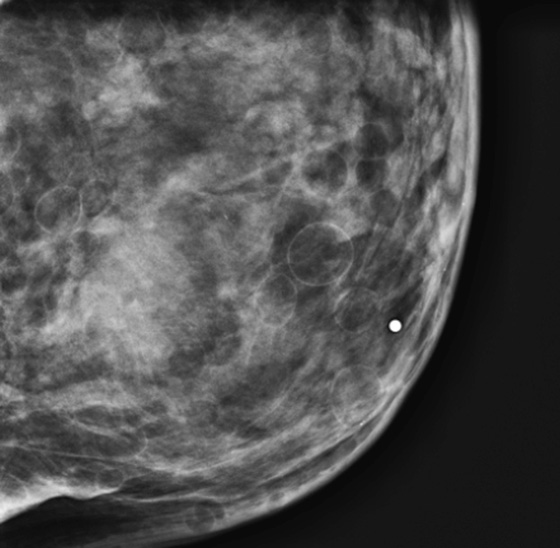
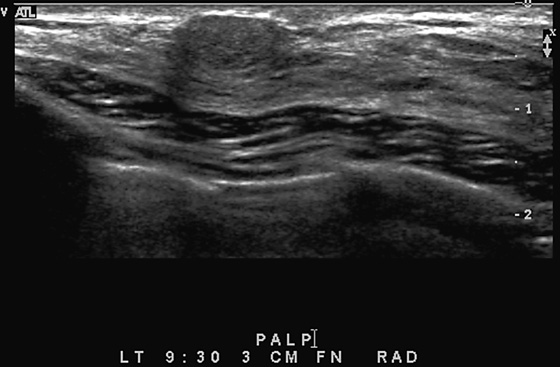
History: A 41-year-old woman presents for a routine screening mammogram.
1. What should be included in the differential diagnosis of the mammogram and ultrasound images shown? (Choose all that apply.)
A. Normal mammogram with multiple lipomas
B. Normal mammogram with multiple oil cysts
C. Normal mammogram in a woman with steatocystoma multiplex
D. Benign mammogram with multiple simple breast cysts
2. Where are the lesions of steatocystoma multiplex located?
3. How does a patient acquire steatocystoma multiplex?
A. It is the sequela of a skin infection.
B. It is the result of trauma, such as a burn.
C. It either arises spontaneously or is an inherited skin condition.
D. It is a reaction to one of many allergens.
4. What is the BI-RADS (Breast Imaging Reporting and Data System) code and recommendation for this mammogram?
A. BI-RADs 1—normal, routine screening recommended
B. BI-RADS 2—benign, routine screening recommended
C. BI-RADS 3—probably benign, short-interval follow-up recommended
D. BI-RADS 4—suspicious, biopsy of one of the lesions recommended
ANSWERS
CASE 37
Steatocystoma Multiplex
1. A, B, and C
2. B
3. C
4. B
References
Cao MM, Hoyt AC, Bassett LW. Mammographic signs of systemic disease. Radiographics. 2011;31(4):1085–1100.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:137.
Comment
Steatocystoma multiplex is a rare skin disorder that is a malformation of the pilosebaceous unit of the mid to deep dermis. The result of this malformation is the development of keratin-filled cysts, which are recognized on a mammogram as oval radiolucent masses with a thin high-density rim. In 40% of cases, the condition is inherited as an autosomal dominant trait. It is seen in men and women. Typically, the cysts involve the upper anterior chest and axilla but can also involve the groin.
The differential diagnosis of a fatty cyst includes lipoma, oil cyst, fat necrosis, and galactocele. The differentiation of this condition from other fatty lesions is not difficult because the others listed do not involve the skin, and they are usually not so numerous. A sebaceous cyst or epidermal inclusion cyst is located in the dermis and can contain fat, but it is unusual to have so many lesions (see the figures).
This condition can lead to palpable findings, and if the patient presents with a palpable finding, ultrasound should be performed. Ultrasound of the lesions of steatocystoma multiplex shows an oval hypoechoic or anechoic mass in the skin, with smooth, sharp margins. Deeper cystic masses may extend into the subcutaneous fat (see the figures).
Patients should be followed routinely because there is no association with breast disease. No work-up is needed.
CASE 38
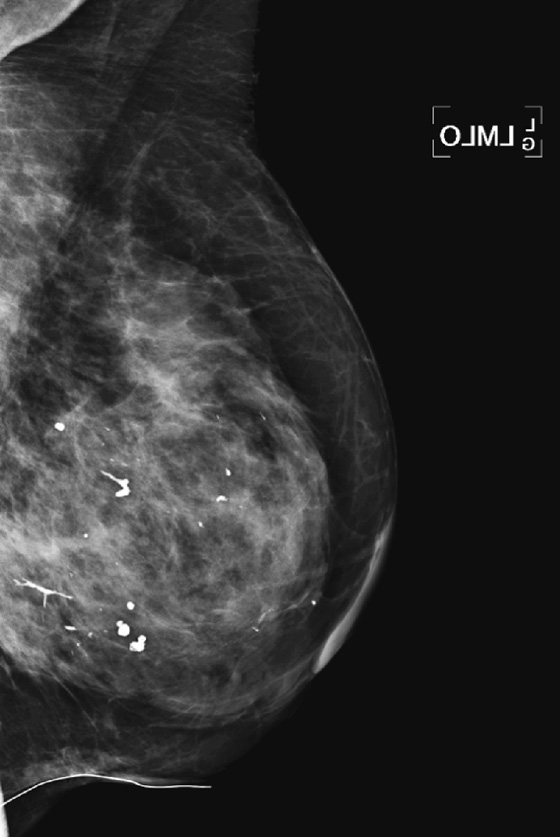
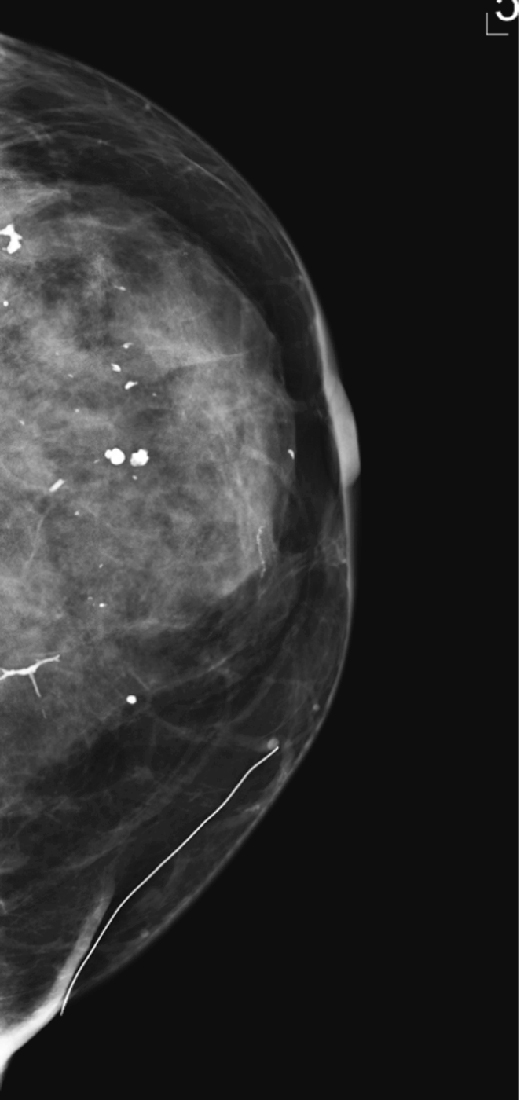
History: A 70-year-old woman with a history of surgery in the left breast.
1. What is the differential diagnosis of the branching calcification in the center of the breast on this view? (Choose all that apply.)
B. Recurrent malignancy, ductal carcinoma in situ (DCIS)
C. Secretory-type calcifications
2. Why is it important to recognize suture calcification?
A. The calcification can mimic recurrence at a lumpectomy site.
B. The surgeon should be alerted to remove this calcification.
C. Calcified suture often explains the clinical finding of palpable mass at the surgical site.
D. It must be differentiated from secretory-type calcifications, which are suspicious.
3. What other types of calcifications are seen on this patient’s mammogram?
A. There are suspicious calcifications near the lumpectomy site.
B. There are coarse, benign calcifications scattered in the breast.
C. There are extensive vascular calcifications in the breast.
D. There are calcifications diagnostic of fat necrosis in this breast.
4. What is the most important consideration in evaluating the mammogram of a patient who has had breast conservation therapy for cancer?
A. To assess for the presence of benign findings and report them
B. To recognize signs of recurrent or residual disease in the breast
C. To report any evidence of fat necrosis.
D. To recommend adjunct ultrasound and MRI for these high-risk patients
ANSWERS
CASE 38
Suture Calcifications
1. B, C, and D
2. A
3. B
4. B
Reference
Davis SP, Stomper PC, Weidner N, Meyer JE. Suture calcification mimicking recurrence in the irradiated breast: a potential pitfall in mammographic evaluation. Radiology. 1989;172:247–248.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:404–407.
Comment
The importance of suture calcification lies in the need to differentiate it from recurrent disease at the lumpectomy site. Recurrence often takes the form of calcifications, which should be recognized as linear, branching, or clustered, as DCIS is recognized anywhere in the breast. It is common for developing calcifications to be seen at the lumpectomy site, especially punctate calcifications or calcifications typical of fat necrosis (rim calcifications and coarse irregular forms). Suture calcification is relatively rare. Benign calcifications at the lumpectomy site can develop early or can take years to develop, and they most often result from fat necrosis due to microvascular damage caused by surgery and radiation.
Suture material is an artifact and causes an abnormal density in the breast. It does not usually calcify, but the shape of the calcification should suggest the diagnosis, especially when a knot is seen (see the figures). In the early stage of development of calcifications, there can be doubt about the etiology, and magnification views should be performed if there is any question about the calcifications, to exclude DCIS from the differential diagnosis. If the calcifications are considered indeterminate after magnification views, a decision needs to be made if they are low suspicion (Breast Imaging Reporting and Data System [BI-RADS] category 3, 6-month follow-up recommended) or higher suspicion (BI-RADS 4, biopsy should be considered). In these patients stereotactic biopsy should be considered as the first choice for biopsy method, rather than re-excision.
CASE 39
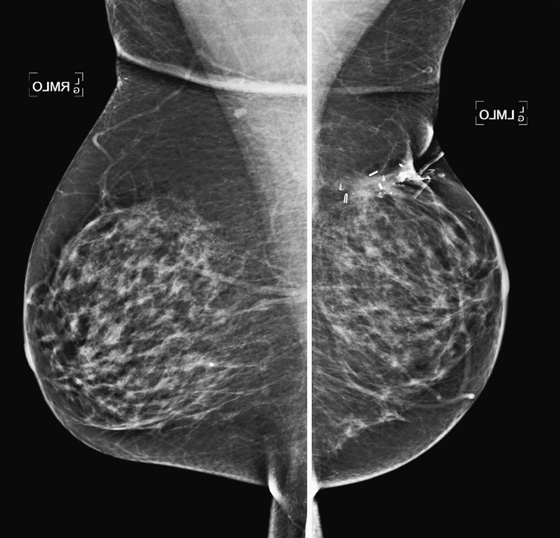
History: A 60-year-old woman who had lumpectomy for breast cancer 4 years ago now presents for routine bilateral mammogram.
1. What should be included in the differential diagnosis for the mammogram? (Choose all that apply.)
A. Normal postlumpectomy mammogram
B. Evidence of left lumpectomy, with expected changes after treatment
C. Evidence of left lumpectomy, with calcifications consistent with fat necrosis
D. Recurrent malignancy at the lumpectomy site
2. What is the next step in management?
A. MRI to check for enhancement at the lumpectomy site
B. Ultrasound to check for evidence of mass at the lumpectomy site
C. Magnification views of the lumpectomy site
D. Short-interval follow-up in 6 months
3. If image-guided biopsy is chosen, how is the finding targeted?
D. Needle localization for surgical excision
4. If the histology of the needle biopsy is fat necrosis, what is the next step?
A. Routine annual follow-up because the finding is concordant
B. Referral for surgery because the biopsy histology is nonconcordant
C. Short-interval follow-up in 6 months for concordant histology
D. MRI of the breast to check for abnormal enhancement
ANSWERS
CASE 39
Postlumpectomy Fat Necrosis
1. C and D
2. C
3. A
4. C
References
Geiss CS, Keating DM, Osborne MP, et al. Comparison of rate of development and rate of change for benign and malignant breast calcifications at the lumpectomy bed. AJR Am J Roentgenol. 2000;175(3):789–793.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:313.
Comment
Breast conservation therapy is a common treatment for breast cancer. Surgery to remove the cancer (lumpectomy) and radiation therapy to the whole breast or as a partial-breast irradiation constitute one local therapy for breast malignancy. This therapy causes changes in the breast—initially a seroma and hematoma, followed by scarring, retraction, and skin thickening and breast edema (see the figures). Calcifications may develop at the lumpectomy site, which may represent recurrence of the malignancy or fat necrosis.
It can be difficult to differentiate fat necrosis from malignancy. The timing of development of the calcifications may suggest an etiology. Studies have shown that benign calcifications typically develop earlier; in one study, the median time was 23 months, and the median time for development of malignant calcifications was 39 months. The morphology is also important: Punctate, or coarse, curvilinear calcifications are typically benign shapes, whether associated with a normal patient or a patient following lumpectomy. Pleomorphic, faint, linear, and branching calcifications are suspicious whether they are seen in a breast that has had surgery or one that has not. If developing calcifications are suspicious or indeterminate, stereotactic biopsy should be performed.
The calcifications developing in this patient 4 years after treatment are pleomorphic and in linear configuration and were thought to be indeterminate (see the figures). Stereotactic biopsy was performed, and the histology was fat necrosis. Two biopsy clips seen on the images were placed at the time of biopsy. The tissue diagnosis was believed to be concordant, and 6-month follow-up magnification views were recommended because there are remaining calcifications that were not sampled.
CASE 40
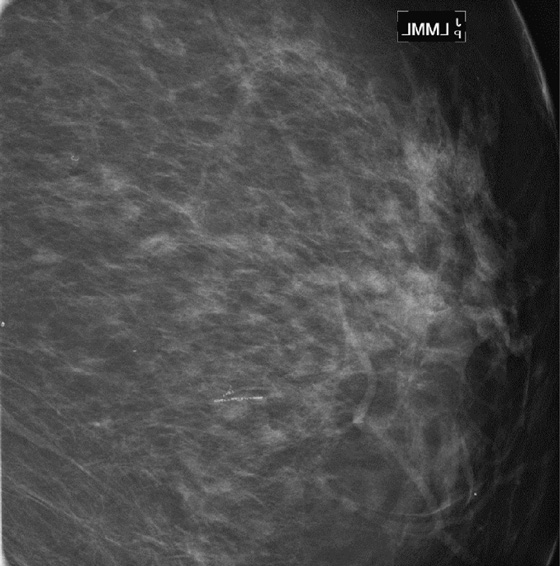
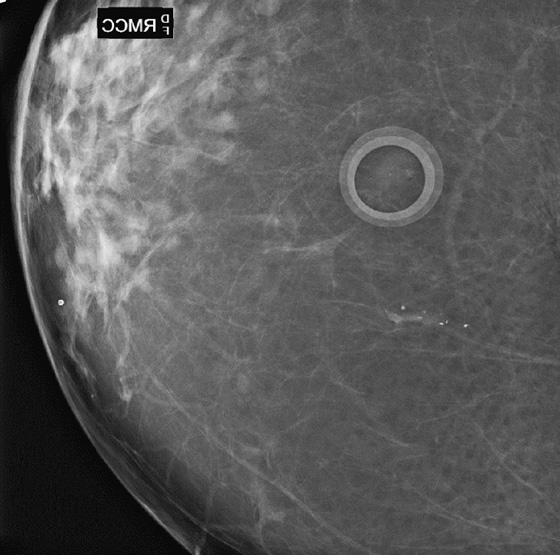
History: Magnification views of microcalcifications in two patients, discovered on screening mammogram, recalled for additional views.
1. What is the differential diagnosis for these calcifications? (Choose all that apply.)
C. Ductal carcinoma in situ (DCIS)
D. Atypical ductal hyperplasia
2. What is the BI-RADS (Breast Imaging Reporting and Data System) designation for these two cases?
B. BI-RADS 5—highly suspicious
3. Is any additional work-up needed prior to biopsy in these two cases?
A. Yes, ultrasound should be performed.
B. Yes, MRI should be performed.
C. Yes, both ultrasound and MRI should be performed.
D. No, biopsy is the next step.
4. You perform the stereotactic biopsy, and the result is DCIS. What is the next step?
A. Nothing. Your diagnosis is complete.
B. You must inform the patient and her referring physician of the diagnosis.
C. You must inform the patient and direct the definitive management of her cancer.
D. Nothing. DCIS is not invasive cancer, so it can be managed expectantly with follow-up mammograms.
ANSWERS
CASE 40
Suspicious Microcalcifications
1. A, C, and D
2. D
3. D
4. B
References
Berg WA, Arnoldus CL, Teferra E, Bhargavan M. Biopsy of amorphous breast calcifications: pathologic outcome and yield at stereotactic biopsy. Radiology. 2001;221:495–503.
Sickles EA. Breast calcifications: mammographic evaluation. Radiology. 1986;160:289–293.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:65.
Comment
In evaluating calcifications, magnification views are essential. When performed with a proper technique, the views provide information to help determine whether the calcifications are more likely to be benign or malignant. If microcalcifications are seen on a conventional screening mammogram, it is important to recall the patient for additional magnification views rather than to try to make a diagnosis on the standard screening images.
Magnification views are often performed in the 90-degree lateral view to check for layering. If the calcifications layer on this view (milk of calcium), they can be reliably characterized as benign, needing no further evaluation.
Differentiating benign from suspicious calcifications is a step-by-step process. The first step is to analyze the magnification views and determine if the calcifications fall into any of the definitely benign categories (round, eggshell, coarse linear, and popcorn). The next step is to decide if the calcifications are suspicious for malignancy. Suspicious calcifications are pleomorphic and can have irregular margins or broken-glass shapes. Calcifications of ductal carcinoma in situ (DCIS) form inside the duct, making casts, and can branch, following the course of the duct (see the figures). In patient one, these cast-type calcifications are seen.
Calcifications that are neither definitely benign nor suspicious may be termed indeterminate for malignancy. In this category are coarse, heterogenous, and amorphous forms, which are faint, small, hazy calcifications. If their distribution is linear or segmental, which are more suggestive of malignancy, biopsy is recommended. In patient two, although the calcifications were not casting, their somewhat linear configuration on both views was considered suggestive for DCIS, and biopsy was performed.
The distribution of the calcifications, as well as the individual morphology, is important. Distribution is categorized as follows (in order of increasing level of suspicion):
1. Diffuse, distributed throughout most of the breast
2. Regional, distributed in an area at least 2 cm of the breast, not in ductal distribution
3. Grouped or clustered (at least five calcifications in less than 1 cm3 of tissue)
The casting-type calcifications in the first patient are DCIS (see the first and second figures). She went on to surgical and oncologic management. The calcifications in patient two (see the third and fourth figures) were benign and were not related to the duct. No further treatment was needed.