CHAPTER 4 The Neural Bases of Acupuncture: Central Mechanisms
BASIC CONCEPTS: BRAIN, ORGANS, AND ACUPUNCTURE
New Imaging Techniques and Understanding the Brain
A vast amount of clinical experience with traditional acupuncture has been gained over thousands of years.1–6 On the other hand, over the last several decades, Western medicine has progressed rapidly in both techniques and physiologic understanding of disease. The mechanisms of treatment have benefited from numerous newly developed medical techniques, such as X-ray computed tomography (XCT) and magnetic resonance imaging (MRI). With these developments it is now possible to visualize the inner organs of our living human body as well as the brain in vivo. More recently the development of functional imaging devices such as positron emission tomography (PET)7–9 and functional MRI (fMRI)10–12 has brought about a revolution in our understanding of the brain. PET and fMRI now allow us to image minute changes in glucose utilization as well as oxygen consumption in different parts of the brain. These techniques allow us to observe the brain’s energy metabolism and oxygenation status with a spatial and temporal resolution never before possible.9 Most importantly these technical advances allow us to investigate cortical responses of the brain as a function of acupuncture stimulation, such as the correlation of a specific stimulation with a specific cortical response.13–15 Direct observation of the human brain in vivo while the body is receiving acupuncture stimulation opens a new era in acupuncture research. In addition this information on cortical activation provides clues about how acupuncture works, that is, the nature of the mechanisms involved in acupuncture treatment. These remarkable developments have the potential to finally unravel the millennia-old enigma of acupuncture.
Neural Substrates of Pain Signal Processing
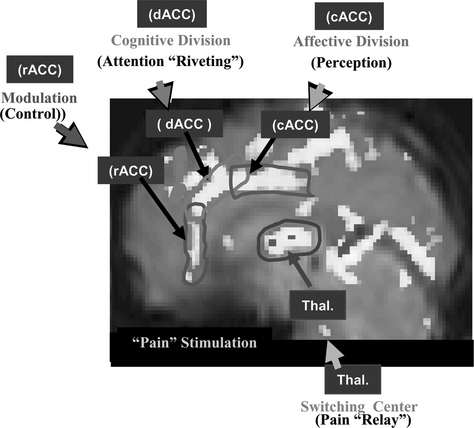
Let us examine the analgesic effect of acupuncture, one of the commonest modalities in acupuncture treatment. First it is necessary to study the basic pain mechanism, from which will follow an explanation of how AA works. In an experiment, pain stimulation was induced by immersion of the index finger into a bath of hot water (about 52° C) for a period of 30 seconds.16 The activation of cortical areas caused by pain stimulation was obtained by fMRI and is shown in Figure 4-1. Three areas are notably involved with pain signal processing and these are indicated by three rectangles (the dorsal anterior cingulate cortex [dACC], the caudal anterior cingulate cortex [cACC], and the rostral anterior cingulate cortex [rACC]) to illustrate more completely the details of the activation pattern. These three areas were chosen because they are believed to be the most significant cortical areas concerned with pain processing, that is, dACC for pain signal “riveting or attention-focusing,” cACC for “perception” of the emotional component of pain, and rACC for “modulation or control” of pain.
The thalamic areas, believed to be the major components of pain signal relay and attention selection centers, are also activated. A most intriguing aspect of this study is that the time-differential activation pattern provides important clues about how various parts of the cingulate cortex are involved in the perception and modulation of pain and in pain processing.16 For instance the rACC is activated during the last minutes of observation, suggesting that some form of the pain modulation process (e.g., the release of endogenous opioids, which initiate the inhibitory process by a stress effect resulting from sustained pain) is taking place at this late period because of sustained pain, as discussed by Zubieta et al.17 Considering the fact that all the cingulate cortices, dACC, cACC, and rACC, together with the thalamus, are activated as a result of pain stimulation, one can hypothesize that the main part of the pain signal processing is a central process rather than a peripheral process. Pain signal relay, attention focusing, pain perception, and modulation or control are all processed in the brain’s center, the cortical and subcortical centers of the brain, including the ACC and the thalamus. This dynamic pain processing pathway appears to provide key information for understanding pain relief mechanisms, such as AA.
ACUPUNCTURE ANALGESIA AND ITS NEURAL SUBSTRATES
Observation of Acupuncture Analgesia by Functional Magnetic Resonance Imaging
Meridian (Homeostatic) Acupoint
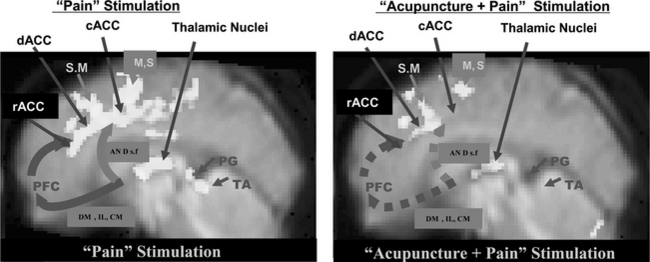
The pain and pain-relief mechanisms involved in AA are complex, and science has yet to provide a convincing physiological explanation of them. Although acupuncture has been used for many centuries,1–6 scientific evidence for the physiology and efficacy of pain treatment has not been established. Possible mechanisms for pain relief in AA have been studied in the West since 1965, beginning with the pioneering work of Melzack and Wall.3 Although rigorous scientific explanations are rare and other evidence is mostly anecdotal, acupuncture has been reported to successfully treat many classes of disease, and be especially effective for the control of pain.1–6,18 As discussed in the previous section, brain imaging tools such as PET and fMRI have made it possible to visualize directly brain function in vivo. fMRI experiments using the new data-processing technique known as dynamic regression analysis are providing the opportunity to study physiologically modulated cortical activation due to “pain stimulation” as well as “pain stimulation after the administration of acupuncture.”16 For the AA study, thermal stimulation was applied in the same way as in the pain study (i.e., immersing the index finger into a bath of hot water with a temperature of 52° C for a duration of 30 seconds). This stimulation resulted in a sequence of different sensations: feelings of heat, unpleasantness, extreme pain, and unbearable pain. Acupuncture stimulus was applied (or administered) to the meridian (homeostatic) acupoints H5 deep fibular (peroneal, Taichong) with manual twirling or rotating of the needle for 30 seconds (approximately one rotation per second), resting for 30 seconds without twirling the needle, and then repeating five times without removing the needle, after which the needle was removed for the remainder of the data acquisition period.19,20 Application of the pain stimulation was continued for 4 minutes after completing the acupuncture stimulation. From this experiment some new observations were made about the relationship between cortical activation and pain as well as the pain-relief mechanism of acupuncture. The experimental data suggest that the cortical areas related to pain signal relaying, attention-focusing or riveting, and perception are the anterior cingulate gyrus and the thalamic areas, as mentioned earlier.16 The experimental results obtained from “pain” and “acupuncture + pain” stimulation are illustrated in Figure 4-2. The activation pattern resulting from pain stimulation alone showed activation of various cortical areas, including the cingulate cortex, the thalamus, and the motor areas (see Figure 4-1). On the other hand, the “acupuncture + pain” experiment revealed a significant decrease in activation of the ACC and the thalamic areas as well as the motor areas. Most of the activations seen in the cingulate cortex and the thalamic nuclei with pain alone become diminished after acupuncture, suggesting that the administration of acupuncture indeed desensitizes or blocks pain perception.20
“Sham” Acupoints: Comparison of Meridian (Homeostatic) and “Sham” Acupoint Stimulation
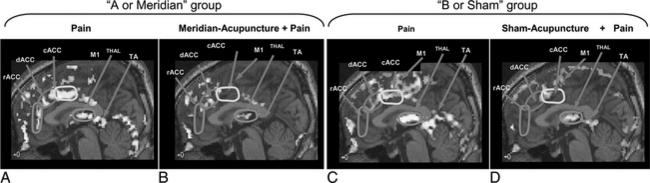
As shown in the previous study on “pain” and “pain with administration of acupuncture,” there appears to be substantial deactivation of the cortical areas when acupuncture is administered compared to when it is not, especially in areas involved with pain signal processing. One of the most interesting aspects of acupuncture, however, is the point specificity, that is, how accurately the acupoint must be localized and how the outcome of the acupuncture treatment is affected by uncertainty in localization. This question of point specificity was studied by comparing the result of traditional “meridian” acupoint stimulation with the result of stimulating “sham” points.19,20 Please note that the “meridian” acupoint Liv 3 Taichong used in these experiments is one of the 24 homeostatic acupoints (HAs) H5 deep fibular (peroneal) described in Chapter 5. All 24 of these HAs are in fact major meridian acupoints in the traditional acupuncture system (see Appendix II). “Sham” points are deliberately chosen as much as a few centimeters away from the traditional meridian acupoints. This study was a comparison of two sets of experiments, namely, one with “pain” versus “meridian acupuncture + pain” and the other with “pain” versus “sham acupuncture + pain.” Figure 4-3 shows the results.19,20
When the results of “pain” versus “sham acupuncture + pain” (see Figure 4-3, C and D) are compared with the traditional “pain” versus “meridian acupuncture + pain” results (see Figure 4-3, A and B), there appears to be very little difference, which suggests that sham and meridian acupuncture might share a common mechanism. This finding also brings into question the point specificity of acupuncture, at least in the case of AA.20
The similarity of the activation patterns in the two cases of meridian and sham acupuncture supports the hypothesis that the effect of acupuncture, at least for AA, is simply the effect of the stress-induced hypothalamic-pituitary-adrenal (HPA) axis response (the pathways are discussed more specifically below). That is, decreased activation may be due to “sustained pain stress” rather than to stimulation of a specific point as is taught in traditional acupuncture.17,21 Acupuncture or acupuncture-like sensory stimulation induces activation of the HPA axis and therefore activation of the endogenous central opiate circuitry, thereby reducing or inhibiting the ascending pain signals.
HYPOTHESIS OF ACUPUNCTURE MECHANISMS
Hypothesis of Acupuncture Mechanisms and Neural Substrates
Humoral, Neural, and Immune Responses Related to Acupuncture
As already described, acupuncture therapy has demonstrated efficacy in several clinical areas; among these is pain. Although the understanding of pain has progressed immensely in the last two decades,1–6,18 the underlying mechanisms of acupuncture in general and its analgesic effect in particular are still not clearly delineated. The leading hypotheses include the effects of local stimulation, neuronal gating, the release of endogenous opiates, and the placebo effect. Accumulating evidence suggests that the CNS is essential for the processing of these effects via its modulation of the autonomic nervous system (ANS), the neuroimmune system, and hormonal regulation. Because these processes are programmed into basic survival mechanisms, it appears vital to seek an understanding of the effects of acupuncture within a framework of neuroscience.
In view of the preceding results, we propose a model that incorporates the stress-induced HPA axis model of Maier et al22 and Akil et al,23 the cholinergic antiinflammatory action model of Tracey et al,24–26 and the recently observed neuroimaging results of Cho et al,19,20 Zubieta et al,17 and Petrovic et al.21 As already mentioned, over the last two decades there have been numerous developments in neuroscience concerning pain, the ANS, the immune system, and functional brain imaging.27–33 The underlying mechanisms of acupuncture, however, have appeared too diverse to be understood in a quantitative and rational manner and require further study. Because acupuncture therapy is claimed to be effective in many conditions, including acute pain, investigators have attempted to explain the underlying mechanism of acupuncture in many different ways: as an effect of local stimuli, a neural reflexive response, a humoral outflow response, and a placebo effect.1–6,34 In addition, physiologic studies using animal models and human subjects have also been developed to explain AA and these have generated increasing scientific support and enhanced the credibility of acupuncture as a treatment option.1–6
One clear fact is that the CNS controls the autonomic processes related to visceral reactivity; cognitive processes related to pain perception, learning, and memory; and other physiologic processes related to pain. Regardless of which pathways are being contemplated, whether it is supposed to work by neural, humoral, or neuroimmunologic interactions, it appears inevitable that the brain is involved in the behavioral effects of acupuncture. The CNS is precisely the network that is essential for information processing between afferent stimuli (inputs) and efferent responses (outputs), as well as control of stress hormones, the ANS, and even the immune system, all of which are essential for survival. On the other hand, recently developed brain imaging techniques, such as PET and fMRI, are beginning to shed light on the workings of the human brain, including acupuncture-related cortical responses.7–15 Because these techniques provide the opportunity to observe neuronal activities directly, both spatially (anatomically) and temporally (in a time-dependent manner), they allow quantitative analysis of the living brain while it is engaged in deliberate actions (e.g., acupuncture or acupuncture-like stimulation). As a result recent acupuncture research has been directed increasingly toward neuroimaging, with molecular science and pharmacokinetics as its bases.16–20
In this section, we briefly review how the scientific understanding of acupuncture has recently evolved based on neuroimmunophysiologic and molecular aspects, and evidence obtained by functional brain imaging.13–21,27–34 In addition, we propose a hypothesis, as well as a model, of the acupuncture mechanism that includes the stress-induced HPA-axis hypothesis,22,23 the neural-immune interaction of the cholinergic antiinflammatory activity hypothesis,24–26 and the recently obtained neuroimaging evidence.17,19–21
Immune Neural Interaction and the Hypothalamic-Pituitary-Adrenal Axis Hypothesis
Let us first look at the recent series of studies conducted by Maier et al and Tracey and colleagues,22–26 which describe the interactions between the immune system and the brain as well as the effects of the ANS on immune functions; they also begin to show how the brain controls certain types of inflammation and therefore pain. In particular, Tracey’s review provides interesting and important clues for the formulation and understanding of acupuncture mechanisms. For example, it explains that inflammatory information is transmitted through sensory nerves to the hypothalamus, where input signals are processed, resulting in an antiinflammatory output via the humoral system and the ANS via the well-known HPA axis, as briefly described earlier. The similar idea that acupuncture is involved in the immune system has recently been supported by observations under various clinical conditions, and it has been suspected that acupuncture might affect immune modulation.27,28 Although actual scientific evidence has yet to be confirmed, the work on neuroimmune and autonomic reflexes by Maier et al (see also Besedovsky et al29) and Tracy and colleagues could form an important basis for understanding the acupuncture mechanism as a neural-immune reflex.24–26 More specifically they have discovered that tumor necrosis factor alpha (TNF-α) and other cytokines (e.g., interleukin-1β) exist in the brain and directly interact with the inflammatory immune system in the periphery or vice versa (i.e., from periphery to brain). By communicating with the brain it produces neural outflows via humoral and ANS pathways.23–26 For example, parasympathetic nerve endings release acetylcholine (ACh), which has a resulting antiinflammatory effect; that is, the immune system interaction appears to suppress the synthesis of inflammatory cytokines, such as TNF-α and interleukin-1β (IL-1β). This observation of the cholinergic suppression of inflammatory cytokines is a new factor that could play an important role in understanding the mechanisms of acupuncture.24
In addition, the blood-borne pathway contributes via the circumventricular (CV) area, including the postrema and subfornical organs.23 The dorsal vagal complex and the dorsal motor nucleus of the vagus nerve are also known to respond to circulating TNF concentrations and activate the HPA axis, thereby inducing the release of glucocorticoids and other hormonal substances (e.g., melanocyte-stimulating hormone [MSH], spermine), which subsequently further suppress inflammatory cytokine synthesis such as IL-1b and TNF-α.24,25
Different Modes of Acupuncture or Acupuncture-like Stimuli
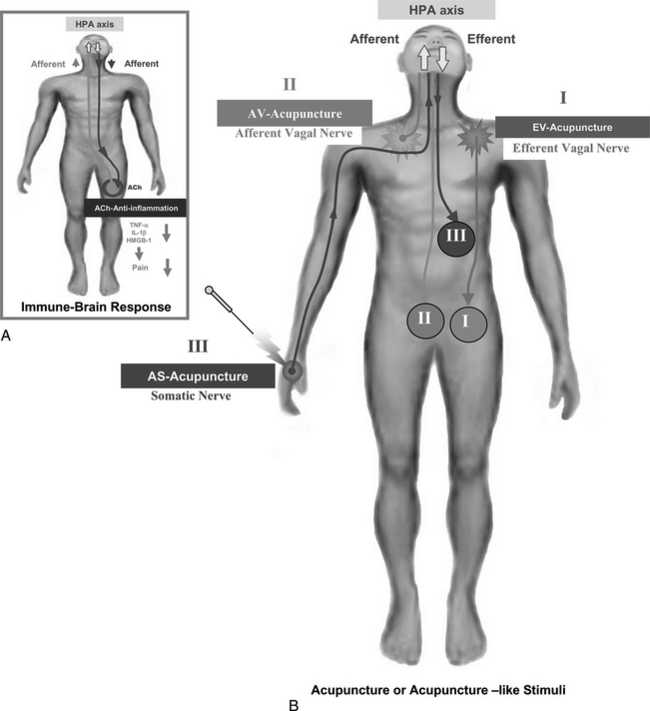
Based on Tracey’s concept of vagus nerve stimulation, and including possible reflexive factors that have an antiinflammatory effect on the inflamed area,24,25,29 the hypothesis of afferent vagus nerve stimulation can be extended to incorporate somatic stimulation. In other words, the afferent visceral and somatic acupuncture signals that can be transmitted to the supraspinal level for the induction of the reflexive antiinflammatory signals, through both humoral and neural mechanisms, could be important components of acupuncture. These are summarized in Figure 4-4. In other words, several different modes of acupuncture or acupuncture-like sensory stimuli can be noted as follows:
Through each different afferent and efferent stimulus, a number of distinctive descending antiinflammatory signals, both direct and reflexive, will be induced (see antiinflammatory efferent shown in the inset of Figure 4-4) as a result of the afferent cytokine signaling of TNF-α, IL-1β, and high-mobility group B-1:
As Tracey and colleagues demonstrated, efferent vagus nerve stimulation (e.g., EV acupuncture) resulted in an increase of efferent vagus nerve cholinergic activity, which had an antiinflammatory effect as a result of suppressing inflammatory cytokines, such as TNF-α, IL-1β, and HMGB-1. These facts suggest that afferent vagus nerve stimulation, or AVA, would work in a similar way, possibly via the supraspinal reflex (the HPA axis), and would have a more global and nonspecific reflexive effect confined mostly to the visceral organs. The most widely used acupuncture stimulation, however, appears to be ASA by the insertion of acupuncture needles into the cutaneous tissues or skin and muscle. One could expect that it might also have a similar effect, possibly with even broader and more diffuse efferent nerve activities, covering both generalized sympathetic and vagus or parasympathetic nerve activities, as shown in the efferent nerve signals, such as signal III (see Figure 4-4). The latter may also work via the HPA axis (see discussion of neural substrates and afferents below, and shown in Figure 4-5).
Afferents to the Paraventricular Nucleus of the Hypothalamus and the Neural Substrates
To substantiate the preceding discussions, it is worthwhile to look at the stress-related circuit, which could provide important clues. The most promising model so far is that of the stress-induced HPA cortical or adrenal medullary (HPA) axis.22,23 We propose to extend this model to a broad-sense HPA (BS-HPA) axis, which includes the neuroimmune interactions, ANS outflows, and other neural projections, such as the projection to the periaqueductal gray-raphe axis. This model appears appropriate because the hypothalamus is also the origin of the ANS as well as other neural outputs.35
It is well known that vagus nerve stimulation may have a reflexive antiinflammatory effect on an inflamed area. This discussion extends the afferent vagus nerve stimulation hypothesis to the somatic stimulation, including the ASA, which will be projected to the supraspinal level for the induction of the reflexive antiinflammatory signals through the BS-HPA axis, that is, via the neuroimmune interactions, humoral system, ANS, and other neural systems.22–26
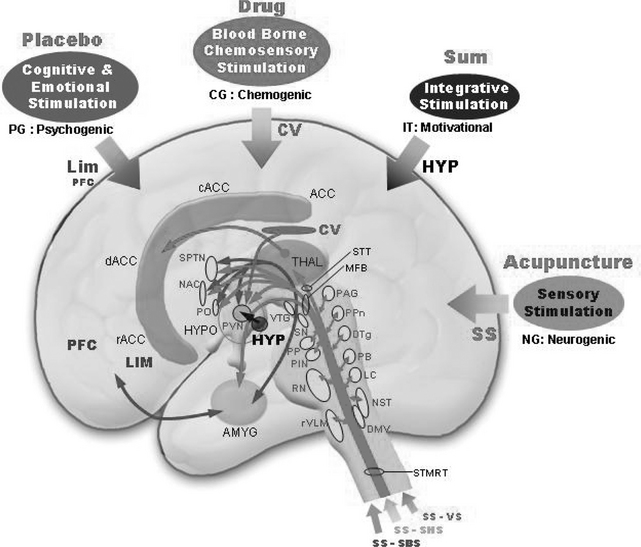
In summary, four major input signals are possibly involved in the stress-induced HPA axis: the sensory stimulus (via the somatic and vagus nerves), the cognitive and emotional stimulus (via the prefrontal area and limbic system), the blood-borne chemosensory stimulus (via the circumventricular area), and the final integrative component (via the hypothalamus). These inputs are believed to be projected to the paraventricular nucleus (PVN; the medial parvocellular part) of the hypothalamus, which would then generate the outflows.23 The cognitive and emotional stimulus might have a relationship to a psychogenic stimulus, such as a placebo,21 whereas the blood-borne chemosensory stimulus is related to drug administration. The somatic and vagus nerve stimulation can be considered neurogenic stimulation, which possibly can be related to acupuncture or acupuncture-like sensory stimuli. The internal hypothalamic inputs to the PVN, such as the daily rhythmic variation of glucocorticoids and the condition of hunger (motivational) or satiety, could be important adjuncts to the modulation of the various effects of input stimuli, including acupuncture.
These four inputs then converge onto the PVN, that is, from the group that consists of the limbic system and the prefrontal cortex, the circumventricular (CV) area, the sensory stimuli pathways (SS), and the hypothalamus itself (HYP) (see Figure 4-5). These four inputs are shown together with various nuclei possibly involved with activation of the stress-induced HPA axis. Because our discussion here is limited to acupuncture, we will also limit it to sensory stimulation. Among the four afferents, the major afferents related to acupuncture needling are the SS pathways. Sensory stimulation consists of three major inputs or afferents, which will be projected to the PVN, possibly via activation of various neurons in the brainstem area. These pathways include one from the body (somatic-body sensory, SS-SBS); one from the head and facial regions (somatic head and special-sensory afferents, SS-SHS, such as the visual and auditory pathways); and the visceral afferent (visceral sensory, SS-VS). These are probably the sensory stimuli that are acupuncture-related, and we call them neurogenic components, except for some parts of the visual and auditory aspects of SS-SHS. Most of the bodily somatic sensory stimuli excite the midbrain periaqueductal gray (PAG), the peduncular pontine (PPn), the dorsal tegmental nucleus (DTg), and the parabrachial nucleus (PB), among others. Some of the nociceptive inputs are believed to activate the PAG from where a projection is made to the PVN. Both the PPn and the DTg neurons are cholinergic and are believed to be projected to the PVN. The facial somatic inputs are also believed to terminate on the same areas (PAG, PPn, DTg). Some of the special sensory stimuli are believed to terminate at the peripeduncular nucleus (PP) and the posterior interpeduncular nucleus (PIN) as well as the PPn and the DTg. Because of the geometric proximity, visceral inputs are believed to terminate in and excite the dorsal and ventral medullary areas, including the nucleus of the solitary tracts (NST, areas A2 and C2) and rostral ventrolateral medulla (rVLM, areas A1, C1, C3). In the dorsolateral medulla, the NST and the LC (locus caeruleus) are the two major noradrenergic neuronal sites, and their outputs are believed to project to the PVN via the parabrachial nucleus following the medial forebrain bundle (MFB). Neurons at the lower and rostral ventrolateral medullary (rVLM) areas are adrenergic (C1, C3) and project to the PVN, again via the MFB. Serotonergic neurons in the dorsal raphe are also believed to be projected to the PVN as well as to the ventral raphe nuclei (B6, B7, B8), although direct involvement of these nuclei with the PVN is not known. In addition, the somatic sensory information conveyed to the thalamus, especially to the ventroposterolateral (VPL) or ventroposteromedial (VPM) nucleus of the thalamus, projects to the corresponding somatosensory cortex and then possibly to the secondary somatosensory cortex.
Parts of the neuronal projections from the PAG and other cholinergic neurons also project to the midline thalamic areas, such as the dorsomedial and intralaminar nucleus as well as the anterior nucleus of the thalamus, and then project to the ACC and also to the prefrontal cortex. The ACC is believed to be the signal processing center for incoming pain signals, including attention focusing, perception of the affective component of pain, and modulation.19,20 Painful stimuli are believed to project to both the somatic and visceral afferents and also to the amygdala, the pain activating center, and finally to the PVN via the stria terminalis as shown in Figure 4-5. The hippocampus (not shown) is believed to play a key role in the negative feedback of glucocorticoids in the HPA axis.29 These inputs, plus emotional and motivational information generated from the hypothalamus itself, are believed to be integrated by various nuclei within the hypothalamus. They project to the PVN, which then generates the outflow, as discussed in the following section.
Those four major inputs (SS, LIM, CV, and HYP) are shown in Figure 4-5. We can conceptualize these connections in common lay terms, such as placebo, drug, motivational, and acupuncture. For example, cognitive and emotional stimulation might have a relationship to more generally known psychogenic stimulation, such as the placebo effect, whereas the blood-borne chemosensory stimulation has a relationship to drug administration. Similarly the somatic and vagus nerve stimulation can be considered a form of related neurogenic stimulation, such as acupuncture needling.
Finally the internal hypothalamic inputs to the PVN, such as the daily rhythmic variation of glucocorticoids and the hunger (motivational) or satiety condition, could be an important adjunct to modulation of the various perceptual and healing effects of acupuncture. When the body has an insufficient level of glucocorticoids, it is most sensitive to input stimuli and also most vulnerable to the changes it encounters. The HPA axis is therefore most sensitive to the change of glucocorticoid level when the level is lowest, such as in the evening.23
Efferents (or Outflows) from the Hypothalamus
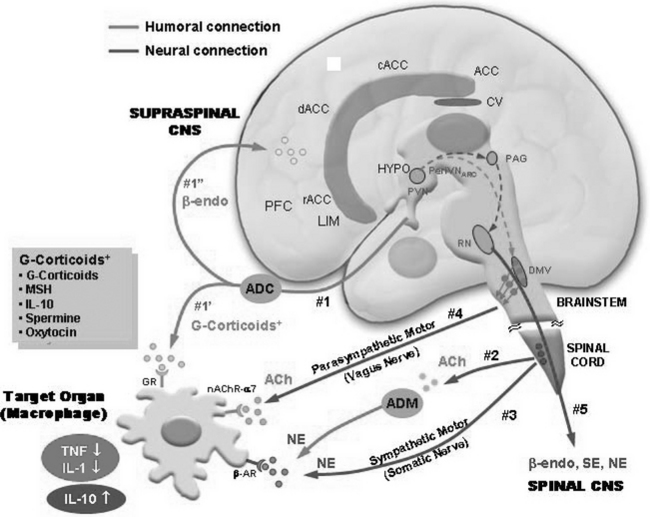
Major efferents or outflows from the PVN (and also perhaps from the PVN and the arcuate nucleus of the hypothalamus) that possibly result from acupuncture or acupuncture-like sensory stimuli may be conveyed via five distinctive pathways. The first group of pathways (group 1) is humoral, originating from the traditional HPA axis and projecting to the various organs in the body and the brain via the bloodstream. One is the pathway to the inflammatory area where immune-related leukocytes, such as macrophages, are induced, and the other is the pathway to the supraspinal CNS, as shown in Figure 4-6. The humoral pathway (no. 1) targets the macrophages in the inflammatory area and releases a number of antiinflammatory cytokines and hormones, including antiinflammatory cytokines such as IL-10, glucocorticoids, melanocyte stimulating hormone, spermine, and oxytocin. These cytokines and hormones act on the macrophages as suppressors of inflammatory cytokine synthesis, such as synthesis of TNF-α, IL-1β, and HMGB-1 at macrophages, thereby reducing inflammation and pain. Another humoral outflow (outflow no. 1) is the β-endorphin-releasing humoral pathway. Because corticotrophin releasing hormone (CRH) from the PVN of the hypothalamus activates proopiomelanocortin (POMC) at the corticotrope in the anterior pituitary, which is the precursor of both adrenocorticotrophic hormone (ACTH) and β-endorphin, it is conceivable that the HPA axis induces the release of β-endorphins, thereby projecting to diffuse areas within the brain. That is, since β-endorphins are likely to be released as a result of activation of the HPA axis, therefore β-endorphins are released as a result of stimulation.
The third group (group 3) is neural, that is, the noradrenergic sympathetic outflow, which probably originates from the hypothalamic autonomic nervous systems of the sympathetic (HAS) axis via the spinal cord (intermediolateral zone). Here again NE is released and expected to produce IL-10 by activation of the β-AR, similarly to pathway 2. NE is usually considered an excitatory neurotransmitter working opposite to ACh in the ANS; this pathway is traditionally known to have numerous adverse effects, such as an increase in heart rate and vasoconstriction. Recent findings suggest, however, that there is also a beneficial effect, such as production of antiinflammatory cytokine, IL-10, as mentioned previously; therefore, NE is also an antiinflammatory agent working in synergy with Ach, which suppresses inflammatory cytokines.24–26
The fourth group (group 4) is the HAP vagus nerve outflow, which is cholinergic. It is directed to macrophages and interacts with nicotinic acetylcholine receptor nAChR-α7, thereby suppressing the synthesis of TNF-α and IL-1β.24 The antiinflammatory effect of this cholinergic pathway is recently discovered and strongly suggests that the vagal nerve outflow activity resulting from the afferent vagus nerve stimulation or other sensory stimuli would be an important antiinflammatory activity and could be related to one of the beneficial effects of acupuncture or acupuncture-like stimuli.24,28
The last group (group 5) is neural. It is neither sympathetic nor parasympathetic and is coupled directly from the PVN, then to the arcuate nucleus of the hypothalamus to the PAG, and then to the ventral raphe nucleus (raphe magnus) and dorsal horn of the spinal cord. This neural pathway is the well-known central descending pain-inhibitory pathway in the spinal cord acting on the ascending pain signal pathway from the periphery.2–6,18,34 This fifth pathway is interesting because its effect can be observed by neuroimaging in conjunction with acupuncture or acupuncture-like stimuli and pain, which was discussed earlier and will be reiterated in the following section.
EVIDENCE SUPPORTING THE BROAD-SENSE HYPOTHALAMIC-PITUITARY-ADRENAL AXIS HYPOTHESIS
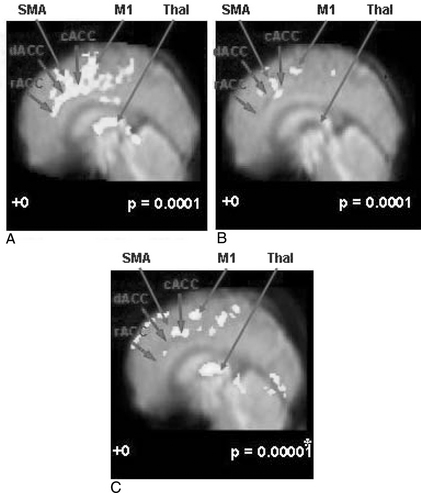
As discussed in the study involving the use of sham acupoints, the fMRI observations of “pain” and “pain with administration of acupuncture” revealed several interesting results and provide clues to an explanation of basic acupuncture mechanisms. The results of another set of experiments similar to the previously discussed fMRI study, which consists of “pain,” “meridian acupuncture + pain,” and “sham acupuncture + pain,” are shown in Figure 4-7.19,20
Comparison of the results from the preceding three experimental paradigms provides the following conclusions. First, as shown in Figure 4-7, A, cortical activation by pain alone clearly demonstrates that pain activates most of the known pain processing centers, such as the dACC, the cACC, and the rACC together with the supplementary and premotor as well as the primary motor areas.16 Also, the thalamic areas are activated as expected; the thalamus is the main relay station of the pain sensory signal to the upper cortical areas, including the cingulate cortex. Although other cortical areas are involved and are activated, this midline view shows clearly the most distinct cortical areas that are activated robustly, including not only the ACC and the thalamus but also the supplementary and the motor areas (SMA and M1), which are also consistently activated.16
Second, Figure 4-7, B, shows an activation pattern resulting from pain stimulation after the administration of acupuncture needling in which activity decreased substantially in most of the areas once activated by pain stimulation alone, namely, the dACC, the cACC, and the rACC, the three most often activated areas together with motor areas (SMA, M1). Again activation of the thalamic area also decreases. These changes in activation on administration of acupuncture based on the traditionally described acupoints (known as the meridian acupuncture points) clearly demonstrate that the pain processing areas are desensitized as a result of acupuncture administration or stimulation. This part of the study is based on traditional acupuncture theory, which designates the acupoints and claims specificity for each acupuncture point. However, the “point specificity” of acupuncture (the idea that the acupoints are specific according to the meridian theory) has been challenged by many Western medical researchers and has also been the subject of great controversy.2,17,21
Third, to test this “point specificity” question, a “sham acupuncture” study was carried out in which the site of needle stimulation was deliberately chosen to be different from the traditional meridian acupuncture points. The result of this study is shown in Figure 4-7, C, and is strikingly similar to that obtained by using the meridian acupoints. This suggests that the point specificity of acupuncture is in question, at least in the case of acupuncture analgesia.20
The implication of these findings is that acupuncture or acupuncture-like sensory stimulation induces the activation of the HPA axis and therefore activation of the endogenous central opiate circuitry, as discussed earlier (e.g., no. 5, shown in Figure 4-6), thereby reducing or inhibiting the ascending pain signals. This descending pain inhibitory theory supports the decreased activation observed in this acupuncture experiment (see and compare Figures 4-7, A to C),19,20 and is consistent with data obtained by Zubieta et al17 and also with the results of Petrovic et al.21 These data again confirm that the effect of acupuncture, especially that of analgesia, is mediated by the BS-HPA axis response to the sensory stimulus; that is, acupuncture’s sustained pain stimulation enhances stress-induced analgesia.
SUMMARY
New molecular and neurophysiological research is increasingly producing the results of stress effect studies,22,23 antiinflammatory immune response studies,24–26 neuroimmune-based acupuncture research,27,28 and more recently neuroimaging-based acupuncture research.16–21
These researches strongly support the view that acupuncture mechanisms can be explained on molecular and neurophysiologic bases, including neuroimmune interactions, specifically via the BS-HPA axis mechanism. The BS-HPA axis hypothesis not only shares the well-known central descending pain-inhibitory theory involving endogenous opioids, but it also suggests that there is a possible antiinflammatory mechanism in conjunction with neuroimmune pathways and the cholinergic antiinflammatory mechanism.23–26,29
Based on the discussion presented here, acupuncture may be working through several different modes within the frame of the BS-HPA axis response, as shown in Figure 4-6. These modes include the following:
1 National Institutes of Health. NIH Consensus Development Statement on Complementary Medicine, 1997. Question 1. Available at: http://odp.od.nih.gov/consensus/statements/cdc/107/107stmt.html.
2 Filshie J, White A, editors. Medical acupuncture: a Western scientific approach. Edinburgh and London: Churchill Livingstone, 1998.
3 Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971-979.
4 Pomeranz B. The scientific basis of acupuncture. In: Stux G, Pomeranz B, editors. The basics of acupuncture. Berlin: Springer-Verlag, 1991.
5 Takeshige C, et al. Descending pain inhibition involved in acupuncture analgesia. Brain Res Bull. 1992;29:617-634.
6 Stux G, Hammerschlag R. Clinical acupuncture. Berlin and Heidelberg: Springer-Verlag, 2000.
7 Cho ZH, Chan JK, Ericksson L. Circular ring transverse axial positron emission camera for 3-D reconstruction of radionuclides distribution. IEEE Trans Nucl Sci. 1976;23:613-622.
8 Phelps ME, Mazziotta JC. Positron emission tomography: human brain function and biochemistry. Science. 1985;228:799-809.
9 Raichle ME. Behind the scenes of functional brain imaging: a historical and physiological perspective. Proc Natl Acad Sci U S A. 1998;95:765-772.
10 Ogawa S, et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992;89:5951-5955.
11 Kwong KK, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89:5675-5679.
12 Cho ZH, Ro YM, Lim TH. NMR venography using the susceptibility effect produced by deoxyhemoglobin. Magn Reson Med. 1992;28:25-38.
13 Cho ZH, et al. New findings of the correlation between acupoints and corresponding brain cortices using functional MRI. Proc Natl Acad Sci U S A. 1998;95:2670-2673.
14 Wu MT, et al. Central nervous pathway for acupuncture stimulation: localization of processing with functional MR imaging of the brain—preliminary experience. Radiology. 1999;212:133-141.
15 Hui K, et al. Acupuncture modulates the limbic system and subcortical gray structures of the human brain: evidence from fMRI studies in normal subjects. Hum Brain Mapp. 2000;9:13-25.
16 Cho ZH, et al. Pain dynamics observed by fMRI using a differential regression analysis technique. J Magn Reson Imag. 2003;18:273-283.
17 Zubieta JK, et al. Regional mu-opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311-315.
18 Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003;26:17-22.
19 Cho ZH, et al. fMRI neurophysiological evidence of acupuncture mechanisms. Med Acupuncture. 2002;14:16-22.
20 Cho ZH, et al. Acupuncture: the search for biologic evidence with functional magnetic resonance imaging and positron emission tomography techniques. J Altern Complement Med. 2002;8:399-401.
21 Petrovic P, et al. Placebo and opioid analgesic: imaging a shared neural network. Science. 2002;295:1737-1740.
22 Maier SF, et al. The role of the vagus nerve in cytokine-to-brain communication. Ann N Y Acad Sci. 1998;840:289-300.
23 Akil H, et al. Overview: thyroid and adrenal axes. Zigmond MJ, et al, editors. Fundamental neuroscience: neuroendocrine systems, vol I. San Diego: Academic Press, 1999.
24 Tracey KJ. The inflammatory reflex. Nature. 2002;420:853-859.
25 Tracey KJ, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662-664.
26 Wang H, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384-388.
27 Son YS, et al. Antipyretic effects of acupuncture on the lipopolysaccharide-induced fever and expression of interleukin-6 and interleukin-1beta mRNAs in the hypothalamus of rats. Neurosci Lett. 2002;319:45-48.
28 Mori H, et al. Unique immunomodulation by electro-acupuncture in humans possibly via stimulation of the autonomic nervous system. Neurosci Lett. 2002;320:21-24.
29 Besedovsky H, et al. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986;233:652-654.
30 Libert C. Inflammation: a nervous connection. Nature. 2003;421:328-329.
31 Derbyshire SWG. Measuring our natural painkiller. Trends Neurosci. 2002;25:67-68.
32 Giardino L, et al. Daily modifications of 3H-naloxone binding sites in the rat brain: a quantitative autoradiographic study. Chronobiol Int. 1989;6:203-216.
33 Jones AK, et al. In vivo distribution of opioid receptors in man in relation to the cortical projections of the medial and lateral systems measured with positron emission tomography. Neurosci Lett. 1991;126:25-28.
34 Han JS, et al. Enkephalin and beta-endorphin as mediators of electro-acupuncture analgesia in rabbits: an antiserum microinjection study. Adv Biochem Psychopharmacol. 1982;33:369-377.
35 Cho ZH, Wong EK, Fallon J. Neuro-acupuncture. Los Angeles: Q-Puncture Inc, 2001.