Foundations and Principles of Pharmacology
Objectives
1. Define the key words used in pharmacology and about giving drugs.
2. Explain the differences between the chemical, generic, official, and brand names of medicines.
3. List the basic types of drug actions.
4. Describe the four basic physiologic processes that affect medications in the body.
5. Discuss the differences between side effects and adverse effects.
Key Terms
absorption (ăb-SŎRP-shŭn, p. 32)
additive effect (ĂD-ĭ-tĭv, p. 36)
adverse reactions (ăd-VŬRS, p. 35)
agonists (ĂG-ō-nĭsts, p. 32)
allergy (ĂL-ĕr-jē, p. 35)
anaphylactic reaction (ăn-ă-fĭ-LĂK-tĭk, p.35)
antagonistic effect (ăn-tăg-ŏ-NĬS-tĭk, p. 36)
antagonists (ăn-TĂG-ŏ-nĭsts, p. 32)
bioequivalent (BĪ-ō-ĭ-KWĬ V-ĭ-lent, p. 36)
biotransformation (BĪ-ō-trăns-fŏr-MĀ-shŭn, p. 34)
chemical name (KĔM-ĭ-kăl, p. 32)
desired action (ĂK-shŭn, p. 35)
displacement (dĭs-PLĀS-mĕnt, p. 36)
distribution (dĭs-trĭ-BŪ-shŭn, p. 33)
drug interaction (ĭn-tĕr-ĂK-shŭn, p. 36)
enteral (route) (ĔN-tĕr-ăl, p. 33)
excretion (ĕks-KRĒ-shŭn, p. 34)
first-pass (effect) (p. 34)
generic name (jĕn-ĔR-ĭk, p. 31)
half-life (p. 34)
hepatotoxic (hĕp-ă-tō-TŎK-sĭk, p. 35)
hypersensitivity (hĭ-pĕr-sĕn-sĭ-TĬV-ĭ-tē, p. 35)
idiosyncratic response (ĭd-ē-ō-sĭn-KRĂ-tĭk, p. 35)
incompatibility (ĭn-kŏm-păt-ĭ-BĬL-ĭ-tē, p. 36)
interference (ĭn-tŭr-FĔR-ĕns, p. 36)
nephrotoxic (nĕf-rō-TŎK-sĭk, p. 35)
official name (ō-FĬSH-ŭl, p. 32)
parenteral (route) (pĕ-RĔN-tĕr-ăl, p. 33)
partial agonists (PĂR-shăl ĂG-ō-nĭsts, p. 32)
percutaneous (route) (pĕr-kū-TĀ-nē-ŭs, p. 33)
pharmacodynamics (FĂRM-ă-kō-dĭ-NĂM-ĭks, p. 31)
pharmacokinetics (FĂRM-ă-kō-kĭNĔT-ĭks, p. 31)
pharmacotherapeutics (FĂRM-ă-kō-thĕr-ă-PŪ-tĭks, p. 31)
receptor site (rē-SĔP-tŏr, p. 32)
side effects (SĪD ĕf-FĔCTS, p. 35)
solubility (sŏl-ū-BĬL-ĭ-tē, p. 33)
synergistic effect (sĭn-ĕr-JĬS-tĭk, p. 37)
trade name (TRĀD, p. 32)
Overview
![]() http://evolve.elsevier.com/Edmunds/LPN/
http://evolve.elsevier.com/Edmunds/LPN/
This chapter provides an overview of very basic information from chemistry, physics, anatomy, and physiology that explains the action of drugs in the body (pharmacokinetics, or what the body does to the drug). It also covers basic information on the effects of drugs on the functions of the body (pharmacodynamics, or what the drug does to the body). This information is vital in understanding pharmacotherapeutics, or the use of drugs in the treatment of disease (Box 4-1).
Drug Names
Medications have several different names that may be confusing when you first learn to work with drugs. It is very important to know the different names of a medication so that the wrong drug is not given to a patient. Sometimes a medication is ordered by one name for the drug and the pharmacist labels it with another name for the same drug. For example, Valium (trade name) is the same as diazepam (generic name). It is important to know whether the medication is the same or a different drug.
The most common drug name used is the generic name. This is the name the drug manufacturer uses for a drug, and it is the same in all countries. It is the name given to a drug before there is an official name, or when the drug has been available for many years and more than one company makes the drug. Examples would be penicillin and tetracycline. The American Pharmaceutical Association, the American Medical Association, and the U.S. Adopted Names Council assign generic names. Generic names are not capitalized when written. An example is warfarin.
Another common drug name is the trade name, or brand name. This name is often followed by the symbol ®, which indicates that the name is registered to a specific drug maker or owner and no one else can use it. This is the drug name used in written or TV advertisements and other marketing, and it is often descriptive, easy to spell, or catchy sounding so that prescribers will remember it easily and be more likely to use it. The first letter of the trade name, and sometimes other letters, are capitalized. Examples of trade names are Dimetapp, Lanoxin, and Augmentin.
Chemical names are often the most difficult to remember, because they include the chemicals that make up the drug. These names are usually long and hyphenated, and they describe the atomic or molecular structure. An example is ethyl 1-methyl-4-phenylisonipecotate hydrochloride, the chemical name for meperidine (Demerol).
The final type of name is the official name, which is given by the Food and Drug Administration. Sometimes this name is similar to the brand or chemical name. The first letter of the official name is also capitalized. An example is Ethacrynic acid.
Types of Drug Actions
Drug Attachment (Drug-Receptor Binding)
Drugs take part in chemical reactions that change the way the body acts. They do this most commonly when the medication forms a chemical bond at a specific site in the body called a receptor site (Figure 4-1). The chemical reactions between a drug and a receptor site are possible only when the receptor site and the drug can fit together like pieces of a jigsaw puzzle or a key fitting into a lock. The drug attaches to the receptor site and activates the receptor; the drug has an action similar to the body’s own chemicals.
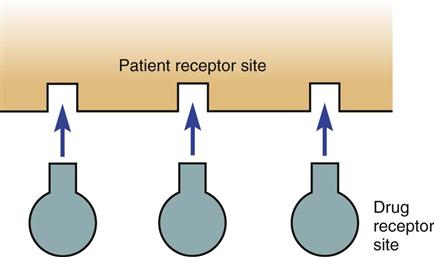
Some drugs attach to the receptor site but produce only a small chemical response. These drugs are called partial agonists. When a drug attaches at a drug receptor site but there is no chemical drug response these are called antagonists. Some partial agonists and antagonists are able to compete with other chemicals or drugs already bonded to a receptor site and replace them. The Memory Jogger box summarizes the various types of receptor site activity.
Basic Drug Processes
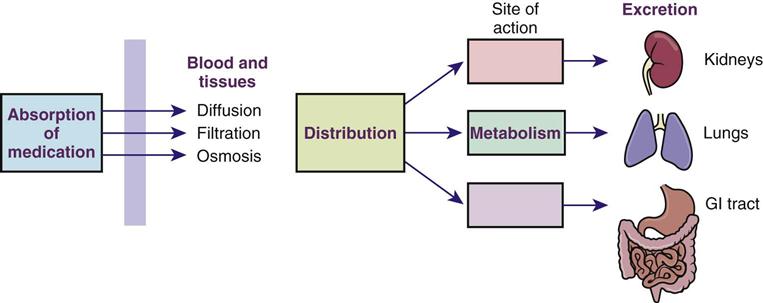
Drugs must be changed chemically in the body to become usable. There are four basic processes involved in drug utilization in the body. These processes are absorption, distribution, metabolism, and excretion. Drugs have different characteristics, or pharmacokinetics, that determine to what extent these processes will be used. To understand how a drug works, the nurse must understand each of these processes for the specific drug being given.
 The Process of Absorption
The Process of Absorption
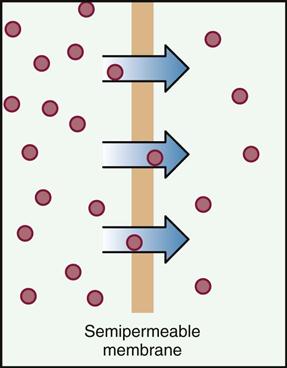
Absorption involves the way a drug enters the body and passes into the circulation. Absorption takes place through processes of diffusion, filtration, and osmosis. These mechanisms of absorption are more fully described in Box 4-2. How fast the drug is absorbed into the body through these processes depends on the solubility of the drug, the route of administration, and the degree of blood flow through the tissue where the medication is found.
All medication must be dissolved in body fluid before it can enter body tissues. The ability of the medication to dissolve is called solubility. To achieve the best possible drug action, sometimes the medication must be dissolved quickly; at other times it should be dissolved slowly. Solubility of the drug is often controlled by the form of the medication; for example, solutions are more soluble than capsules because a liquid is absorbed faster than a tablet, which must dissolve. An injection with an oil base must be chemically changed before absorption can take place, and this holds the drug in the tissues longer, which may be the desired action. When the patient takes water with a tablet, it not only helps in swallowing but also helps dissolve the medication.
The route of administration also influences absorption. The most common medication routes are enteral (directly into the gastrointestinal [GI] tract through oral, nasogastric tube, or rectal administration); parenteral (directly into dermal, subcutaneous, or intramuscular tissue, epidurally into the cerebrospinal fluid, or into the bloodstream through intravenous [IV] injections); and percutaneous (through topical [skin], sublingual [under the tongue], buccal [against the cheek], or inhalation [breathing] administration).
In areas where the blood flow through tissues is very high, medication is rapidly absorbed. Examples of this include placing a nitroglycerin tablet under the tongue right next to blood vessels or spraying steroids into the nose and lungs through a nebulizer. IV medications injected into the bloodstream have the fastest action. Oral or rectal medications usually take much longer because they must dissolve and diffuse across the barrier tissue in the GI tract (the gastric mucosa) before being carried to the body tissues where they will have their action.
 The Process of Distribution
The Process of Distribution
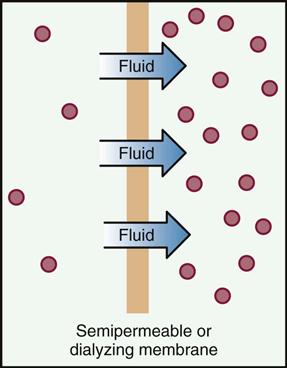
Once the medication is absorbed, it must travel throughout the body. The term distribution refers to the ways that drugs move by means of circulating body fluids to their sites of action in the body. The bloodstream and lymphatic system usually carry the drug throughout the whole body. The organs that have the biggest blood supply receive the medication faster, and areas of skin and fat receive the medication more slowly. Some drugs do not pass well through cell membranes with very small passages, such as those covering the placenta and the brain. These are referred to as placental and blood-brain barriers, although the barrier is not a complete barrier, because some drugs and some conditions make it possible for drugs to easily pass through these areas. The various types of tissues, including bone, fat, and muscle, do not absorb equal amounts of the drug. Thus the distribution is different for different drugs.
The chemical properties of a drug also affect how the drug is distributed. Some chemicals bind together with proteins such as albumin (found in the blood plasma), which serve as carriers giving a ride to drugs that are not easily dissolved. The ratio of bound chemical compared with free chemical remains the same in the blood. As more of the free chemical diffuses into the tissues, more of the bound chemical becomes unlocked and thus also available to diffuse.
Some medications are attracted to tissues other than the target receptor sites. For example, medications that dissolve easily in lipids (fats) prefer adipose, or fat tissue, and stores of the medication may build up in these areas. As the medication moving throughout the body binds at the receptor sites, more of the medication stored in adipose tissue will gradually be given up by those cells. Thus a drug that can be stored in the fat cells may remain in the body for a long time while it is slowly released.
 The Process of Metabolism
The Process of Metabolism
Once the medication is absorbed and distributed in the body, the body’s enzymes use it in chemical reactions through the process of metabolism. Some drugs that are breathed into the lungs or injected into the tissue may go directly into the bloodstream and be carried quickly to the site of action. But many medications have to be stimulated or have activation of pro-drugs before they become usable, while drugs are broken down into smaller usable parts, primarily in the liver, through a series of complex chemical reactions until they become chemically inactive. This process is called biotransformation. When most of a medicine goes very quickly to the liver, a lot of the medication is inactivated on its “first-pass” through the liver before it can be distributed to other parts of the body. That is why some medicines are given sublingually or intravenously; otherwise, patients do not get the amount of medication they require. (For example, only 1 mg of propranolol is required intravenously, but 40 mg are required when the drug is given by mouth.)
Genetic differences in the enzyme pathways in the liver also explain why people respond differently to a drug—whether they grow tolerant of the drug and seem to need larger doses, or whether they are sensitive to the drug and only need a small dose. These enzyme pathways, known as the cytochrome P-450 system, play an important role in the adverse drug reactions patients may have when taking several drugs at the same time or when there are drug-food interactions.
 The Process of Excretion or Elimination
The Process of Excretion or Elimination
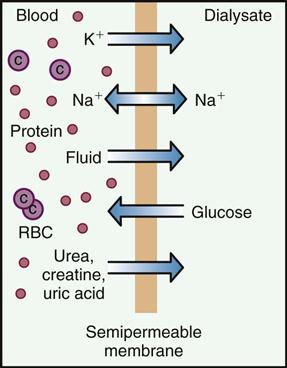
All inactive chemicals, chemical by-products, and waste (often referred to as metabolites) finally break down through metabolism and are removed from the body through the process of excretion. Fibrous or insoluble waste is usually passed through the GI tract as feces. Chemicals that may be made water soluble are dissolved and filtered out as they pass through the kidneys and then lost in the urine. Some chemicals are exhaled from the lungs through breathing or lost through evaporation from the skin during sweating. Very small amounts of medication may also escape in tears, saliva, or milk of breastfeeding mothers.
The major processes involved in drug utilization in the body are shown in Figure 4-2. These four major processes are basic to understanding how medications are used in the body.
Half-Life.
Some drugs enter and leave the body very quickly; other drugs remain for a long time. The standard method of describing how long it takes to metabolize and excrete a drug is the half-life, or the time it takes the body to remove 50% of the drug from the body. Because the rates of metabolism and excretion are usually the same for most people, the half-life helps explain the dose (how much medicine should be taken), the frequency (how often it should be taken), and the duration (how long it will last) for different drugs. If a drug has a long half-life, it may need to be taken only once a day. If a person takes too much medication with a long half-life, this may cause a serious problem because the action of the drug lasts for such a long time. If the half-life of a drug is short, such as for many antibiotics, the person must take frequent doses to keep the correct level in the blood. If a person’s liver or kidneys do not function correctly, medications may not be properly metabolized or excreted, and this would mean that higher doses of the medication will circulate for a longer time and produce symptoms of overdosage. Drugs are often dosed based on renal and hepatic function for this reason. Therefore the function of the kidneys and liver are watched by doing repeated renal and hepatic tests.
Basics of Drug Action
When a drug is given to a patient, it is usually possible to predict the chemical reaction that will be seen. However, because each patient is different, some unexpected chemical reactions are also seen. With each patient, giving a medication is somewhat of an experiment, so patients must be watched closely to monitor their reaction to the medication.
The expected response of the medication is called the desired action. This is when the medication does what is desired, and the therapeutic goal is reached (for example, meperidine [Demerol] relieves pain).
Because a medication may influence many body systems at the same time, the effect of the medication is often not restricted to the desired action. Other actions called side effects or adverse reactions may also take place. Side effects are usually seen as mild but annoying responses to the medication. Adverse reactions, or adverse effects, usually imply more severe symptoms or problems that develop because of the drug. Some adverse effects may require the patient to be hospitalized or may even be life threatening. Some side effects, such as drowsiness, may go away after the patient takes the medication for a time. Certain side effects, such as nausea, may be stopped if the dosage is reduced. Some side effects are such a problem that the medication must be changed or stopped. An example of this might be insomnia (inability to sleep) or making the patient pass out. Certainly, if severe adverse effects such as damage to the kidney (nephrotoxic drug) or liver (hepatotoxic drug) or bleeding develop, the medication must often be stopped.
Occasionally a patient may have a drug reaction that is a surprise. Strange, unique, or unpredicted responses are called idiosyncratic responses. These reactions may be the result of missing or defective metabolic enzymes caused by a genetic or hormonal variation. They often produce either an unexpected result, such as pain or bleeding, or an overresponse to the drug. These types of reactions are usually rare. One type of idiosyncratic response is called a paradoxical response. In this situation, the patient’s reaction may be just the opposite of what would be expected. For example, pseudoephedrine hydrochloride, a chemical that was used until a few years ago in decongestants for children, usually produces sedation or drowsiness as a side effect. However, some children respond in the opposite way, having insomnia and tachycardia (rapid heartbeat) and are overly stimulated.
A second type of unexpected reaction is an increased reaction to a drug (hypersensitivity) or a sensitivity caused by antibody response to a drug (allergy). Some medications (sulfa products, aspirin, penicillin) and some conditions (asthma) are more likely to produce allergic reactions than others. Allergic reactions usually occur when an individual has taken the drug and the body has developed antibodies to it. When the patient takes the drug again, the antigen-antibody reaction produces hives, rash, itching, or swelling of the skin. This type of allergic reaction is very common, so ask all patients about whether they have ever had a drug reaction. Patients with an allergy to one medication may be more likely to develop an allergy to another medication, but individuals may also develop a reaction to medications they have taken before without problems.
Occasionally, the allergic reaction is so severe the patient has trouble breathing, and rarely the heart may stop. This life-threatening response is called an anaphylactic reaction. A patient who has a mild allergic reaction to a medication is much more likely to develop the more severe anaphylactic reaction if the medication is given again. An anaphylactic reaction is a true medical emergency because the patient may suffer paralysis of the diaphragm and swelling of the oropharynx that interfere with breathing. Patients who have anaphylactic reactions should always be warned about their allergy so they will not take the drug again, and they should wear a Medic Alert bracelet or necklace or carry identification about their allergy.
Patients often confuse an allergy with side effects—both of which may produce unpleasant symptoms. If a patient reports an “allergy” to a drug, make sure you ask the right questions to understand the exact past reaction to the drug. If the patient had nausea or stomach pain when taking aspirin, that is a side effect, but not an allergy. If the patient reported sedation when taking an antihypertensive medication, that is also not an allergy. Because patients often do not understand the difference between an allergy and a side effect, it is important to clarify the difference when patients say they have had an allergic reaction
The common responses to medications are listed in Box 4-3.
Bioequivalence
After a new drug enters the market, a patent protects the financial interest of the drug company for some time, usually 17 years, by limiting other companies from producing that drug. After the patent ends, other companies may file an application to make the same drug under a generic name. Brand-name drugs are usually more expensive than generic drugs because the maker of the brand-name drug is attempting to recover the huge sums of money spent on research and making of the drug. Thus generic products are often less expensive.
Drug products seen as identical with respect to their active ingredients are known as generic equivalents. However, slight differences in processing or formulation may mean that the action of the generic drug in the body is slightly different from that of the brand-name product. These differences most commonly cause variations in absorption, distribution, or metabolism. Thus what product the patient actually purchases when a prescription is written for a drug may vary according to what specific brand the pharmacist dispenses. Some products are chemically the same or identical and thus bioequivalent. Some products are quite different, and thus the medication that is given by the pharmacist should not be changed from what the prescriber has written. This may be particularly important for some cardiac or antiseizure medications.
 Drug Interactions
Drug Interactions
When one drug changes the action of another drug, a drug interaction is present. These reactions often take place during the process of metabolism (or biotransformation) in the liver and are a result of the cytochrome P-450 enzyme pathways each person inherits. The actions of a number of drugs may be altered when they are taken with other drugs; these drugs include many antidepressants, theophylline, warfarin, cimetidine, ciprofloxacin, isoniazid, ketoconazole, phenytoin, zafirlukast, phenobarbital, rifampin, codeine, and morphine. Some medications are given together on purpose because the drug interactions are helpful. For example, probenecid is given with penicillin to increase the amount of penicillin that is absorbed, which is important in treating venereal disease. Other drug interactions produce adverse effects. For example, some antibiotics make birth control pills less effective, thus making it more likely a woman will get pregnant.
Several types of effects are seen with drug interactions (Box 4-4). When two drugs are given together, and the combined effect of the drugs is equal to either that of the most active drug or the sum of the effects of the individual drugs, an additive effect is seen. If one drug interferes with the action of another drug, it is described as an antagonistic effect. At times, one drug may replace another drug at a receptor site, increasing the effect of the first drug. This is called displacement. Sometimes incompatibility occurs when drugs do not mix well chemically. Attempts to mix them together in a syringe may cause a chemical reaction, so neither of the drugs can be given. Interference is seen when one drug promotes the rapid excretion of another drug, thus reducing its activity. Finally, if the effect of two drugs taken at the same time is greater than the sum of the effects of each drug given alone, the drugs have a synergistic effect. This is often seen when individuals are exposed to pollutants and toxins. Potentiation is one type of synergistic effect in which a drug that might produce only a small effect by itself produces a larger effect when given with another specific drug.
Food, Alcohol, and Drug Interactions
Food, alcohol, and most medications taken by mouth must travel through the liver for chemical changes before they can be used by the body. Thus the risk for drug interaction with food or alcohol is high. When taken together, food or alcohol and drugs may alter the body’s ability to use a particular food or drug. Part of these interactions may be due to activation of the P-450 enzyme system or competition for receptor sites. Monoamine oxidase inhibitors are some of the drugs most noted for drug-food interactions, because they cannot be taken with aged cheese or many processed foods. Information every patient should know about possible drug interactions includes the following:
Alcohol-Medication Interactions.
The amount of alcohol use is very high in the U.S. population. It has been estimated that approximately 70% of adults consume alcohol at least occasionally, and up to 10% of people may drink daily. Many patients may not be aware that alcohol is one of the products that reacts most commonly with drugs. The extent to which a dose of medicine reaches its site of action is called availability. Alcohol can influence whether a drug is effective by changing its availability.
In an alert from the National Institute on Alcohol Abuse and Alcoholism* it was estimated that alcohol-medication interactions may be a factor in at least 25% of all emergency department admissions. An unknown number of less serious interactions go unrecognized and unrecorded. One group of individuals at high risk for alcohol-drug interactions is older adults, who take 25% to 30% of all prescription medications and also frequently drink alcohol. Older adults are more likely to suffer medication side effects compared with younger persons, and these effects tend to be more severe with advancing age.
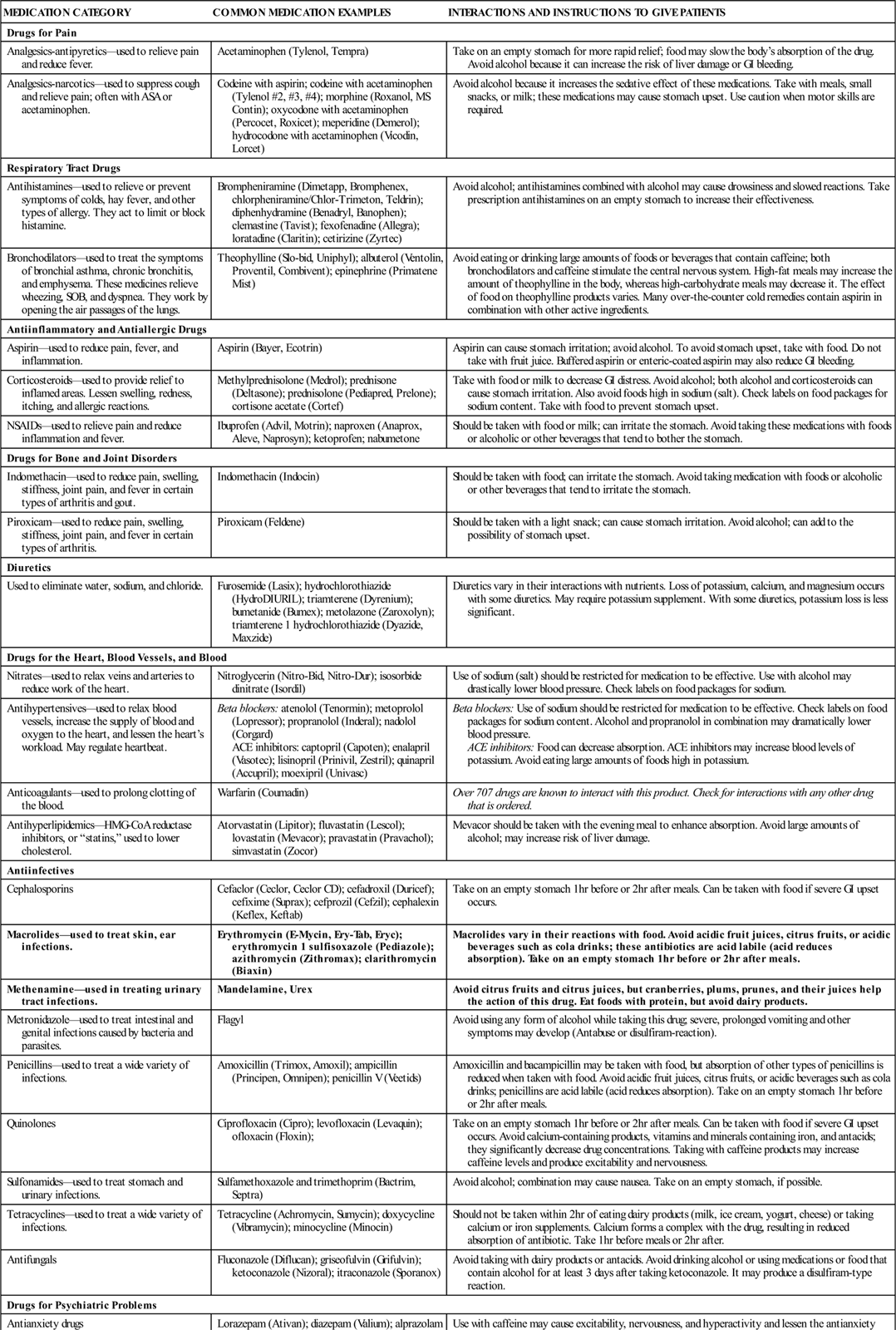
Table 4-1 provides information about food-drug-alcohol interactions patients should know.
![]() Table 4-1
Table 4-1
Specific Food-Alcohol-Drug Interactions by Drug Category
| MEDICATION CATEGORY | COMMON MEDICATION EXAMPLES | INTERACTIONS AND INSTRUCTIONS TO GIVE PATIENTS |
| Drugs for Pain | ||
| Analgesics-antipyretics—used to relieve pain and reduce fever. | Acetaminophen (Tylenol, Tempra) | Take on an empty stomach for more rapid relief; food may slow the body’s absorption of the drug. Avoid alcohol because it can increase the risk of liver damage or GI bleeding. |
| Analgesics-narcotics—used to suppress cough and relieve pain; often with ASA or acetaminophen. | Codeine with aspirin; codeine with acetaminophen (Tylenol #2, #3, #4); morphine (Roxanol, MS Contin); oxycodone with acetaminophen (Percocet, Roxicet); meperidine (Demerol); hydrocodone with acetaminophen (Vicodin, Lorcet) | Avoid alcohol because it increases the sedative effect of these medications. Take with meals, small snacks, or milk; these medications may cause stomach upset. Use caution when motor skills are required. |
| Respiratory Tract Drugs | ||
| Antihistamines—used to relieve or prevent symptoms of colds, hay fever, and other types of allergy. They act to limit or block histamine. | Brompheniramine (Dimetapp, Bromphenex, chlorpheniramine/Chlor-Trimeton, Teldrin); diphenhydramine (Benadryl, Banophen); clemastine (Tavist); fexofenadine (Allegra); loratadine (Claritin); cetirizine (Zyrtec) | Avoid alcohol; antihistamines combined with alcohol may cause drowsiness and slowed reactions. Take prescription antihistamines on an empty stomach to increase their effectiveness. |
| Bronchodilators—used to treat the symptoms of bronchial asthma, chronic bronchitis, and emphysema. These medicines relieve wheezing, SOB, and dyspnea. They work by opening the air passages of the lungs. | Theophylline (Slo-bid, Uniphyl); albuterol (Ventolin, Proventil, Combivent); epinephrine (Primatene Mist) | Avoid eating or drinking large amounts of foods or beverages that contain caffeine; both bronchodilators and caffeine stimulate the central nervous system. High-fat meals may increase the amount of theophylline in the body, whereas high-carbohydrate meals may decrease it. The effect of food on theophylline products varies. Many over-the-counter cold remedies contain aspirin in combination with other active ingredients. |
| Antiinflammatory and Antiallergic Drugs | ||
| Aspirin—used to reduce pain, fever, and inflammation. | Aspirin (Bayer, Ecotrin) | Aspirin can cause stomach irritation; avoid alcohol. To avoid stomach upset, take with food. Do not take with fruit juice. Buffered aspirin or enteric-coated aspirin may also reduce GI bleeding. |
| Corticosteroids—used to provide relief to inflamed areas. Lessen swelling, redness, itching, and allergic reactions. | Methylprednisolone (Medrol); prednisone (Deltasone); prednisolone (Pediapred, Prelone); cortisone acetate (Cortef) | Take with food or milk to decrease GI distress. Avoid alcohol; both alcohol and corticosteroids can cause stomach irritation. Also avoid foods high in sodium (salt). Check labels on food packages for sodium content. Take with food to prevent stomach upset. |
| NSAIDs—used to relieve pain and reduce inflammation and fever. | Ibuprofen (Advil, Motrin); naproxen (Anaprox, Aleve, Naprosyn); ketoprofen; nabumetone | Should be taken with food or milk; can irritate the stomach. Avoid taking these medications with foods or alcoholic or other beverages that tend to bother the stomach. |
| Drugs for Bone and Joint Disorders | ||
| Indomethacin—used to reduce pain, swelling, stiffness, joint pain, and fever in certain types of arthritis and gout. | Indomethacin (Indocin) | Should be taken with food; can irritate the stomach. Avoid taking medication with foods or alcoholic or other beverages that tend to irritate the stomach. |
| Piroxicam—used to reduce pain, swelling, stiffness, joint pain, and fever in certain types of arthritis. | Piroxicam (Feldene) | Should be taken with a light snack; can cause stomach irritation. Avoid alcohol; can add to the possibility of stomach upset. |
| Diuretics | ||
| Used to eliminate water, sodium, and chloride. | Furosemide (Lasix); hydrochlorothiazide (HydroDIURIL); triamterene (Dyrenium); bumetanide (Bumex); metolazone (Zaroxolyn); triamterene 1 hydrochlorothiazide (Dyazide, Maxzide) | Diuretics vary in their interactions with nutrients. Loss of potassium, calcium, and magnesium occurs with some diuretics. May require potassium supplement. With some diuretics, potassium loss is less significant. |
| Drugs for the Heart, Blood Vessels, and Blood | ||
| Nitrates—used to relax veins and arteries to reduce work of the heart. | Nitroglycerin (Nitro-Bid, Nitro-Dur); isosorbide dinitrate (Isordil) | Use of sodium (salt) should be restricted for medication to be effective. Use with alcohol may drastically lower blood pressure. Check labels on food packages for sodium. |
| Antihypertensives—used to relax blood vessels, increase the supply of blood and oxygen to the heart, and lessen the heart’s workload. May regulate heartbeat. | Beta blockers: atenolol (Tenormin); metoprolol (Lopressor); propranolol (Inderal); nadolol (Corgard) ACE inhibitors: captopril (Capoten); enalapril (Vasotec); lisinopril (Prinivil, Zestril); quinapril (Accupril); moexipril (Univasc) |
Beta blockers: Use of sodium should be restricted for medication to be effective. Check labels on food packages for sodium content. Alcohol and propranolol in combination may dramatically lower blood pressure. ACE inhibitors: Food can decrease absorption. ACE inhibitors may increase blood levels of potassium. Avoid eating large amounts of foods high in potassium. |
| Anticoagulants—used to prolong clotting of the blood. | Warfarin (Coumadin) | Over 707 drugs are known to interact with this product. Check for interactions with any other drug that is ordered. |
| Antihyperlipidemics—HMG-CoA reductase inhibitors, or “statins,” used to lower cholesterol. | Atorvastatin (Lipitor); fluvastatin (Lescol); lovastatin (Mevacor); pravastatin (Pravachol); simvastatin (Zocor) | Mevacor should be taken with the evening meal to enhance absorption. Avoid large amounts of alcohol; may increase risk of liver damage. |
| Antiinfectives | ||
| Cephalosporins | Cefaclor (Ceclor, Ceclor CD); cefadroxil (Duricef); cefixime (Suprax); cefprozil (Cefzil); cephalexin (Keflex, Keftab) | Take on an empty stomach 1 hr before or 2 hr after meals. Can be taken with food if severe GI upset occurs. |
| Macrolides—used to treat skin, ear infections. | Erythromycin (E-Mycin, Ery-Tab, Eryc); erythromycin 1 sulfisoxazole (Pediazole); azithromycin (Zithromax); clarithromycin (Biaxin) | Macrolides vary in their reactions with food. Avoid acidic fruit juices, citrus fruits, or acidic beverages such as cola drinks; these antibiotics are acid labile (acid reduces absorption). Take on an empty stomach 1 hr before or 2 hr after meals. |
| Methenamine—used in treating urinary tract infections. | Mandelamine, Urex | Avoid citrus fruits and citrus juices, but cranberries, plums, prunes, and their juices help the action of this drug. Eat foods with protein, but avoid dairy products. |
| Metronidazole—used to treat intestinal and genital infections caused by bacteria and parasites. | Flagyl | Avoid using any form of alcohol while taking this drug; severe, prolonged vomiting and other symptoms may develop (Antabuse or disulfiram-reaction). |
| Penicillins—used to treat a wide variety of infections. | Amoxicillin (Trimox, Amoxil); ampicillin (Principen, Omnipen); penicillin V (Veetids) | Amoxicillin and bacampicillin may be taken with food, but absorption of other types of penicillins is reduced when taken with food. Avoid acidic fruit juices, citrus fruits, or acidic beverages such as cola drinks; penicillins are acid labile (acid reduces absorption). Take on an empty stomach 1 hr before or 2 hr after meals. |
| Quinolones | Ciprofloxacin (Cipro); levofloxacin (Levaquin); ofloxacin (Floxin); | Take on an empty stomach 1 hr before or 2 hr after meals. Can be taken with food if severe GI upset occurs. Avoid calcium-containing products, vitamins and minerals containing iron, and antacids; they significantly decrease drug concentrations. Taking with caffeine products may increase caffeine levels and produce excitability and nervousness. |
| Sulfonamides—used to treat stomach and urinary infections. | Sulfamethoxazole and trimethoprim (Bactrim, Septra) | Avoid alcohol; combination may cause nausea. Take on an empty stomach, if possible. |
| Tetracyclines—used to treat a wide variety of infections. | Tetracycline (Achromycin, Sumycin); doxycycline (Vibramycin); minocycline (Minocin) | Should not be taken within 2 hr of eating dairy products (milk, ice cream, yogurt, cheese) or taking calcium or iron supplements. Calcium forms a complex with the drug, resulting in reduced absorption of antibiotic. Take 1 hr before meals or 2 hr after. |
| Antifungals | Fluconazole (Diflucan); griseofulvin (Grifulvin); ketoconazole (Nizoral); itraconazole (Sporanox) | Avoid taking with dairy products or antacids. Avoid drinking alcohol or using medications or food that contain alcohol for at least 3 days after taking ketoconazole. It may produce a disulfiram-type reaction. |
| Drugs for Psychiatric Problems | ||
| Antianxiety drugs | Lorazepam (Ativan); diazepam (Valium); alprazolam (Xanax) | Use with caffeine may cause excitability, nervousness, and hyperactivity and lessen the antianxiety effect. Use with alcohol may impair mental and motor functions. |
| Antidepressants | Paroxetine (Paxil), sertraline (Zoloft), fluoxetine (Prozac) | Avoid concurrent use with alcohol. These medications can be taken with or without food. |
| Lithium carbonate—regulates changes in chemical levels in the brain. | Various names | Follow the dietary and fluid intake instructions of the health care provider to avoid very serious toxic reactions. |
| MAO inhibitors—antidepressants | Phenelzine (Nardil); tranylcypromine (Parnate) | A very dangerous, potentially fatal interaction can occur with foods containing tyramine, a chemical in alcoholic beverages (particularly wine) and many foods (e.g., hard cheeses, chocolate, beef or chicken livers, sour cream, yogurt, raisins, bananas, avocados, soy sauce, yeast extract, meat tenderizers, sausages, anchovies). Patient may develop severe headache, nosebleed, chest pain, photosensitivity, or severe hypertension with hypertensive crisis. |
| Sedative-hypnotics | Various names | Do not use alcohol with any sleep medications; oversedation occurs. |
| Antacids, Antiulcer Medications, and Histamine Blockers | ||
| Work to reduce acid in the stomach. | Cimetidine (Tagamet); famotidine (Pepcid); ranitidine (Zantac); nizatidine (Axid) | Follow specific diets given by health care provider. Avoid large amounts of caffeine; dairy products such as milk or cream may increase acid secretion. If calcium carbonate is used as a calcium supplement, avoid bran and whole-grain breads or cereals that reduce absorption of calcium. |
| Laxatives | ||
| Stimulate intestine, soften stool, add bulk or fluid to stool. | Various names | Excessive use of laxatives can cause loss of essential vitamins and minerals and may require replenishment of potassium, sodium, and other nutrients through diet. Mineral oil can cause poor absorption of vitamins A, D, E, and K, and calcium. Take 2 hr before eating food. |
Modified from Food & Drug Interactions, Washington DC, 2004, National Consumers League (available at http://www.nclnet.org); McKenry LM, Tessier E, Hogan MA: Mosby’s pharmacology in nursing, ed 22, St Louis, 2006, Mosby.
Drug Effects on Laboratory Tests and Blood Substances.
Although medications exert a therapeutic effect in the body, they may also have effects on various natural substances in the blood, or they may alter the results of some laboratory tests. For example, the drug may increase the blood glucose level or affect the clotting time. Nurses should be aware of these changes as they look at the results of laboratory tests and try to monitor the action of a drug.
Chronotherapy.
Research has shown that certain drugs are more effective at different times of the day, and drug treatment may work best when it is linked to the normal human circadian rhythm (a repetitive cycle based on a 24-hour clock). The circadian clock controls rhythms in endocrine gland secretion, metabolic processes, and behavioral activity. Certain diseases, such as asthma, angina, diabetes mellitus, and hypertension, get better or worse throughout the day according to the circadian cycle. Chronotherapy is a process that attempts to time the drug action so that it occurs when that action is most needed by the body.
Patient Variables Affecting Drug Use
Special knowledge and sometimes special medications are required for neonates, small children, adults, and older adult patients. Women who are pregnant have special risks when they take any type of medication. People of different cultures also have different attitudes about medication use. When giving medications, all these factors are important for the nurse to know and may affect the nursing care plan. (These variables are discussed in greater detail in Chapters 5 and 6.)