CASE 4
Richard is a 12-month-old infant with a severe gram-negative kidney infection (pyelonephritis). This is his third such infection in 6 months, although investigation of the urogenital system for congenital defects (e.g., ureteral valvular defects) revealed no abnormality. The mother insists that “something” must be wrong because he has had recurrent sinopulmonary infections and repeated episodes of diarrhea. Richard has three healthy sisters aged 1, 3, and 4 years. An uncle died at 10 years of age with bacterial pneumonia. Despite the fact that he had received childhood immunizations, blood tests performed at a hospital elsewhere revealed low serum IgG, IgA, and IgE levels but high serum levels of IgM. B and T cell numbers were normal, as was the function of T cells. There was no evidence for defective neutrophil functioning. How would you proceed?
QUESTIONS FOR GROUP DISCUSSION
RECOMMENDED APPROACH
Implications/Analysis of Laboratory Investigation
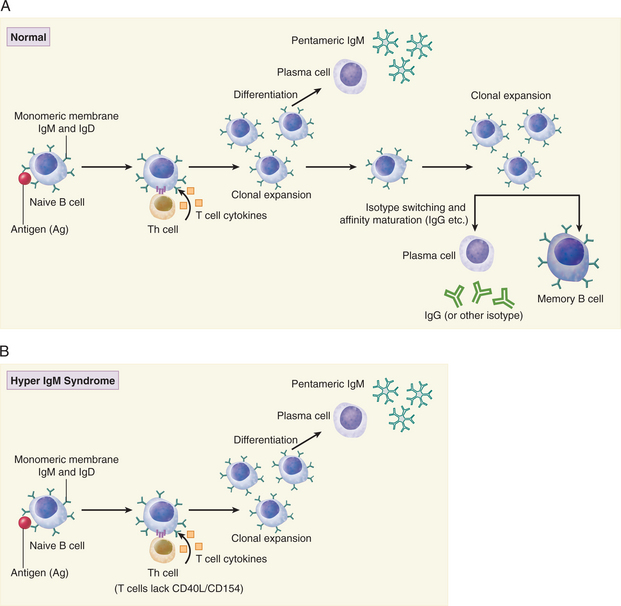
Richard’s initial blood work consisting of a complete blood cell count and differential was normal so this is not a problem in T cell or B cell development (see Case 1). Serum immunoglobulin measures (see Case 3) indicated little to no IgG, IgA, or IgE, but IgM was present in higher concentrations than the normal reference value, suggesting that there is a problem in isotype switching. The normal numbers of B cells and the high concentrations of IgM rule out X-linked (Bruton’s) agammaglobulinemia (XLA) as a possible diagnosis.
B cell activation leading to isotype switching requires T cell–derived cytokines as well as cognate interaction with activated T cells (Fig. 4-1A). As such, a deficiency or defect in T cells could account for the observed levels of IgG, IgA, and IgE. However, we are told that T cell function is normal. In light of normal T cell function, the molecular interactions between B and T cells should be investigated.
DIAGNOSIS
The lack of CD154 expression on activated T cells is a strong indication that Richard has X-linked hyper IgM syndrome because CD40-CD154 interaction is required for isotype switching of activated B cells (see Fig. 4-1B). On the basis of the clinical history and laboratory investigations it is possible to rule out XLA as a cause of these infections. In XLA there are few B cells and even IgM is deficient. Transient hypogammaglobulinemia of infancy is also ruled out because in this disorder IgM levels are normal. Additionally, there is published evidence for a maturational defect in CD4+ Th1 cells. Both cognate (T-B cell) interaction and T cell–derived cytokines are required for isotype switching in B cells.
ETIOLOGY: HYPER IGM SYNDROME
Role of CD28 and CD152
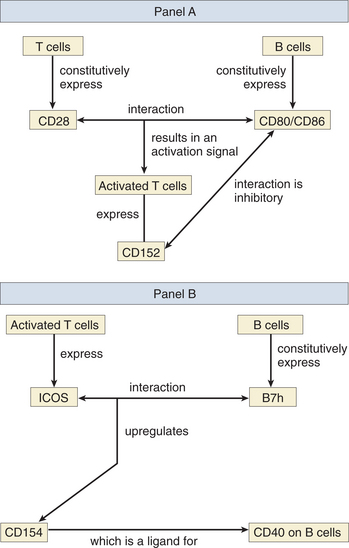
CD80 and CD86 (formerly known as B7-1 and B7-2) are expressed on antigen-presenting cells, including dendritic cells, macrophages, and B cells (see Fig. 4-2A). They are both ligands for CD28, which is constitutively expressed on T cells, and CD152 (formerly CTLA-4), which is induced after T cell activation. It has long been recognized that T cell activation requires at least two signals, the first being delivered via the T cell antigen receptor and the second via CD28, whose interaction with CD80/86 triggers a signal transduction cascade that results in the stabilization of mRNA for IL-2, a growth factor for T cells. CD152 is induced on activated T cells and probably plays a more important role in downregulating the T cell response after binding to the same ligands CD80 and CD86. Because CD80 and CD86 have a higher affinity for CD152, the inhibitory signal plays the dominant role once CD152 is expressed on the cell surface.
Inducible Co-stimulator and CD154 Expression
Studies with knockout mice for the ICOS (inducible co-stimulator) gene also show profound defects in isotype switching after B cell activation. ICOS is expressed only on the surface of activated T cells and has been shown to upregulate CD154 on T cells following ICOS-B7h interaction (Fig. 4-2B). B7h is constitutively expressed on naive B cells and is a newly discovered member of the B7 (CD80/86) family. This defect in isotype switching is reversed when anti-CD40 antibodies are used to stimulate CD40 directly (instead of binding CD154). Cytokine secretion is also affected in the ICOS knockout mice. T cells from such animals show increased production of IL-2 and interferon gamma (IFNγ) but a decrease in IL-4 and IL-10 after immune stimulation. IL-4 is required for isotype switching to IgE. Note: The reader should beware of some potential confusion caused by redundant nomenclature. B7h (the ligand for ICOS) has also been named B7RP-1, GL-50, ICOSL, and LICOS. The inducible co-stimulator (ICOS) is also known as activation-inducible lymphocyte immunomodulator (AILIM).