CASE 39
FD, a 3-month-old HIV-positive patient who is stable on triple therapy with essentially normal CD4 counts, is referred to your transplant team with fulminant hepatitic failure (FHF). A complete blood cell count with differential was requested. Rapidly progressing liver failure has been apparent over the past 2 days, during which laboratory tests (bilirubin, aspartate aminotransferase [AST], alanine aminotransferase [ALT], albumin, international normalized thromboplastin ratio [INR], prothrombin time [PT], and activated partial thromboplastin time [aPTT]) were ordered. Hepatitis B infection was confirmed when enzyme-linked immunosorbent assay (Elisa) revealed anti-HBc IgM antibodies. Following the results of these tests, abdominal computed tomography [CT] and ultrasonography were also requested.
QUESTIONS FOR GROUP DISCUSSION
RECOMMENDED APPROACH
Implications/Analysis of Family History
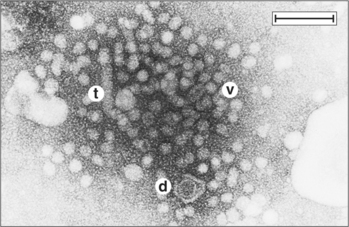
FD’s mother is a hepatitis B virus (HBV) carrier who was identified during maternal screening and was found to have anti-HBc and anti-HBe IgG antibodies specific for the HBV core and HBe (pre core) antigens (HBcAg and HBeAg, respectively). HBeAg is associated with HBcAg (Fig. 39-1). Additional enzyme-linked immunosorbent assays (ELISAs) revealed that the mother was positive for the HBs antigen (HBsAg) but negative for HBeAg. Had the mother been HBeAg positive, the infant would have been given gamma globulin (anti-HBV antibodies) and the first of the three required HBV vaccine antigens. Because the mother was negative for HBeAg, the child had not been given either therapy, according to the regulations in place. HBsAg may also be detected in liver biopsies (Fig. 39-2).

FIGURE 39-1 Serologic and clinical patterns observed during acute and chronic hepatitis B infection.
(From Murray P, Rosenthal K, Kobayashi Y, Pfaller M: Medical Microbiology, 4th ed. St Louis, Mosby, 2002; redrawn from Hoofnagle JH: Annu Rev Med 32:1–11, 1981.)
In some regions, however, different regulations are in place and infants born to mothers who are HBsAg positive are immunized at birth. Otherwise, the first dose of the HBV vaccine is deferred until the infant is 2 to 6 months of age. There are reported cases when vertical transmission has occurred in the first months post partum if the mother becomes infected during this time or if she is a chronic carrier. Additionally, development of FHF in infants, despite appropriate prophylaxis, has been documented.
Implications/Analysis of Laboratory Tests
The infant’s blood cell count indicated a leukocytosis (see Appendix for normal reference values) and thrombocytopenia. Biochemical tests revealed high serum bilirubin, increased ALT and AST levels, decreased albumin, an increased INR (>4; normal ˜1.0), and an increase in PT (normal: 12 to 14 seconds) and aPTT (normal: 25 to 38 seconds). Overall, the results of these tests indicated severe liver damage. Patients with severe FHF have an increased PT because the liver makes the majority of the clotting factors. Consequently, bleeding is common. A diagnosis of HBV infection was confirmed when the ELISA revealed anti-HBc IgM antibodies in FD’s serum, as well as anti-HBc IgG antibodies, transferred via the placenta ELISA. On the basis of the results of these tests, abdominal CT and ultrasonography were requested.
Note: The PT refers to the time required for thrombin to convert plasma fibrinogen to fibrin. The aPTT measures the clotting time of plasma as a result of the activation by factor XII after serum contact with a negatively charged surface (e.g., silica).
OVERVIEW OF XENOTRANSPLANTATION
Challenges of Xenotransplants: Hyperacute Rejection
Transplantation across species barriers (xenotransplantation) is fraught with risks not seen in allotransplants. The first problem that arises from major species barriers is a ubiquitously expressed carbohydrate structure Galα1-3-Galβ1-4GlcNAc-R (α-galactosyl epitope). Humans and Old World monkeys do not express in their genome the gene encoding α1-3-galactosyltransferase (α1-3GT), an enzyme that synthesizes α-galactosyl epitopes on glycoproteins and glycolipids (using uridine diphosphate galactose [UDP-Gal]). Hence we, unlike multiple other species, including pigs, cats, dogs, horses, and New World primates, do not link Galα1-3 to the ends of carbohydrates expressed at the surface of cells. Accordingly, lacking tolerance to this α-galactosyl epitope, we have abundant antibodies (anti-Gal) that target this particular epitope (induced in response presumably to exposure from commensal bacteria, expressing this on their cell walls). Transplantation of organs expressing the α-galactosyl epitope on the surface leads to rapid antibody deposition at the cell surface, complement fixation, and thrombosis, with graft loss. Consequently, it has been thought that if complement activation could be controlled, this form of rejection (hyperacute rejection) would be minimized.