CASE 37
WT has been a “sun worshipper” all his life. Over the past several months he has noticed some worrisome changes in some freckles on his legs and arms, and one in particular seems to have grown substantially, with a significant change in coloration and an irregular contour. You recognize all of these signs as being potentially indicative of malignant melanoma and send him to a local dermatologist for biopsy. The results come back positive. WT has heard about some recent positive data using immunotherapy in melanoma and asks your advice.
QUESTIONS FOR GROUP DISCUSSION
RECOMMENDED APPROACH
DIAGNOSIS
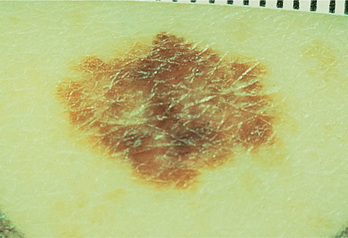
WW was diagnosed with malignant melanoma (Fig. 37-1). The most widely used measurement system (after skin biopsy) to predict 5-year survival is the “Breslow” measurement. In this system a defined melanoma thickness is associated with a 5-year survival in 97% of patients. At the other end of the scale a 10-fold increase in thickness is associated with a 5-year survival of only 32% of the patients. Another system, the “Clark Level of Invasion” can also be used to determine prognosis. There are five Clark levels of invasion, with level one indicating that the melanoma is confined to the outermost layer of skin and level five indicating penetration of the melanoma into fat cells beneath the dermis. This is then correlated with 5-year survival rate after surgical removal of the melanoma.
ETIOLOGY: MALIGNANT MELANOMA
Bedrosian I, et al. Intranodal administration of peptide-pulsed mature dendritic cell vaccines results in superior CD8+ T cell-function in melanoma patients. J Clin Oncol. 2003;21:3826.
Berubstein N. Carcinoembryonic antigen as a target for therapeutic anticancer vaccines: A review. J Clin Oncol. 2002;20:2197.
Cameron EC, et al. Human papillomavirus-specific antibody status in oral fluids modestly reflects serum status in human immunodeficiency virus-positive individuals. Clin Diagn Lab Immunol. 2003;10:431.
Hensin T, et al. Case 7-2004: A 48-year-old-woman with multiple pigmented lesions and a personal and family history of melanoma. N Engl J Med. 2004;350:924.
Choyke PL, et al. Hereditary renal cancers, target for therapeutic anticancer vaccines: A review. Radiology. 2003;226:33.
Clarke MF. Chronic myelogenous leukemia–identifying the hydra’s head. N Engl J Med. 2004;351:634.
Dighiero G, Binet JL. When and how to treat chronic lymphocytic leukemia. N Engl J Med. 2000;343:1799.
Druker BJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031.
Faderl S, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164.
Giannoudis A, et al. Variation in the E2-binding domain of HPV 16 is associated with high-grade squamous intraepithelial lesions of the cervix. Br J Cancer. 2001;84:1058.
Goldman JM, Melo JV. Chronic myeloid leukemia–advances in biology and new approaches to treatment. N Engl J Med. 2003;349:1451.
Hamblin TJ. Predicting progression–ZAP-70 in CLL. N Engl J Med. 2004;351:856.
Hausen HZ. Papillomaviruses and cancer: From basic sciences to clinical application. Nat Rev. 2002;2:342.
Hsueh EC, et al. Active specific immunotherapy of melanoma with a polyvalent vaccine and recombinant human GM-CSF: An immunogenicity study. Proc Am Soc Clin Oncol. 2003;22:176.
Igarashi T, et al. Effect of tumor-infiltrating lymphocyte subsets on prognosis and susceptibility to interferon therapy in patients with renal cell carcinoma. Urol Int. 2002;69:51.
Jamieson CHM, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast crisis CML. N Engl J Med. 2004;351:657.
Karem KL, et al. Optimization of a human papillomavirus-specific enzyme linked immunosorbent assay. Clin Diagn Lab Immunol. 2002;9:577.
Kim CJ, et al. Immunotherapy for melanoma. Cancer Control. 2002;9:22.
Laughlin MJ, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265.
Law TM, et al. Phase III randomized trial of interleukin-2 with or without lymphokine-activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer. 1995;76:824.
Liu KJ, et al. Generation of carcinoembryonic antigen (CEA)-specific T cell responses in HLA-*0201 and HLA-A*2402 late stage colorectal cancer patients after vaccination with dendritic cells loaded with CEA peptides. Clin Cancer Res. 2004;10:2645.
Lode HN, Xiang R, Ursula P. Melanoma immunotherapy by targeting Il-2 depends on CD4+ T cell help mediated by CD40/CD40L interaction. Clin Invest. 2000;105:1623.
Margolin KA. Interleukin-2 in the treatment of renal cancer. Semin Oncol. 2000;27:194.
Mateo L, et al. An HLA-A2 polyepitope vaccine for melanoma immunotherapy. J Immunol. 1999;163:4058.
Matsukura T, Sugase M. Relationship between 80 human papillomavirus genotypes and different grades of cervical intraepithelial neoplasia: Association and causality. J Virol. 2001;283:139.
Menzies HP, et al. Phase I/II study of treatment with dendritic cell vaccines in patients with disseminated melanoma. Cancer Immunol Immunother. 2003;53:125.
Nagayama H, et al. Results of a phase I clinical study using autologous tumour lysate-pulsed monocyte-derived mature dendritic cell vaccinations for stage IV malignant melanoma patients combined with low dose interleukin-2. Melanoma Res. 2003;13:521.
O’Brien SG, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994.
O’Rouke MG, et al. Durable complete clinical responses in a phase I/II trial using an autologous melanoma cell/dendritic cell vaccine. Cancer Immunol Immunother. 2003;52:387.
Pfister DG, Benson AB, Somerfield MR. Surveillance strategies after curative treatment of colorectal cancer. N Engl J Med. 2004;350:2375.
Rai KR, Chiorazzi N. Determining the clinical course and outcome in chronic lymphocytic leukemia. N Engl J Med. 2003;348:1797.
Ravaud A, et al. Subcutaneous interleukin-2 and interferon alpha in the treatment of patients with metastatic renal cell carcinoma–less efficacy compared with intravenous interleukin-2 and interferon alpha. Results of multicenter Phase II trial from the Groupe Francais d’Immunotherapie. Cancer. 2002;95:2324.
Rocha V, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276.
Russo P. Renal cell carcinoma: Presentation, staging, and surgical treatment. Semin Oncol. 2000;27:160.
Saeterdal I, et al. Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc Natl Acad Sci U S A. 2001;98:13255.
Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999;340:1330.
Smith IIJW, et al. Immune effects of escalating doses of granulocyte-macrophage colony-stimulating factor added to a fixed dose, in patient interleukin-2 regimen: A randomized phase I trial in patients with metastatic melanoma and renal cell carcinoma. J Immunother. 2003;26:130.
Ullenhag GJ, Frodin JE. Durable carcinoembryonic antigen (CEA)-specific humoral and cellular immune responses in colorectal carcinoma patients vaccinated with recombinant CEA and granulocyte/macrophage colony-stimulating factor. Clin Cancer Res. 2004;10:3273.