Elizabeth A. Ayello and Joy E. Schank
DEFINITION AND INCIDENCE
Persons at end-of-life are at risk for skin injury and ulcerations due to immobility, inadequate nutrition, incontinence, and underlying disease processes. In particular, pressure ulcers, skin tears, and malignant cutaneous wounds (MCWs) create physical and psychoemotional challenges for patients.
Palliative wound care is an emerging concept. It takes a holistic approach to improving the quality of life and relieving suffering for persons with chronic wounds. Because wound treatment goals and priorities are based on the person’s changing health status, the aims of interventions may shift from wound closure to wound stabilization and prevention of wound deterioration and infection (Ferris, Khateib et al., 2004) (Table 35-1).
| Staging Classification | |||||
|---|---|---|---|---|---|
| Stage 1 | Stage 1N | Stage 2 | Stage 3 | Stage 4 | |
| Wound | |||||
| Closed wound/intact skin | X | ||||
| Closed wound/superficially open to drain then close/hard and fibrous | X | ||||
| Open wound/dermis and epidermis tissue involved | X | ||||
| Open wound/full thickness skin loss involving subcutaneous tissue | X | ||||
| Open wound/invasive to deep anatomic tissues and structures | X | ||||
| Predominant Color | |||||
| Red/pink | X | X | X | ||
| Red/pink/yellow | X | X | |||
| Hydration | |||||
| Dry | X | ||||
| Both moist and dry | X | ||||
| Moist | X | X | X | ||
| Drainage | |||||
| None | X | ||||
| Clear/purulent | X | ||||
| Serosanguineous/bleeding | X | ||||
| Purulent/serosanguineous | X | ||||
| Serosanguineous/bleeding/purulent | X | ||||
| Pain | |||||
| No | X | ||||
| Pain possible | X | X | X | ||
| Yes | X | ||||
| Odor | |||||
| No | X | X | |||
| Yes | X | X | X | ||
| Tunneling/Undermining | |||||
| No | X | X | X | X | |
| Yes | X | ||||
Both pressure ulcers and MCWs can have devastating physical and psychosocial effects. These wounds can be a source of pain, anemia, and infection. These sometimes unsightly and foul-smelling lesions can cause self-concept disturbance, social isolation due to shame or embarrassment, anxiety, fear, and depression (Foltz, 1980; Goodman, Ladd, & Purl, 1993; Miller, 1998; Waller & Caroline, 2000). The family of a patient receiving palliative care at home may not be aware of how to prevent and treat pressure ulcers; therefore, a palliative patient may have to be admitted to the hospital against his or her wishes.
ETIOLOGY AND PATHOPHYSIOLOGY
Pressure Ulcers
Pressure ulcers are wounds caused by “unrelieved pressure resulting in damage of underlying tissue” (National Pressure Ulcer Advisory Panel [NPUAP] et al., 2001). Usually located over bony prominences (e.g., sacrum, heels), pressure ulcers develop when the soft tissue is compressed between a bony prominence and an external surface, disrupting the blood supply and resulting in cellular death. Risk factors for the development of pressure ulcers include immobility, incontinence, inadequate nutrition, friction and shearing forces, and altered level of consciousness (Agency for Healthcare Research and Quality [AHRQ] [formerly the Agency for Health Care Policy and Research], 1992), as well as a previous healed pressure ulcer.
The incidence of pressure ulcers in any particular practice setting is somewhat difficult to determine, since study designs vary significantly. For example, some studies include stage I pressure ulcers and some do not, and as a result study outcomes vary greatly. In the acute care setting, the incidence of pressure ulcers ranges from 0.4% to 38%, with increased incidence in higher-risk groups, such as the elderly and persons with paralysis. The incidence of pressure ulcers in the nursing home setting is 2.2% to 23.9%. Beginning data from home care studies provide a range of 0% to 17% (NPUAP, Cuddigan, Ayello et al., 2001).
Malignant Cutaneous Wounds
MCWs are ulcerating skin lesions that develop when malignant cells infiltrate the epithelium (Foltz, 1980). MCWs may occur as single lesions or in groups (Haisfield-Wolfe & Baxendale-Cox, 1999). Approximately 5% to 10% of persons with metastatic malignancies have MCWs, usually during the last 3 to 6 months of life (Crosby, 1998; Foltz, 1980; Thiers, 1986; Waller & Caroline, 2000). The malignant processes associated with the development of cutaneous metastases include breast cancer, malignant melanoma, lung cancer, and colorectal cancer (Crosby, 1998). These wounds are sometimes called fungating tumor wounds, which refers to the tendency of these wounds to both ulcerate and proliferate (Mortimer, 1998).
MCWs may result from primary skin lesions or metastasis from other malignant processes. As mentioned, breast cancer is the most common cause of MCWs; up to 25% of persons with breast cancer experience skin metastasis (Waller & Caroline, 2000). The malignancies associated with the development of MCWs are listed in Box 35-1.
Box 35-1
Butterworth-Heinemann
Primary Skin Malignancies
Untreated basal cell carcinoma
Untreated squamous cell carcinoma
Malignant melanoma
Metastatic Spread from Primary Malignancy
Breast
Head and neck
Lung
Stomach
Kidney
Uterine
Ovarian
Colon
Bladder
Lymphoma
Melanoma
Data from Mortimer, P.S. (1998). Management of skin problems: Medical aspects. In D. Doyle, G.W.C. Hanks, & N. MacDonald (Eds.). Oxford textbook of palliative medicine (2nd ed., pp. 617-627). New York: Oxford University Press; Crosby, D.L. (1998). Treatment and tumor-related skin disorders. In A.M. Berger, R.K. Portenoy, & D.E. Weissman (Eds.). Principles and practice of supportive oncology (pp. 251-264). Philadelphia: Lippincott-Raven; and Waller, A. & Caroline, N.L. (2000). Handbook of palliative care in cancer. Boston: Butterworth-Heinemann.
The initial appearance of a primary skin malignancy may be a sore that does not heal. Metastatic MCWs may begin as hard dermal or subcutaneous nodules and may be fixed to underlying tissue (Crosby, 1998; Thiers, 1986). These lesions occur most commonly in the vicinity of the primary tumor (Crosby, 1998). However, MCWs are also seen at a secondary site related to metastatic disease (Goodman et al., 1993; Miller, 1998). They may vary in color from flesh-toned to red and are usually asymptomatic at early stages (Crosby, 1998).
Malignancies spread to the cutaneous tissues via direct extension or embolization into the vascular or lymph channels (Goodman et al., 1993; Mortimer, 1998). Eventually these lesions infiltrate the epithelium and supporting lymph and blood vessels, interfering with blood flow and the supply of oxygen and nutrients to the tissues, leading to ulceration (Haisfield-Wolfe & Baxendale-Cox, 1999; Mortimer, 1998). Capillary rupture, necrosis, and infection are common, leading to a purulent, friable, and malodorous ulceration (Foltz, 1980; Mortimer, 1998).
Infections in MCWs may be caused by both anaerobic and aerobic pathogens. Anaerobic organisms proliferate in necrotic tissue. The foul-smelling odor of many of the MCWs is due to the release of malodorous volatile fatty acids as metabolic end-products of anaerobic activity (Mortimer, 1998). These wounds can readily become infected with aerobic organisms and produce yellow to green purulent discharge.
Skin Tears
Skin tears are acute traumatic wounds that occur when the epidermis separates from the dermis (Malone, Rozario, Gavinski et al., 1991). Most skin tears are found on the arms and legs, especially over areas of senile purpura (areas where blood vessels become more purple as an individual ages) (Malone et al., 1991; McGough-Csarny & Kopac, 1998; Payne & Martin, 1990). Skin tears can be very distressing for the patient. This can occur from something as simple as tape used to hold an intravenous line. With age, the subcutaneous tissue decreases and leaves the skin very thin and easy to tear. The main concern is often pain control.
ASSESSMENT AND MEASUREMENT
Risk Assessment
Two validated risk assessment tools that are used in the United States to identify persons at risk for the development of pressure ulcers are the Braden Scale (www.bradenscale.com/braden.pdf) and the Norton Scale (AHRQ, 1992). Although the onset of risk score differs, both scales produce risk scores based on known risk factors, such as mobility, mental status, and moisture. The Centers for Medicaid and Medicare Services (CMS) (2004) also advises that prevention strategies must be implemented for persons with low scores in any risk assessment subscale. These risk scales are readily available in the published guidelines from the AHRQ as well as the newer pressure ulcer guidelines from the Wound Ostomy and Continence Nurses Society (WOCN) (2003). It is important to reassess risk on admission and at intervals, especially in the palliative care setting, where declining functional status and nutritional impairment are anticipated.
Skin Assessment
Do not confuse a skin assessment with a wound assessment. The CMS has provided clinicians with a minimal standard for skin assessment in the recently revised guidance to surveyors for long-term care facilities, Tag F 314 (CMS, 2004). This includes assessing the skin for temperature, color, moisture, turgor, and integrity. Skin assessment should be done on admission, discharge, and weekly. Skin tears and stage I ulcers may be in places that cannot be seen on quick assessment and can be missed. It is important not only to look at the patient’s skin but also to assess pressure areas related to, for example, the wheelchair, bed, linens, stockings, and so on.
Wound Assessment
When any type of ulceration is present, documentation of the degree of tissue destruction is an important aspect of wound assessment. Pressure ulcers, MCWs, and skin tears each have their own classification systems. The current staging system for pressure ulcers from the NPUAP 2001) is as follows:
▪ Stage I: Observable, pressure-related alteration of intact skin whose indicators as compared to an adjacent or opposite area on the body may include changes in one or more of the following parameters: skin temperature (warmth or coolness), tissue consistency (firm or boggy feel), and sensation (pain, itching). The ulcer appears as a defined area of persistent redness in lightly pigmented skin; in darker skin tones, the ulcer may appear with persistent red, blue, or purple hues.
▪ Stage II: Partial-thickness skin loss involving epidermis and/or dermis. The ulcer is superficial and presents clinically as an abrasion, a blister, or a shallow crater.
▪ Stage III: Full-thickness skin loss involving damage or necrosis of subcutaneous tissue that may extend down to, but not through, underlying fascia. The ulcer presents clinically as a deep crater with or without undermining of adjacent tissue.
▪ Stage IV: Full-thickness skin loss with extensive destruction, tissue necrosis, or damage to muscle, bone, or supporting structures (e.g., tendon or joint capsules). Undermining and sinus tracts also may be associated with stage IV pressure ulcers.
Because the depth of tissue destruction cannot be seen in necrotic pressure ulcers, these wounds are categorized as “unstageable” in acute or home care. CMS requires necrotic pressure ulcers to be documented as stage IV in long term care (CMS, 2004).
NPUAP raised the issue of problems with the stage I and II definitions at their 2005 consensus conference (Ankrom, Bennett, Sprigle et al., 2005). At present, NPUAP recommends that a new type of pressure ulcer, deep tissue injury (DTI), does not fit into the current staging system and is working on how best to stage it (Black & NPUAP, 2005). The NPUAP Website (www.npuap.org) provides up-to-date information about DTI.
A special type of pressure ulcer that is seen during the last few weeks of life and signals impending death is called the Kennedy terminal ulcer (Kennedy, 1989). Based on research findings, it is pear shaped with irregular borders; the tissue is red, yellow, or black; onset is sudden; it is usually found on the coccyx or sacrum; and death is imminent (occurring within 2 weeks to several months) (Kennedy, 1989; www.kennedyterminalulcer.com).
Staging of Malignant Cutaneous Wounds
Recognizing that the staging system for pressure ulcers does not necessarily apply to assessment of MCWs, Haisfield-Wolfe and Baxendale-Cox (1999) developed and tested a specific staging system for these lesions, which is presented in Table 35-1. The purpose of this scale is to clarify communication among health care professionals to improve patient care, consultation, and comparison of research results. Haisfield-Wolfe and Baxendale-Cox (1999) used digital photography as an adjunct to observation to assess and record the MCW accurately on initial assessment and follow-up.
Classifying Skin Tears
Skin tears have a separate classification system that has three categories based on the amount of tissue lost. In category I skin tears, there is no loss of the epidermal flap. Category II skin tears have partial loss of the epidermal flap, while in category III there is complete loss of the epidermal skin flap (Payne & Martin, 1990, 1993).
Wound Assessment Variables
Staging or classification of wounds is only one part of wound assessment. Photographic documentation may be helpful in assessing and monitoring all types of ulcerative lesions (Miller, 1998). In addition to the stage of pressure ulcer, CMS (2004) requires the following minimal assessment of the wound:
▪ Location
▪ Size of the wound
Length and width are measured, using established landmarks for measurement.
Depth is noted at the deepest point. This is measured by inserting a sterile applicator into the deepest area, holding or marking the applicator stick at the skin surface level, and measuring from the tip of the stick to the mark.
▪ Exudate
Color and consistency of the drainage are documented. Drainage may be serous, sanguineous, serosanguineous, or purulent.
Amount of drainage is noted. It may be described (1) verbally, as scant, moderate, large, or copious; (2) in terms of the number of soaked dressings; or (3) by weight of the dressings.
▪ Pain
Pain at the site may indicate infection, tissue destruction, or vascular insufficiency. The absence of pain may indicate nerve damage (Hess, 1999).
▪ Color and type of wound bed tissue
Red or pink color generally indicates clean, healthy granulation tissue.
Yellow may be the result of infection-related exudate or necrotic slough.
Black tissue indicates eschar from necrosis.
Some wounds have mixed colors. Clinicians use one of two approaches in documenting the color of mixed wounds: (1) use the least desirable color or (2) estimate the percentage of each color within the wound (Hess, 1999). For clear communication, the palliative care team should adopt one of these two approaches as a standard.
▪ Description of wound edges and surrounding tissue
Color and condition of the skin surrounding the pressure ulcer are noted (Hess, 1999).
Temperature of the intact skin is also noted. Heat is a sign of pressure ulcer formation and can also indicate an underlying infection (Hess, 1999).
Some clinicians go beyond these minimal documentation requirements and include the following additional wound characteristics:
▪ Tunneling, which is tissue destruction under intact skin, is also noted and measured. It is measured by inserting a sterile applicator into tunneled areas, holding or marking the applicator stick at the wound edge, and measuring from the tip of the stick to the mark.
▪ Odor is also noted. Wound odor may be described as pungent, strong, foul, fecal, or musty.
HISTORY AND PHYSICAL EXAMINATION
Medical history identifies those persons who have diseases that create skin integrity risks, such as heart disease, peripheral vascular disease, diabetes, and cancer. Past treatments that may affect skin integrity and wound healing, such as radiation therapy or extensive surgery, are also important aspects of the patient’s history. In addition, the advanced practice nurse (APN) assesses the patient’s activity level, mobility, level of consciousness, nutritional status, and hydration status. Many of these factors are included in the risk assessment tools described.
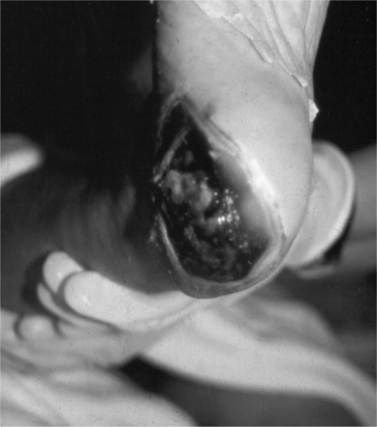
The routine skin inspection includes observing for any areas of discomfort, redness, edema, or ulceration, paying special attention to bony prominences, heels (Figure 35-1), and elbows. Some patients with cancer can have a dehiscence of their wound. In addition, for persons at risk for MCWs, the skin is inspected for the presence of any new nodules, especially in the same general region as the primary tumor. The presence of incontinence or excessive diaphoresis must also be noted for all patients.
 |
| Figure 35-1 |
When an ulcerative lesion is present at initial assessment, a history of the lesion is important, including how long it has been present, previous treatments for the lesion, the effectiveness of these treatments, and the psychosocial effect of the lesion on the patient and family (Miller, 1998).
DIAGNOSTICS
On occasion, wound cultures may be appropriate to determine the exact organism causing an infection, to guide subsequent more-specific interventions. Orders must be written to obtain cultures for both aerobic and anaerobic organisms. It is often appropriate to forgo cultures and treat the likely infection on the basis of the appearance and odor of the exudate as recommended in the Clinical Signs and Symptoms checklist developed to identify infection in chronic wounds (Gardner, Frantz, Troia et al., 2001).
INTERVENTION AND TREATMENT
At a CMS meeting (www.cms.hhs.gov/transmittals/downloads/R4SOM.pdf), it was recommended that the usual care of chronic wounds include the following elements:
1. Debridement
2. Cleansing
3. Dressings
4. Compression (venous ulcers only)
5. Antibiotics
6. Pressure redistribution and offloading
7. Team care
8. Nutrition
Prevention of Pressure Ulcers
▪ Document the risk assessment and initiate prevention protocols based on levels of risk (Ayello & Braden, 2002).
▪ Assess skin routinely; the frequency depends on the risk assessment. For persons who are relatively inactive, daily inspection by the nurse and/or primary caregiver is recommended.
▪ Keep the skin clean and free of excessive external moisture:
▪ Use mild cleansers that minimize irritation and dryness.
▪ Treat dry skin with moisturizers.
▪ If there is incontinence, perspiration, or drainage, use padding or dressing materials that whisk moisture away from skin rather than keeping the moisture against the skin, causing maceration (WOCN, 2003).
▪ Use skin barriers (creams, ointments, pastes, films, hydrocolloids, and film-forming products) to protect and maintain the skin’s integrity (WOCN, 2003).
▪ Prevent friction and shear injuries:
Use proper positioning and careful transfer and turning techniques. Two-person lifts using the bed sheets or a pad for turning and lifting are very helpful. Overhead trapezes can also be of assistance if the patient is able to use this device.
Friction can also be reduced by using lubricants, such as corn starch or petroleum jelly, polyurethane thin film dressings, or hydrocolloids, over heels and elbows or protective padding, such as sleeves and heel or elbow protectors.
▪ Encourage dietary intake with sufficient protein, calories, vitamins, minerals, and fluids. Vitamin and zinc supplements are indicated when a deficiency is present.
▪ Reduce pressure on tissues:
Teach the patient and caregiver the importance of turning and repositioning at least every 2 hours. Pain management may be an important part of getting a patient to turn and reposition. If a patient and/or caregivers decide that turning and repositioning cause too much discomfort for the patient, it should be clearly explained to them the consequences to the skin with pressure ulcer formation. An understanding of this by the patient and family needs to be documented.
Active or passive range-of-motion exercises can also be used to relieve pressure and to maintain muscle tone.
Prevent positioning on the trochanter.
Use supports, such as wedges, pillows, and heel supports.
Persons who are at high risk may benefit from using a special pressure-redistributing device, such as air-filled overlays, alternating air-filled mattress overlays, foam-, gel- or water-filled mattress overlays, and specialty mattresses and beds (AHRQ, 1992; Hess, 1999).
Prevent the pressure of chair sitting by teaching patients to shift their weight every 15 minutes, if they are able, or by instructing caregivers to reposition the patient in the chair at least every hour. Pressure-reducing devices, such as foam, gel, or air chair pads, may also be used.
Instruct caregivers not to use doughnut-type devices.
▪ Prevent other mechanical tissue damage. Evidence suggests that massage over bony prominences may actually lead to deep tissue trauma and increase the risk of pressure ulcers (AHRQ, 1992). All caregivers need to be instructed not to massage any reddened areas.
Preventing and Treating Skin Tears
There is no universal protocol for preventing skin tears (Baranoski, 2000, 2001). Clinicians (Baranoski, 2000, 2001; Baranoski & Ayello, 2004) have suggested the following interventions to help prevent skin tears:
▪ Have patient wear long sleeves and pants to protect the skin over these at-risk areas.
▪ Pad the chair arm and wheelchair leg supports.
▪ Use the least potentially disruptive method of securing a dressing or drain to a person’s skin. This might include paper tape or nonadherent dressings, gauze wrap, and tube securing devices.
▪ Avoid rubbing or scrubbing the skin during bathing and cleansing activities.
▪ Use no-rinse bathing products with emollients; limit use of nonemollient soap and alcohol products on the skin.
▪ Pat the skin dry rather than rubbing the skin dry.
▪ Apply moisturizing products to treat xerosis (dry) skin.
▪ Gently handle skin when turning and positioning patients; lift rather than drag skin.
Skin tears can be painful. Various treatments are used for skin tears, including the following:
▪ Initially the tear is cleansed with normal saline, wound cleanser or other solution, then patted dry.
▪ Another approach to skin tears includes applying hydrogel to minimally exuding wounds. Sometimes health care providers cover this with an Adaptic (nonadhering dressing; Johnson & Johnson)-type product, to decrease chances of the wound adhering to the dressing. This can also decrease pain. Others top the hydrogel with gauze if there is no worry of further skin damage. Telfa (nonadhering dressing; Kendall)-type products have also been used, often in conjunction with antibiotic ointment or hydrogel. At times, Telfa is the only treatment. If there is increased drainage, hydrofiber or calcium alginate dressings have been used and topped with gauze. Dressings are usually changed daily with these approaches and more often if necessary.
Treatment of Malignant Cutaneous Wounds
An ulceration that is confined to local recurrence may be treated by one or a combination of the following: surgery, radiation therapy, chemotherapy, or hyperthermia. However, lesions that recur only locally are rare in end-of-life care; most skin ulcerations are a manifestation of a disseminated disease. Hormonal manipulation may shrink some lesions associated with metastatic breast cancer. Most often, the care is directed to minimizing infection, bleeding, and odor (Foltz, 1980; Goodman et al., 1993; Miller, 1998).
Care and Dressing of Ulcerations
All ulcerative lesion treatment plans include instructions for the cleansing solution, frequency of cleansing, type of dressing materials, and frequency of dressing changes. In addition, each includes a time frame for reevaluation (Hess, 1999). Consultation with a wound, ostomy, continence nurse (formerly known as an enterostomal therapist) is helpful when treating particularly challenging wounds.
▪ Irrigate the wound to flush out cellular debris and drainage. Wounds are irrigated at time of dressing changes.
Use normal saline solution or water for a wound with no signs of infection. A 35-ml syringe with a 19-gauge needle or angiocatheter delivers adequate pressure. Bulb syringe, gravity drip through intravenous tubing, or piston syringe with rubber catheter attached may be used but may not deliver adequate pressure for thorough irrigation.
Use one of the following for short-term treatment of infected and foul-smelling wounds. All of these solutions are toxic to fibroblasts, inhibiting wound healing (Hess, 1999). The solutions must be rinsed from the wound with normal saline solution. Use these solutions for infected wounds only and discontinue after infection is adequately treated:
• Acetic acid solution is effective for Pseudomonas infection (Hess, 1999). This solution can be made from equal parts of vinegar and water (Foltz, 1980).
• Hydrogen peroxide works for mechanical cleansing, helping to dissolve and remove crusted exudate.
• Povidone-iodine can be used for its broad-spectrum antimicrobial action (Hess, 1999).
• Sodium hypochlorite solution (Dakin’s solution) is effective for staphylococcal and streptococcal infections (Hess, 1999). It may also be helpful for managing odor. This solution can be made by using one part of household bleach to nine parts of water (Foltz, 1980).
▪ Debridement is performed to remove necrotic tissue and promote healing (Walker, 1996; Hess, 1999). When healing is anticipated, this is a necessary step. The clinician must, of course, be cautious of the wound with poor arterial flow and consult a vascular surgeon as appropriate. In the palliative care setting, complete healing of a pressure ulcer is not always possible, and healing of an MCW is next to impossible. Consider the purpose and potential outcomes of debridement for each individual. When removal of necrotic tissue will decrease infection, reduce inflammation, and improve comfort, debridement is appropriate. If the debridement procedure itself causes significant discomfort, the removal of the “protective” layer of eschar causes prolonged pain or active bleeding, or the patient is not likely to live long enough to derive benefit, debridement may not be appropriate. Different types of debridement are available, and often a combination of them is used to achieve adequate wound debridement and wound bed preparation.
Surgical debridement is the quickest method but may be the most uncomfortable. It may require general anesthesia. Pain management is an important issue.
Mechanical debridement includes the use of wet-to-dry dressings to pull necrotic tissue from the wound. It may be helpful for removing encrusted, purulent materials. The disadvantage of this method is that it is nonselective: healthy tissue is also removed with the dry dressings and it is painful. Hydrotherapy and wound irrigation are other examples of mechanical debridement.
Enzymatic debridement is the use of topical enzymes to digest necrotic tissue. These products can be very effective. Some enzymes are selective and are specific to collagen, whereas others are nonselective and will break down many types of proteins. These medications can be expensive, so the ability of the family to pay for the prescription may be a consideration. Enzymes that have been used for this purpose include collagenase (Santyl) and papain-urea ointments (Accuzyme [also available as a spray], Gladase, Kovia, and others). It is important to read the manufacturer’s directions specific to each type of enzyme for correct method and frequency of application (Ayello & Cuddigan, 2004).
Autolytic debridement is the use of the body’s enzymes and white blood cells to remove the necrotic tissue. Many dressings are designed to promote this process (Ayello & Cuddigan, 2004; Hess, 1999; Walker, 1996), including transparent films, hydrocolloids, semipermeable polyurethane foams, and hydrogels.
Maggot debridement therapy (MDT) has been revived as a debridement option after being out of use for many years. Beginning evidence points to better patient outcomes when free-range technique rather than contained maggots are used (Steenvoorde, Jacobi & Oskam, 2005).
▪ Clean the skin around the wound with normal saline solution or plain water.
▪ Pack deep wounds, being careful not to pack too tightly; the dressing needs to be flexible enough to touch all wound surfaces:
Any solution used on the packing materials must be nontoxic to cells. A possible exception is the use of wet-to-dry dressings moistened with Dakin’s solution for infection and odor control. However, as mentioned, both the procedure and the solution are cytotoxic and its use should be limited (CMS, 2004).
Wounds that require packing or fillers of any kind generally require a secondary dressing.
▪ Select a dressing that promotes a moist, but not wet, environment. The wound characteristics (stage, depth, location, and the amount of drainage) help guide the selection of the appropriate dressing:
Use moisture-retentive dressings for wounds that have light to moderate drainage. Examples for stage I or II ulcers include polyurethane transparent film dressings, hydrocolloids, and foam. Hydrofiber or calcium alginate may be used under these dressings to control moderate exudate. For stage III and IV ulcers, both a primary and a secondary dressing are often required. The primary dressing comes in contact with the wound first. Hydrogels may be used for the dry wound, whereas hydrofiber and calcium alginate are used to absorb exudate from the draining wound. These are then topped with the secondary dressing, which may be a hydrocolloid, foam, composite, or other type of cover dressing. Gauze or abdominal pad dressings are also used as the secondary dressing.
Use absorbent dressings such as foam, calcium alginates, or hydrofiber for wounds with moderate to heavy drainage. There are many excellent products on the market, both absorbent wound fillers and absorbent dressings:
• If wound drainage is less than 50 ml/day, an absorbent dressing may be sufficient.
• If wound drainage is greater than 50 ml/day, a wound drainage bag or pouch may be helpful. Use of a drainage collection system allows accurate measurement of drainage, decreases dressing changes, decreases contamination, improves comfort, and protects surrounding skin (Hess, 1999). A good seal around any appliance used to collect drainage is important. Stomahesive paste or a similar product may be helpful to fill in creases or indentations in the skin and improve the seal (Miller, 1998). Pouching is an effective way of managing patients who develop fistulas. A WOCN nurse can provide insight about the most cost-effective type of drainage bag and sealant to use.
Dry wounds may benefit from use of one of the many hydrogels available. Most are amphorous gels and others are available as sheets of dressing materials.
A new method of wound dressing is the wound vacuum-assisted closure. This is intended for pressure ulcers, flaps, diabetic ulcers, and chronic wounds. It is not appropriate for MCWs. The purpose of this device is to promote wound closure by exerting a continuous and/or intermittent negative pressure on the wound. This pressure is typically kept on 22 of 24 hours each day (KCI, 2005).
▪ Protect surrounding skin.
Remove all dressings carefully to prevent damage to surrounding tissue. An acetone-free adhesive remover or baby oil may be helpful in removing old adherent dressings.
When significant drainage necessitates frequent dressing changes, avoid using tape to secure dressings if possible. Options include gauze wrap bandages, flexible netting or stretch elastic bands (e.g., tube tops), and Montgomery straps. Another option is to place strips of hydrocolloid strategically around the wound and tape to the hydrocolloid rather than to the patient’s skin.
Skin sealants, petroleum-based products, and other water-resistant products such as protective barrier ointments may be used to protect the surrounding skin from wound drainage.
▪ Control bleeding. MCWs are especially prone to bleeding as a result of the disruption of the capillary bed. The selection of an intervention depends on the degree of oozing and patient tolerance.
Reduce any trauma to the tissue by keeping the wound moist and using nonadherent dressings if needed. If dressings become too dry, it may be helpful to moisten with saline solution before removal (Goodman et al., 1993). Another option may be to have the patient shower in order to loosen the dressing. Calcium alginate and hydrofiber dressings may be effective in stopping minor bleeding. Wet-to-dry dressings should be avoided.
Pressure may be applied to visible bleeding vessels, but only if the underlying structures can support the force (Foltz, 1980; Waller & Caroline, 2000).
Silver nitrate sticks can be used for pinpoint capillary oozing (Foltz, 1980).
Gauze soaked in 1:1000 epinephrine can be applied over areas of bleeding (Waller & Caroline, 2000).
Coagulant dressings, such as absorbable gelatin (Gelfoam), or QR Powder (www.biolife.com) are helpful for multiple areas of oozing.
Sucralfate paste is another option for widespread oozing. Crush a 1-g sucralfate tablet and mix with 2 to 3 ml of water or soluble gel (Waller & Caroline, 2000).
Radiation therapy is also an option for some patients (Mortimer, 1998).
▪ Control odor, which is often a problem with MCWs. This is most likely caused by infection in necrotic tissue and can be minimized if the lesion can be adequately cleansed and debrided or the infection treated with a bacteriostatic agent. Additional measures that filter or mask the odor are beneficial when other interventions do not eliminate it:
Topical metronidazole is active against anaerobic bacterial infections and has been demonstrated to be helpful in controlling wound odor (Finlay, Bowszyc, Ramlau et al, 1996; Poteete, 1993; Rice, 1992). Most studies use metronidazole 0.75% to 0.8% gel. Some practitioners report that use of metronidazole (parenteral) solution to irrigate wounds controls odor well (McMullen, 1992). Metronidazole irrigation has not been studied to compare its effectiveness and cost with those of topical gel.
Sodium hypochlorite solution can be used for its debriding and bacteriostatic properties, either in irrigation or on wet-to-dry dressings (Foltz, 1980; Hess, 1999). Use with caution, however, as it is very irritating to healthy tissues (both in and around the wound) and interferes with blood clotting.
A chlorophyll-containing ointment applied to the wound or chlorophyll tablets taken orally may be helpful (Goodman et al., 1993), although no studies have compared the effectiveness of this intervention with that of the others discussed.
Some dressings are designed to filter odors with a carbon-and-charcoal layer. Some products on the market are combination dressings, with both absorptive and carbon-filtering layers. Until comparative studies are available, the APN must determine the best product with respect to amount of drainage, size of wound, ease of use, and cost. The following are dressings with carbon layers (Hess, 1999):
• Lyofoam C Polyurethane Foam Dressing with Activated Carbon (ConvaTec) is designed to neutralize odors on wounds with light or moderate exudate.
• CarboFlex Odor Control (ConvaTec) is designed for odor control on wounds with light or moderate exudate.
• Odor-Absorbent Dressing (Hollister) is designed for odor control on dry wounds or as a secondary dressing over an absorbent dressing if there is exudate.
Home remedies for controlling odor include honey, sugar, yogurt, or buttermilk in wounds (Goodman et al., 1993; Miller, 1998). No studies have evaluated the effectiveness of these treatments.
Deodorizers can be used to mask odors. These often have odors of their own, which may or may not be desirable to the patient:
• Some deodorizers (e.g., Banish, Hexon) can be used sparingly on dressings.
• Room deodorizers, including commercial products, scented candles, and scented oils (e.g., spirit of wintergreen), may be used.
• Some clinicians report using pans of charcoal briquettes under the bed to assist in absorbing room odor. No studies have reported on the effectiveness of this approach.
Treat pain. Depending on the severity of the pain, the APN may select a local treatment, a systemic analgesic, or a combination of several interventions.
• Aluminum hydroxide–magnesium hydroxide suspension (Maalox) or yogurt applied to the wound may relieve burning sensations (Waller & Caroline, 2000). However, Maalox can be drying, and yogurt can be messy and require frequent dressing and linen changes. The reports of using these interventions are anecdotal; no research has documented their effectiveness.
• Since some of the pain is due to inflammation, systemic nonsteroidal antiinflammatory drugs (NSAIDs) are appropriate. For moderate to severe pain, an opioid analgesic may be required.
PATIENT AND FAMILY EDUCATION
The patient and family require instruction in the prevention and management of ulcerative lesions (Tomaselli, 2005). A comprehensive teaching plan includes the following:
▪ Teach general assessment and skin care measures:
Inspect the skin daily for redness, irritation, or breakdown.
Keep the skin clean and prevent excessive dryness:
• Avoid hot water, use a mild cleanser, and minimize force and friction on skin (AHRQ, 1992).
• Keep air humidified.
• Use a moisturizer on the skin.
• Clean the patient as soon as possible if there is urinary or bowel incontinence.
Avoid massage over bony prominences.
Keep the bed and bed clothing clean and dry. Use incontinence pads as necessary.
▪ Teach measures to reduce pressure and friction:
Use heel and elbow protectors.
Use a recommended special mattress and overlay.
Position the patient to prevent pressure, using pillows, wedges, or other padding. Prevent positioning of the patient on the trochanter.
Keep the head of the bed at its lowest comfortable position to prevent shearing that can occur when the patient slides down in bed.
Use good technique when lifting, using two-person lifts whenever possible. Use lift pads instead of dragging the patient. Add an overhead trapeze to the bed when feasible.
▪ Teach appropriate wound cleansing and dressing techniques for those situations in which the family will be providing some of the wound care. Teaching should include demonstration, verbal instruction, and return demonstration. Ensure that the caregiver is comfortable with the procedure. Issues to be addressed in the teaching plan include the following:
Preparation and storage of cleansing solution
Cleansing techniques for wound and surrounding skin
Use of all dressing products, including fillers and secondary dressings
Appropriate disposal of soiled dressing materials
▪ Teach the patient and family to report any changes to their health care team:
Changes in wound size, color, drainage, or odor
Changes in the appearance of surrounding skin
Signs of discomfort
EVALUATION AND PLAN FOR FOLLOW-UP
The APN monitors for changes in any of the wound assessment variables from the baseline and changes the intervention plan accordingly. For pressure ulcers, the Pressure Ulcer Scale for Healing (PUSH) (www.novartisnutrition.com/pdfs/us/moreproductinfo/PUSH_Tool_03.pdf) can be a way of monitoring wound progress. The goal of care is determined by the underlying cause and the patient’s overall condition. It may not be appropriate to expect wound healing because of the poor nutritional status and impaired circulation many patients have at end-of-life. Under these circumstances, the goals may be to prevent or minimize progression and to prevent infection. For patients with MCWs in end-of-life care, the goal is to promote physical and psychoemotional comfort by managing pain, drainage, infection, and odor.
The entire team is involved in the planning, intervention, and evaluation process. Support from the physical therapist, occupational therapist, pharmacist, social workers/counselors, home health aides, clergy, and volunteers is required to address the many needs of the patient and family. Consultation with a certified wound specialist is extremely helpful when facing particularly challenging wounds.
Mrs. J. is 83 years old and has a diagnosis of metastatic breast cancer. She has an extensive fungating tumor wound covering most of the right side of her chest. The skin is reddish purple from the clavicle to the bottom of the rib cage and from the axilla to the sternum, and there are multiple raised, hard nodules across the chest. There are multiple areas where the skin appears to have surface abrasions, and there is an open wound located about 5 cm from the sternum and 15 cm from the clavicle that measures 10 cm across and 8 cm down. The wound bed is light red around the edges, but about 60% of the wound is covered by a yellowish, stringy, purulent material. The wound is deepest at the sternal margin and measures 1.5 cm. No tunneling is observed and there is no bleeding. The patient reports that the wound is not painful, but the foul odor is “driving her and her family crazy.” She has been cleaning the wound with soap and water using a gauze pad and covering it with a gauze dressing. There is a moderate amount of serosanguineous drainage noted. Mrs. J. reports the gauze gets soaked and needs to be changed four or five times each day. She also says that the surface abrasions surrounding the wound were caused by the tape.
The clinician determines that this is a stage III MCW. She recommends initially using Dakin’s solution for infection control followed by rinsing with normal saline solution for 3 days. After the first 3 days, saline solution only is to be used for wound cleansing. The surrounding skin is cleaned with normal saline solution and a protective skin barrier is applied around the wound to protect the skin from drainage. Metronidazole 0.75% gel is applied topically to the wound bed. A wet-to-damp gauze dressing is selected, and saline solution is used to wet the gauze, which is then fluffed to fill the wound lightly. This dressing is covered with a gauze pad, which is reinforced with a sanitary napkin. An elastic “tube top” is used to secure the dressing in place to prevent tape from touching the sensitive skin.
After 1 week the clinician reassesses the wound and finds that only about 20% of it shows signs of slough and the odor has decreased. The padding has been sufficient to control the drainage during the day, but the dressing is sometimes soaked through by morning. The cleansing and dressing procedures are continued and additional padding by sanitary napkins used at night. After another week, the metronidazole gel is discontinued as the wound odor is no longer a problem. The clinician changes the dressing procedure to cleansing with normal saline solution, filling the wound bed with an alginate because of the amount of exudate, covering the alginate with a gauze pad to fit the wound, and covering the gauze with a pad. The dressing is secured with the tube top, and the caregiver is instructed to change the dressing every 3 days or when there are signs of moisture on the pad. (If the surrounding tissue was healthy and could tolerate the adhesive, a hydrocolloid wound filler and dressing might have been selected at this point. This type of dressing might allow for more time between dressing changes.)
A month later the wound is larger, now measuring 11 cm by 8.5 cm. The wound bed is 90% pink and drainage is moderate. The dressing requires changing every 2 days, but these procedures are becoming more difficult because Mrs. J. is weaker and maneuvering the tube top for dressing changes requires much effort by both Mrs. J. and her caregiver. To make securing the dressing easier, the clinician applies four 2 × 5-cm strips of hydrocolloid dressing wafers to the healthy skin at the four corners of the wound and tapes the dressing in place, using the hydrocolloid wafer as the tape anchor.
REFERENCES
Agency for Healthcare Research and Quality (AHRQ), Pressure ulcers in adults: Prediction and prevention: Clinical practice guideline number 3. ( 1992)Author, Rockville, Md..
Ankrom, M.; Bennett, R.; Sprigle, S.; et al.National Pressure Ulcer Advisory Panel, Pressure-related deep tissue injury under intact skin and the current ulcer staging systems, Adv Skin Wound Care 18 (1) ( 2005) 35–42.
Ayello, E.A.; Braden, B., How and why to do pressure ulcer risk assessment, Adv Wound Care 15 (3) ( 2002) 125–131.
Ayello, E.A.; Cuddigan, J.E., Conquer chronic wounds with wound bed preparation, Nurse Pract 29 (3) ( 2004) 8–25.
Baranoski, S., Skin tears: The enemy of frail skin, Adv Skin Wound Care 13 (3) ( 2000) 123–126.
Baranoski, S., Skin tears: Guard against this enemy of frail skin, Nurs Manage 32 (8) ( 2001) 25–31.
Baranoski, S.; Ayello, E.A., Wound care essentials: Practice principles. ( 2004)Lippincott Williams & Wilkins, Springhouse, Pa..
Black, J.; National Pressure Ulcer Advisory Panel, Moving toward consensus on deep tissue injury and pressure ulcer staging, Adv Skin Wound Care 18 (8) ( 2005) 415–421.
Centers for Medicare and Medicaid Services (CMS), CMS manual for guidance to surveyors for long term care facilities, Tag F 314, Retrieved August 10, 2006, from www.cms.hhs.gov/mcd/viewmcac.asp?where=&what=&from=&mid=28&basketitem=mcac:28:%20Usual%20Care%20of%20Chronic%20Wounds:3/29/2005 ( 2004).
Crosby, D.L., Treatment and tumor-related skin disorders, In: (Editors: Berger, A.M.; Portenoy, R.K.; Weissman, D.E.) Principles and practice of supportive oncology ( 1998)Lippincott-Raven, Philadelphia, pp. 251–264.
Finlay, I.G.; Bowszyc, J.; Ramlau, C.; et al., The effect of topical 0.75% metronidazole gel on malodorous cutaneous ulcers, J Pain Symptom Manage 11 (1996) 158–162.
Foltz, A.T., Nursing care of ulcerating metastatic lesions, Oncol Nurs Forum 7 (2) ( 1980) 8–13.
Gardner, S.E.; Frantz, R.A.; Troia, C.; et al., A tool to assess clinical signs and symptoms of localized chronic wound infection: Development and reliability, Ostomy Wound Manage 47 (1) ( 2001) 40–47.
Goodman, M.; Ladd, L.A.; Purl, S., Integumentary and mucous membrane alterations, In: (Editors: Groenwald, S.L.; Frogge, M.H.; Goodman, M.; et al.) Cancer nursing: Principles and practice ( 1993)Jones & Bartlett, Boston.
Haisfield-Wolfe, M.E.; Baxendale-Cox, L.M., Staging of malignant cutaneous wounds: A pilot study, Oncol Nurs Forum 26 (1999) 1055–1064.
Hess, C.T., Clinical guide: Wound care. 3rd ed. ( 1999)Springhouse Corporation, Springhouse, Pa..
KCI, How V.A.C. therapy works, Retrieved July 25, 2006, from www.kci1.com/82.asp ( 2005).
Kennedy, K.L., The prevalence of pressure ulcers in an intermediate care facility, Decubitus 2 (2) ( 1989) 44–45.
Malone, M.L.; Rozario, N.; Gavinski, M.; et al., The epidemiology of skin tears in the institutionalized elderly, J Am Geriatr Soc 39 (6) ( 1991) 591–595.
McGough-Csarny, J.; Kopac, C.A., Skin tears in institutionalized elderly: An epidemiological study, Ostomy Wound Manage 44 (3A suppl) ( 1998) 14S–24S.
McMullen, D., Topical metronidazole: Part 2, Ostomy Wound Manage 38 (3) ( 1992) 42–46.
Miller, C., Skin problems in palliative care: Nursing aspects, In: (Editors: Doyle, D.; Hanks, G.W.C.; MacDonald, N.) Oxford textbook of palliative medicine2nd ed. ( 1998)Oxford University Press, New York, pp. 642–656.
Mortimer, P.S., Management of skin problems: Medical aspects, In: (Editors: Doyle, D.; Hanks, G.W.C.; MacDonald, N.) Oxford textbook of palliative medicine2nd ed. ( 1998)Oxford University Press, New York, pp. 617–627.
In: (Editors: National Pressure Ulcer Advisory Panel (NPUAP); Cuddigan, J.; Ayello, E.A.; et al.) Pressure ulcers in America: Prevalence, incidence, and implications for the future ( 2001)Author, Reston, Va..
Payne, R.L.; Martin, M.L., The epidemiology and management of skin tears in older adults, Ostomy Wound Manage 26 (1) ( 1990) 26–37.
Payne, R.L.; Martin, M.L., Defining and classifying skin tears: Need for a common language, Ostomy Wound Manage 39 (5) ( 1993) 16–20; 22-24, 26..
Poteete, V., Case study: Eliminating odors from wounds, Decubitus 6 (4) ( 1993) 43–46.
Rice, T.T., Metronidazole use in malodorous skin lesions, Rehabil Nurs 17 (1992) 244–245; 255..
Steenvoorde, P.; Jacobi, C.E.; Oskam, J., Maggot debridement therapy: Free-range or contained? An in-vivo study, Adv Skin Wound Care 18 (8) ( 2005) 430–435.
Thiers, B.H., Dermatologic manifestations of cancer, CA: Cancer J Clin 36 (1986) 130–148.
Tomaselli, N.L., Teaching the patient with chronic wound, Adv Skin Wound Care 18 (7) ( 2005) 379–387.
Walker, D., Choosing the correct wound dressing, Am J Nurs 96 (9) ( 1996) 35–39.
Waller, A.; Caroline, N.L., Handbook of palliative care in cancer. ( 2000)Butterworth-Heinemann, Boston.
Wound Ostomy and Continence Nurses Society (WOCN), Guideline for prevention and management of pressure ulcers. Number 2 WOCN Clinical Practice Guideline Series. ( 2003)Author, Glenview, Ill..


