Debra E. Heidrich and Peg Esper
DEFINITION AND INCIDENCE
The International Association for the Study of Pain (IASP) defines pain as an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage (IASP, 1979). Pain, however, is a highly personal and subjective experience. McCaffery (1968) proposed the definition most applicable to clinical practice: “Pain is whatever the experiencing person says it is, existing whenever he/she says it does.” The patient’s self-report of pain is its single most reliable indicator. In other words, the patient’s report must be accepted at face value (American Pain Society, 2003).
Pain may be classified based on duration or by inferred pathophysiology. The interventions selected to treat pain will be influenced by both of these types of classification (McCaffery & Pasero, 1999). These classifications are as follows:
Types of Pain Based on Duration
▪ Acute pain is relatively brief in duration. There is a recognized cause of the pain, and the pain diminishes as healing takes place. With acute pain, there may or may not be observable autonomic signs of discomfort (e.g., increased pulse rate and blood pressure) and nonverbal signs (e.g., tense muscles, facial grimace).
▪ Chronic pain is described as pain that persists after the resolution of the initial injury. It is perceived as irreversible and meaningless. Due to physiologic and behavioral adaptation, there are few or no autonomic or nonverbal signs of discomfort.
▪ Breakthrough pain (also called episodic pain) is defined by Portenoy and Hagen (1990) as a “transitory exacerbation of pain that occurs on a background of otherwise stable pain in a patient receiving chronic opioid therapy” (p. 273). Breakthrough pain tends to occur in persons with higher pain severity and is associated with distress and disability (Caraceni, Martini, Zecca et al., 2004; Zeppetella, O’Doherty, & Collins, 2000). The three subtypes of breakthrough pain are listed next (Mercadante & Portenoy, 2001; Portenoy & Hagen, 1990). Assessment of the onset, duration, frequency, and precipitating factors assists in determining the type and guides the treatment.
End-of-dose failure occurs when the blood levels of analgesics are declining. This indicates a need to increase the dose or the frequency of administration of the analgesic.
Nonincident breakthrough pain has no identified precipitant.
Types of Pain Based on Inferred Pathophysiology
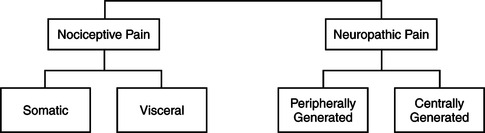
▪ Nociceptive pain arises from direct stimulation of the afferent nerves in the skin, soft tissue, or viscera. This type of pain may be further classified as somatic pain, caused by stimulation of nociceptors in the skin, joints, muscle, bone, or connective tissue; or visceral pain, caused by stimulation of nociceptors in the visceral organs (Figure 32-1).
 |
| Figure 32-1 |
▪ Neuropathic pain results from abnormal processing of sensory input due to nerve damage. This type of pain may be further classified as centrally generated pain or peripherally generated pain.
Patients may experience several different types of pain at the same time; some of these pains may be somatic, some visceral, some neuropathic, and some mixed. Because interventions are based on the type and severity of pain, thorough assessment of each site of pain is critical to appropriate pain management.
Pain is a common symptom with advanced diseases, affecting at least up to 90% of people with metastatic cancer, 90% of those with acquired immunodeficiency syndrome (AIDS), 65% of people with multiple sclerosis, 78% of people with cardiac disease, 8% of people with cerebrovascular disease, and 20% of people with diabetes (Anderson, Vestergaard, Ingeman-Nielsen et al., 1995; Biovie, 1999; Breitbart, Passik, & Rosenfeld, 1999; Corbett, 2005; Levenson, McCarthy, Lynn et al., 2000; McCarthy, Lay, & Addington-Hall, 1996; Ogle & Hopper, 2005). Breakthrough pain occurs in up to 89% of persons with advanced cancer (Zeppetella et al., 2000). The incidence of breakthrough pain in nonmalignant diseases is not investigated as extensively but is reported with many different disease processes (Svendsen, Andersen, Arnason et al., 2005). Zeppetella, O’Doherty, & Collins (2001) reported breakthrough pain in 63% of persons admitted to a hospice with noncancer diagnoses. Important to note and just as significant is the fact that many persons also experience pains unrelated to their primary diagnosis, such as arthritis and chronic low back pain.
Pain control at the end-of-life remains an important, yet often neglected, issue. Data from the SUPPORT study showed a high incidence of uncontrolled pain (from 74% to 95%) in very ill and dying hospitalized adults despite planned interventions from nurses encouraging physicians to attend to pain control (SUPPORT Study Principal Investigators, 1995). A recent study shows that patient satisfaction with pain control has improved only slightly since the implementation of pain standards by the Joint Commission on the Accreditation of Healthcare Organizations in 2001 (Leddy & Wolosin, 2005).
Uncontrolled pain causes physical, psychosocial, emotional, spiritual, and financial burdens and decreases quality of life. Pain interferes with activities of daily living, decreases strength and endurance, stimulates nausea, impairs appetite, interferes with sleep, and impairs immune response (Page, 2005); it interferes with social and intimate relations, contributing to isolation. Pain is also associated with high emotional distress (Vachon, 2004). A study by Sela, Bruera, Conner-Spady et al. (2002) showed gender differences in the affective responses to cancer pain: men most often reported frustration, anger, and exhaustion due to pain, and women described exhaustion, helplessness, frustration, hopelessness, and anger as their top concerns. Spiritual distress may be a cause or an effect of pain (Georgesen & Dungan, 1996). In addition, pain increases caregiver burden and diminishes important social supports for both the patient and the family. Finally, some pain medications and other treatment regimens are expensive, causing economic stress at a particularly vulnerable time.
ETIOLOGY AND PATHOPHYSIOLOGY
Nociceptive pain occurs when a pain stimulus is generated from either somatic or visceral structures. Pain from somatic structures, including bone, joints, muscle, skin, and connective tissue, is usually described as “aching” or “throbbing,” and the patient can often point to the exact area where the painful stimulus is occurring (i.e., it is well localized). Stimuli from visceral tissues (mainly thoracic, abdominal, and pelvic organs) cause pain that is described as gnawing and aching. Visceral pain may or may not be well localized. In fact, visceral pain may be felt in areas other than the original site, a phenomenon known as referred pain (Hudspith, Siddall, & Munglani, 2006). Figure 32-2 illustrates some commonly reported sites where pain may be referred from visceral organs.
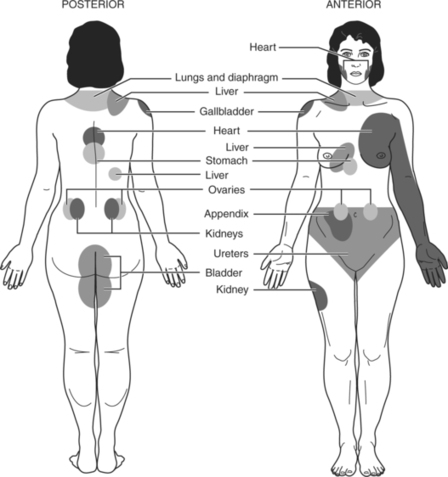 |
| Figure 32-2
(From Brockrath, M. [1985]. Fundamentals. Nursing Now [p. 18]. Springhouse, Pa.: Springhouse.)
Springhouse
|
Neuropathic pain is not as clearly understood as nociceptive pain. Peripherally generated neuropathic pains involve abnormal processing of sensory input from the peripheral nerves and may be described as “aching,” “burning,” “tingling,” or “shocklike.” Centrally generated neuropathic pains involve abnormal processing of sensory input at the spinal cord level, leading to hyperexcitability. This causes an abnormal continuation of pain impulses, even in the absence of further pain stimuli, or an abnormal processing of stimuli such that normally nonpainful stimuli are perceived as painful (allodynia). Neuropathic pain syndromes can be difficult to treat. The words used to describe an individual’s pain assist in determining the type of pain and inferring a cause (Table 32-1).
| Pain Type | Subtype | Descriptors | Examples |
|---|---|---|---|
| Nociceptive | Somatic |
Aching
Throbbing
Well-localized
|
Acute somatic pain:
Surgical incisions
Muscle or joint sprain
Chronic somatic pain:
Arthritis
Metastatic cancer to the bone
|
| Nociceptive | Visceral |
Aching
Gnawing
Deep and squeezing
Intermittent cramping
Poorly localized; referred
|
Acute visceral pain:
Angina
Bladder irritation (e.g., infection)
Acute bowel obstruction
Chronic visceral pain:
Pancreatic cancer
Metastatic cancer to the liver
|
| Neuropathic: centrally generated | Deafferentation |
Burning
Aching
Lancinating
Pricking
Lacerating
Pressing
|
Phantom limb pain (may have both central and peripheral mechanisms)
Spinal cord injury
Stroke
PHN
|
| Sympathetically maintained |
Burning
Hyperalgesia
Allodynia
Accompanied by excessive sweating and vasomotor changes
|
Complex regional pain syndrome types I and II | |
| Neuropathic: peripherally generated | Polyneuropathies |
Deep aching
Superficial burning, stinging, or prickling
Shocklike; lancinating
Pain felt along distribution of many peripheral nerves
|
Diabetic neuropathy
Drug-induced neuropathy (e.g., due to vinca alkaloid chemotherapy)
|
| Mononeuropathies |
Burning
Severe aching
Intermittent stinging or electric shocklike
Pain along distribution of single nerve or dermatome
|
Mononeuropathies:
Nerve root compression
Trigeminal neuralgia
Multiple mononeuropathies: Postherpetic neuralgia
|
Nociceptive Pain Processes
There are four processes involved in nociceptive pain: transduction, transmission, perception, and modulation. Transduction begins when mechanical, thermal, or chemical stimuli cause tissue damage. The damage itself and the inflammatory response to the damage release substances that stimulate or sensitize the pain fibers (Figure 32-3). In the presence of sufficient stimulation, the nerve membrane becomes permeable to sodium, leading to depolarization. An efflux of potassium causes repolarization. Repeated depolarization and repolarization generate an impulse and transduction is complete (Hudspith et al., 2006; McCaffery & Pasero, 1999; Wilke, 1995). In addition to transduction, peripheral pain fibers have the ability to release substances in response to injury (including substance P) that contribute to the inflammatory process and further increase the sensitization of the pain fibers (Hudspith et al., 2006). This explains why light touch of an inflamed area is perceived as painful.
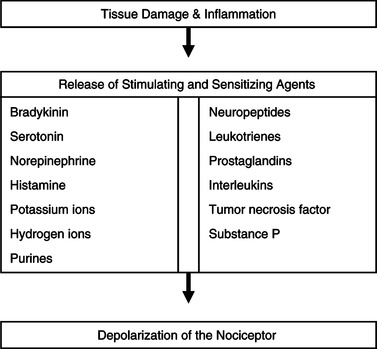 |
| Figure 32-3
(Data from Wilke, D.J. [1995]. Neural mechanisms of pain: A foundation for cancer pain assessment and management. In D.B. McGuire, C.H. Yarbro, & B.R. Ferrell [Eds.]. Cancer pain management [2nd ed., pp. 61-87]. Boston: Jones & Bartlett; and Hudspith, M.J., Siddall, P.J., & Munglani, R. [2006]. Physiology of pain. In H.B. Hemmings & P.M. Hopkins [Eds.]. Foundations of anesthesia: Basic sciences for clinical practice [2nd ed., pp. 267-285]. St. Louis: Mosby.)
|
The second phase of nociception is transmission of the impulse from the site of injury to the dorsal horn of the spinal cord, up to the brainstem, and out to the thalamus and cortex. Nociceptive fibers carry the impulse to the dorsal horn of the spinal cord, where these fibers end. In order to transmit the impulse to dorsal horn neurons, neurotransmitters are required. These neurotransmitters include glutamate, substance P, neurokinin A, and calcitonin gene–related peptide (CGRP). Glutamate plays a major role in nociceptive transmission by binding with α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors. AMPA receptors are responsible for fast transmission of nociceptive impulses. Sustained activation of AMPA and/or sustained activation of neurokinin receptors primes the NMDA receptor so that it reaches a state ready for activation. Activation of NMDA receptors causes large and prolonged depolarization; prolonged activation initiates processes that contribute to changes observed in chronic pain states, including central sensitization (Hudspith et al., 2006). Thus, prolonged exposure to nociceptive pain may lead to neuropathic pain.
Once transmitted across the synaptic space in the dorsal horn, the impulse is transmitted through several different ascending pathways to the brainstem, thalamus, and higher brain centers. This ends the transmission phase of nociception and begins the phase of perception. Precisely where pain is perceived in the brain is not clear, but this is thought to occur at several cerebral levels. Processing by the somatosensory cortex allows an individual to localize and characterize the pain. The emotional and behavioral responses to pain occur when the pain stimulus is processed in the limbic system. The reticular activating system is responsible for the autonomic responses to pain (Hudspith et al., 2006; McCaffery & Pasero, 1999; Wilke, 1995).
The fourth process in nociception is modulation. This involves the descending pathways from the brain to the dorsal horn of the spinal cord. Substances that inhibit the transmission of pain may be released by these descending fibers. Among these substances are endogenous opioids (enkephalin), serotonin, norepinephrine, γ-aminobutyric acid (GABA), α 2-adrenergic substances, acetylcholine, thyrotropin-releasing hormone, and somatostatin (Hudspith et al., 2006).
Neuropathic Pain Processes
As mentioned, neuropathic pain processes are not as clearly understood but involve abnormal processing of sensory input. Peripheral neuropathic pains may be caused by injury to the nerve that causes a spontaneous generation of an action potential (Coderre & Melzack, 1992; Paice, 2003). The pain pathway is typically along a single dermatome, affected nerve, or plexus. Combined motor and sensory involvement is suspected if the individual is found to have altered reflexes, muscle atrophy, or weakness (Paice, 2003). There are three categories of damage that cause neuropathic pain: physical (e.g., surgery, trauma), chemical (e.g., neurotoxic medications, hyperglycemia), and viral (e.g. HIV, herpes zoster).
Centrally generated neuropathic pain may occur as a result of repetitive transmission of nociceptive signals to the dorsal horn, causing changes in the processing of these impulses. Hypersensitivity and hyperexcitability result. As mentioned, prolonged activation of NMDA receptors is likely involved in this process. Prevention and prompt treatment of pain may prevent these dorsal horn changes.
Physiologic Rationale for Pain Medications
Medications used to treat pain are discussed in detail in the “Intervention and Treatment” section of this chapter. It is important to understand the rationale for the use of these medications, which is based on the physiologic characteristics of pain. Most medications used to manage pain function by interrupting transduction or transmission or by enhancing modulation, as summarized in Table 32-2. Nociceptive pains tend to respond to traditional analgesics, nonopioids, and opioids. The interplay of the inflammatory process in, particularly, the somatic pains indicates that antiinflammatory medications may be helpful. Neuropathic pains are less responsive to traditional analgesics; often an adjuvant medication, such as an antidepressant or an anticonvulsant, is needed alone or in combination with an opioid.
| Medication Class | Mechanism of Action |
|---|---|
| Nonsteroidal antiinflammatory drugs | Block production of prostaglandins |
| Anticonvulsants | Block influx of sodium ions, preventing depolarization and generation of an action potential |
| Local anesthetics | Block influx of sodium ions, preventing depolarization and generation of an action potential |
| Corticosteroids | Block production of prostaglandins |
| Opioids | Bind with opioid receptors and block release of substance P |
| N-Methyl-D-aspartate receptor antagonists | Inhibit binding of excitatory amino acids, such as glutamate, preventing transmission |
| Tricyclic antidepressants | Prevent reuptake of serotonin and norepinephrine |
| GABA agonists | Enhance release of GABA |
| α-Adrenergic agonists | Inhibit release of substance P |
MYTHS AND MISCONCEPTIONS ABOUT PAIN
The treatment of pain is wrought with myths, misconceptions, and erroneous beliefs. Understanding these myths and correcting them are essential to the assessment and treatment of pain. Unfortunately, these issues are quite common among patients, families, health care professionals, and the general public.
Many patients and families hesitate to report pain or use analgesics because of misperceptions, misinformation, and fear. Ward, Goldberg, Miller-McCauley et al. (1993) identified eight barriers:
1. Fear of opioid side effects
2. Fear of addiction
3. Belief that increasing pain signifies disease progression
4. Fear of injections
5. Concern about drug tolerance
6. Belief that “good” patients do not complain about pain
7. Belief that reporting pain may distract the physician from treating or curing the cancer
8. Fatalism, the belief that pain is inevitable with cancer and that it cannot be relieved
Likewise, health care professionals retain erroneous attitudes and beliefs about pain and the use of medications that negatively affect pain assessment and management:
1. The patient’s self-report of pain is not to be believed; health care professionals are the best judge of pain. The patient’s report of pain must be accepted because there are no diagnostic or “objective” tests for pain. Pain is whatever the experiencing person says it is, existing whenever he or she says it does (McCaffrey, 1968).
2. Addiction to pain medication is common. When pain medications are used to treat pain and there is no history of substance abuse, addiction is rare (Weissman, Burchman, Dahl et al., 1994). To clear up confusion over the meanings and application of these terms, addiction, tolerance, and physical dependence are defined as follows:
▪ “ Addiction is a primary, chronic, neurobiologic disease, with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving” (American Academy of Pain Medicine, American Pain Society, & American Society of Addiction Medicine, 2001, p. 6).
▪ “ Tolerance is a state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time” (American Academy of Pain Medicine et al., 2001, p. 7).
▪ “ Physical dependence is a state of adaptation that is manifested by a drug class specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist” (American Academy of Pain Medicine et al., 2001, p. 8).
Tolerance and physical dependence are physiologic responses to prolonged use of many substances and medications, including caffeine (e.g., caffeine withdrawal headache), and are not signs of addiction. Requests for pain medication, “clock watching,” and other “drug-seeking” behaviors due to poorly controlled pain are often mistaken for addiction. This is referred to as opioid pseudo-addiction.
▪ Opioid pseudo-addiction is an iatrogenic (meaning “health care system– acquired”) syndrome in which certain behavioral characteristics of psychologic dependence develop as a consequence of inadequate pain treatment (American Academy of Pain Medicine et al., 2001).
3. Respiratory depression is a frequent, serious complication with opioid use. Respiratory depression is feared and misunderstood by patients, families, and health care professionals. Tolerance develops rapidly to respiratory depression; thus, it is rarely a problem except in the opioid-naïve patient. Keep in mind that respiratory rate alone is not an indicator of respiratory depression; careful assessment of the whole patient, not just respiratory rate, is required. Most important, adequate pain management does not shorten life or hasten death (Fohr, 1998; Sykes & Thorns, 2003).
There are other barriers to adequate pain management including the “antidrug” culture and perceived regulatory barriers that vary from state to state. Such restrictions include triplicate prescription programs or other systems that monitor prescribing patterns or a lack of laws facilitating pain management in end-stage illness, including partial filling of scheduled medications. Be familiar with the state’s nurse practice act, regulations, and controlled substances laws.
ASSESSMENT AND MEASUREMENT
If pain is not assessed, appropriate management is impossible. Pain is a multidimensional human experience and thus requires a comprehensive, holistic evaluation. Each new pain report requires a systematic assessment, appropriate treatment, and follow-up. A complete pain assessment includes the following:
▪ Site: The patient identifies primary sites as well as sites of radiation on his or her body or a diagram. Remember also that the patient may have several sites of pain; it may be helpful to number pains to organize assessment, interventions, and evaluations.
▪ Character: Use the patient’s own words; a careful description will lead to the diagnosis of pain type and assist in determining appropriate analgesics (i.e., sharp, shooting describes neuropathic pain syndromes).
▪ Onset: When did it start? Did (or does) a specific event trigger the pain? Carefully distinguish between new and preexisting pain (i.e., arthritis, chronic low back pain syndromes).
▪ Duration and frequency: How long has the pain persisted? Is it constant or intermittent?
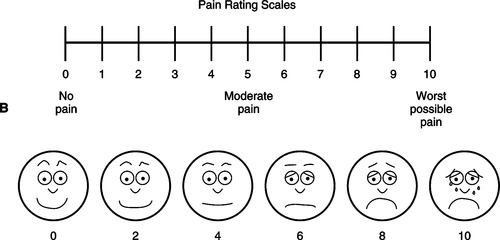
▪ Intensity: Intensity is commonly defined on a scale, most frequently of 0 to 10. The 0-to-10 scale has been validated in international populations (Serlin, Mendoza, Nakamura et al., 1995). However, the pain intensity measure must be adapted to the patient. Have an alternate scale available for patients unable or unwilling to use the 0-to-10 scale. For example, some patients prefer a verbal descriptor scale or a modification of a smile-to-sad scale. Find a scale that is meaningful to the patient. Ask patients to rate their pain intensity “now,” at its “worst,” and at its “least.” Note that ratings of pain intensity are the most important pain assessment data to collect if time is short; pain intensity directly correlates with interference with the patient’s quality of life.
▪ Exacerbating factors: What times, activities, or other circumstances make the pain worse?
▪ Associated symptoms: What other symptoms occur before, with, or after the pain? Nausea is frequently associated with pain and often attributed to pain medications and not the pain itself. If nausea occurs at the peak of the drug, it may be drug related; if it occurs at the end of the dose, it may be pain related.
▪ Alleviating factors: What makes the pain better? What treatments have been successful in the past? What treatments have been unsuccessful? Remember that treatments that did not work in the past may work now if the previous dose was inappropriate or the type of pain problem is different. Include a thorough medication history, especially for the past 24 hours.
▪ Effect on quality of life: How does the pain affect the patient’s ability to perform activities of daily living? How does the pain affect relationships with close others? What does the pain mean to the patient and family? How has this pain affected them? How much do the patient and family know about pain? Do they have the expectation that it can be relieved? Are there emotional or spiritual components to the pain? Does unrelieved pain lead to increased fear or anxiety, or to fears that death is imminent?
▪ Patient’s goal for relief: Consider using either a pain intensity score or a functional goal, for example, the ability to walk without pain. What would you like to do that your pain is preventing you from doing? What pain score would allow you to perform that activity?
▪ Physical examination: Observe the site of the pain, and validate with the patient the pain’s location. Note skin color, warmth, irritation, integrity, and any other unusual findings (Berry, Eagan, Eighmy et al., 1999).
▪ Other effects of pain: Assess for the presence of depression, anxiety, and other emotional aspects of the pain experience.
There are multiple pain assessment forms and scales available. Figure 32-4, one example, presents a 0-to-10 scale and other alternate scales.
Assessment of the Cognitively Impaired
Assessment of patients who lack verbal skills because of cognitive impairment requires astute observation of behavior. A change in behavior is the gold standard for suspecting pain or discomfort in a cognitively impaired person. Several pain rating scales have been proposed for use with nonverbal patients; most include monitoring for changes in breathing patterns, negative vocalizations, frowning or grimacing, tense body language or agitation, and the ability to console the person (Warden, Hurley, & Volicer, 2003). Kovach, Weissman, Griffie et al. (1999) suggest the following protocol for assessing discomfort in patients with dementia who exhibit a change in behavior:
▪ Perform a physical assessment for a cause of discomfort and pain. If a cause is found, treatment is initiated.
▪ Review the patient’s history for possible causes of pain through medical records and family members.
▪ Trial nonpharmacologic interventions appropriate to the circumstances; levels of environmental stimuli should also be evaluated and adjusted for the patient’s comfort.
▪ If the preceding steps are unsuccessful, trial of a nonopioid analgesic is indicated.
▪ If the nonopioid is unsuccessful, choose a stronger medication, using the World Health Organization (WHO) analgesic ladder as a guide (Figure 32-5); for example, a smaller dose of an opioid in combination with acetaminophen such as hydrocodone (WHO, 1990).
The importance of including a caring approach and the use of nonpharmacologic interventions that enhance the dignity and self-esteem of the individual cannot be overstressed in the care of persons with cognitive impairment.
HISTORY AND PHYSICAL EXAMINATION
Physical examination, appropriate to each identified location of pain, should be performed. Additional history may be suggested by physical findings.
DIAGNOSTICS
Radiologic examinations may be appropriate to direct treatment of the underlying cause of pain within the patient’s goals of care. As discussed in the previous chapters, decisions to pursue further diagnostics are based on the patient’s place on the disease trajectory and the goals of care.
Laboratory evaluation may or may not be appropriate. In any case, the most recent laboratory values should be reviewed. Blood urea nitrogen (BUN) and creatinine levels may be indicated to ascertain renal function before the initiation of opioid therapy if renal insufficiency is suspected. Likewise, liver function tests may be indicated if hepatic insufficiency is suspected.
INTERVENTION AND TREATMENT
Basic Principles of Pain Management
The following principles serve as a guide to the basics of pain management (American Pain Society, 2003; Gordon, Dahl, Miaskowski et al., 2005; National Cancer Institute, 2005; National Comprehensive Cancer Network [NCCN], 2005).
1. Document the pain assessment data so that pain syndrome can be identified and appropriately treated.
2. Match the choice of drug with the intensity and type of pain.
4. Use the oral route whenever possible. If the patient is unable to swallow oral medications, buccal, sublingual, rectal, and transdermal routes are considered before parenteral routes. The intramuscular route is avoided.
5. Continuous pain requires treatment with a scheduled sustained-release (modified-release) or long-acting opioid and a short-acting medication for breakthrough pain. Patients and families, however, need to be educated about taking the medication when the pain is first perceived—not when it has become severe or unbearable.
6. Educate patients and families about side effects of opioids to avoid the perception that these are allergic reactions.
7. Use an adequate rescue dose for breakthrough pain: 10% to 20% of the 24-hour dose every 1 to 2 hours is the customary rescue dose.
8. Increase the baseline dose if the patient needs more than three rescue doses in 24 hours.
9. If an increase in the baseline dose is required, it can be done safely every 2 hours with immediate-release preparations, every 12 hours with long-acting preparations, and every 48 hours (2 days) with a fentanyl transdermal patch (Davis, Weissman, & Arnold, 2004).
10. Order only one analgesic for breakthrough pain.
11. Order only one long-acting opioid for constant pain.
12. Increase doses of opioids commensurately with the patient’s report of pain:
For mild to moderate pain, increase by 25% to 50%.
For moderate to severe pain, increase by 50% to 100%, although at higher opioid doses, increases of 20% to 30% are deemed safer.
Increases of less than 25% are meaningless.
13. The appearance of analgesic tolerance may require switching to a different opioid. Consider reducing the calculated equianalgesic dose by 25% to 50% to address issues of incomplete cross-tolerance.
14. Use equianalgesic conversions when changing medications or routes.
15. Use adjuvant medications for opioid-resistant neuropathic pain.
16. Nonpharmacologic approaches should always be a part of any pain management plan.
17. Order an appropriate preventative bowel regimen at the initiation of opioid therapy.
Commonly used analgesics can be found in TABLE 32-3, TABLE 32-4, TABLE 32-5 and TABLE 32-6. The following medications should be avoided:
| *Aspirin is not recommended for long-term use due to risk of bleeding and gastrointestinal ulceration. |
||||
| †Choline magnesium trisalicylate is the only nonsteroidal antiinflammatory drug that does not interfere with platelet aggregation. |
||||
| ‡Cyclooxygenase-2 inhibitors should be used with caution in persons with impaired renal or hepatic functioning. |
||||
| Data from McCaffery, M. & Pascero, C. (1999). Pain: Clinical manual (2nd ed.). St. Louis: Mosby; and Hogdson, B.G. & Kizior, R.J. (2004). Saunders nursing drug handbook 2004. St. Louis: Saunders. | ||||
| Chemical Class | Generic Name | Starting Dose (Oral) in Adults | Dosing Schedule | Maximal Daily Dose |
|---|---|---|---|---|
| p-Aminophenol derivatives | Acetaminophen | 325 to 650 mg | Every 4 to 6 hr | 4000 mg |
| Salicylates | Aspirin* | 650 mg | Every 4 to 6 hr | 4000 mg |
| Choline magnesium trisalicylate† | 1500 mg × 1, then 1000 mg | Every 12 hr | 4000 mg | |
| Proprionic acids | Ibuprofen | 200 to 400 mg | Every 4 to 6 hr | 3200 mg |
| Naproxen | 500 mg | Every 8 to 12 hr | 1500 mg | |
| Acetic acids | Diclofenac | 25 to 50 mg | Every 8 to 12 hr | 150 mg |
| Nabumentone | 1000 mg | Every 24 hr | 2000 mg | |
| Pyranocarboxylic acids | Etodolac | 200 to 400 mg | Every 6 to 8 hr | 1200 mg |
| Cyclooxygenase-2 inhibitors‡ | Celecoxib | 100 mg | Every 12 to 24 hr | 400 mg |
| *Caution: The first number of the dosage listed is the milligram dosage of the opioid; the second is the milligram dosage of the nonopioid analgesic that is used in combination. APAP is the pharmacologic designation for acetaminophen. The acetaminophen dosage should not exceed 4 g/24 hr. ASA is the pharmacologic designation for aspirin. | |
| Opioid | Proprietary Name and Combination Agent |
|---|---|
| Codeine | Tylenol #3 30/300 APAP |
| Tylenol #4 60/300 APAP | |
| Hydrocodone | Lorcet HD 5/500 APAP |
| Lorcet Plus 7.5/650 APAP | |
| Vicodin 5/500 APAP | |
| Vicodin HP 10/650 APAP | |
| Vicodin ES 7.5/750 APAP | |
| Vicoprofen 7.5/200 ibuprofen | |
| Zydone 5/400, 7.5/400, 10/400 APAP | |
| Co-Gesic 5/500 APAP | |
| Norco 5/325, 7.5/325, 10/325 APAP | |
| Lortab 2.5/500, 5/500, 7.5/500, 10/500 APAP | |
| Lortab ASA 5/500 ASA | |
| Oxycodone | Percocet 5/325, 7.5/325, 10/325, 7.5/500, 10/650 APAP |
| Percodan 4.5/325 ASA | |
| Percodan-Demi 2.25/325 ASA | |
| Roxicet 5/325, 5/500 APAP | |
| Roxilox 5/500 APAP | |
| Roxiprin 4.5/325 ASA | |
| Endocet 5/325, 7.5/325, 10/325, 7.5/500, 10/650 APAP | |
| Tylox 5/500 APAP | |
| Opioid | Formulations | Available Dosages |
|---|---|---|
| Short-Acting Opioids | ||
| Morphine | Tablet | 15, 30 mg |
| Solutab | 10, 15, 30 mg | |
| Liquid | 10 mg/5 ml, 20 mg/5 ml, 20 mg/ml | |
| Suppository | 5, 10, 20, 30 mg | |
| Oxycodone | Tablet | 5, 10 mg |
| Liquid | 5 mg/5 ml, 20 mg/ml | |
| Hydromorphone | Tablet | 2, 4, 8 mg |
| Suppository | 3 mg | |
| Long-Acting Opioids | ||
| Morphine | ||
| Oramorph SR | Tablet | 15, 30, 60, 100 mg every 8 to 12 hr (initial starting interval: 12 hr) |
| MS Contin | Tablet | 15, 30, 60, 100, 200 mg every 8 to 12 hr (initial starting interval: 12 hr) |
| Kadian | Capsule | 20, 50, 100 mg every 12 to 24 hr (starting interval: 24 hr) |
| Avinza | Capsule | 30, 60, 90, 120 mg every 24 hr |
| Fentanyl | ||
| Duragesic-25,-50,-75,-100 patch | Transdermal | 25, 50, 75, 100 mcg/h every 72 hr |
| Oxycodone | ||
| OxyContin | Tablet | 10, 20, 40, 80, 160 mg every 12 hr |
| GABA, Gamma-aminobutyric acid; NMDA, N-methyl-D-aspartate; NSAID, nonsteroidal antiinflammatory drug. | |||||
| *All tricyclic antidepressants exhibit anticholinergic properties. Use with caution in the elderly. Desipramine and nortriptyline have the lowest incidence of anticholinergic effects of this class. |
|||||
| †Some specialists titrate gabapentin up to 6000 mg/day. |
|||||
| ‡Dexamethasone starting dose is dependent on severity of symptom. Avoid administration late in day as may interfere with sleep. |
|||||
| Data from American Pain Society. (2003). Principles of analgesic use in the treatment of acute pain and cancer pain (5th ed.). Glenview, Ill.: Author; Hogdson, B.G. & Kizior, R.J. (2004). Saunders nursing drug handbook 2004. St. Louis: Saunders; Hugel, H., Ellershaw, J.E., & Dickman, A. (2003). Clonazepam as an adjuvant analgesic in patients with cancer-related neuropathic pain. J Pain Symptom Manage, 26(6), 1073-1074; and Kuebler, K., Varga, J., & Davis, M.P. (2005). Medications by disorder. In K.K. Kuebler, M.P. Davis, & C.D. Moore (Eds.). Palliative practices: An interdisciplinary approach (pp. 418-441). St. Louis: Elsevier. | |||||
| Class | Uses | Generic Name | Starting Dose in Adults | Dosing Schedule | Titration, Maximal Dose, Comments |
|---|---|---|---|---|---|
| Tricyclic antidepressants* | Neuropathic pain | Despiramine | 10 to 25 mg orally | Every 24 hr | Titrate every 3 to 4 days to a maximum of 150 mg/day |
| Nortriptyline | |||||
| Anticonvulsants | Neuropathic pain | Carbamazepine | 100 to 200 mg orally | Every 12 hr | Titrate to a maximum of 400 mg every 8 hr; monitor liver function |
| Gabapentin | 100 mg orally | Every 8 hr | Titrate to effectiveness; reported maximal daily dose of 3600 mg† | ||
| Clonazepam | 0.5 mg orally | Every 12 hr | Titrate every 2 to 3 days; maximal dose cited between 2 to 8 mg/day | ||
| Corticosteroids | Inflammation not responsive to NSAIDs; increased intracranial pressure; spinal cord compression | Dexamethasone | 1 to 10 mg orally‡ | Every 6 to 24 hr | Maximum of 96 mg/day; taper dose slowly to lowest effective dose |
| Prednisone | 5 to 10 mg orally | Every 24 hr in the morning | Taper slowly to lowest effective dose | ||
| Bisphosphonates | Bone pain associated with hypercalcemia | Pamidronate | 60 to 90 mg intravenously over 1 to 2 hr | Every 4 wk | Monitor for hypocalcemia |
| Zolendronic acid | 3-4 mg intravenously over 15 min | Every 4 wk | Monitor for hypocalcemia | ||
| Use renal dosing | |||||
| GABA agonist | Neuropathic pain | Baclofen | 5 mg orally | Three times daily | May increase by 15 mg/day at 3-day intervals; maximum dose |
| NMDA receptor antagonist | Neuropathic pain | Ketamine | 100 to 200 mg subcutaneously over 24 hr | Narrow therapeutic index; high risk for psychomimetic effects | |
| Local anesthetics | Neuropathic pain | Lidocaine 5% patch | 1 to 3 transdermal patches | 12 hr on; 12 hr off | |
▪ Meperidine is short acting with a duration of 2 to 3 hours; active excitatory metabolites accumulate with chronic use.
▪ Propoxyphene has modest analgesic efficacy and is biotransformed to potentially toxic central nervous system and cardiac metabolites (Ulens, Daenens, & Tytgat, 1999).
▪ Agonist-antagonists (e.g., buprenorphine, butorphanol, nalbuphine, pentazocine) have a ceiling effect (i.e., above a certain dose there is no more gain in analgesia) and can generate an acute withdrawal syndrome if used with opioids.
Selection of an Opioid Starting Dose
The starting dose of an opioid is determined by the patient’s pain intensity and the results of the history and physical examination, including the patient’s age, circulatory status, and hepatic and renal function. The WHO analgesic ladder (Figure 32-5) may also be used as a guide. Be aware, however, that successful pain management requires an individualized approach, frequent monitoring, and appropriate dosage escalation (as outlined in “Basic Principles of Pain Management”) until the patient is comfortable.
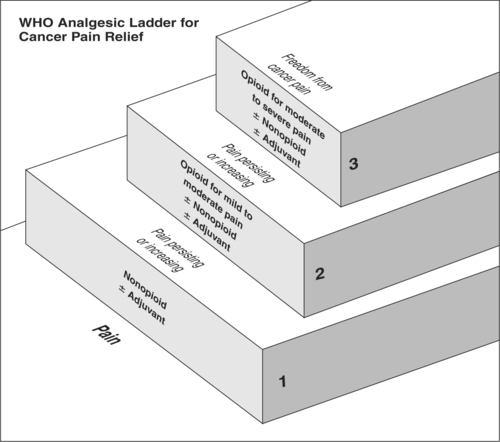 |
| Figure 32-5
(From World Health Organization [1996]. Cancer pain relief [2nd ed.]. Geneva: Author.)
Author
|
Equianalgesic Conversions
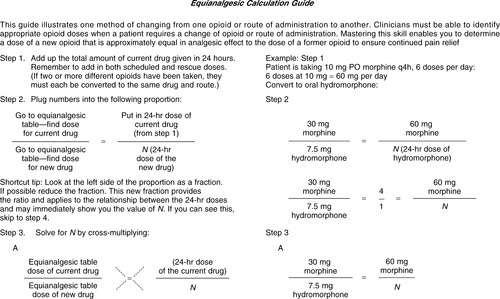
Understanding opioid pharmacodynamics requires an understanding of the concepts of potency and equianalgesia. Refer to an equianalgesic conversion table (Table 32-7) when converting from one route to another or from one opioid to another. Note that 10 mg of parenteral morphine sulfate has the same analgesic effect as 30 mg of oral morphine sulfate. Clinicians should use such tables with caution, in that the dosages stated are not standard starting doses. Starting doses should be based on pain intensity combined with other information from the patient assessment. The doses on the table represent ratios of one drug to another, and one route to another. These ratios do not take into account individual differences in response to various opioids; use these ratios as estimates for a new starting dose and evaluate the patient’s response (American Pain Society, 2003). Doses may need to be titrated up or down after the initial dose. One dose can be converted to another by following a four-step procedure. Figure 32-6 works through four basic steps for calculating an equianalgesic conversion.
 |
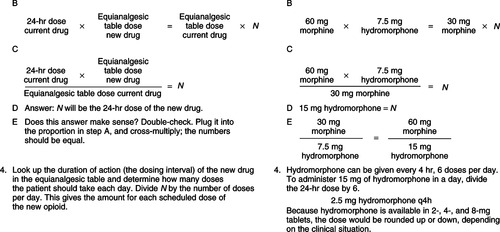 |
| Figure 32-6
(From Gordon, D., Stevenson, K., Griffie, J., et al. [1999]. Opioid equianalgesic calculations. J Palliat Med, 2[2], 209-218.)
|
| *Equianalgesic tables are intended to be a guide. Each individual patient must be evaluated for the appropriateness of the opiate dose used based on prior opiate use, comorbidities, and concomitant medications. |
||||
| †The following drugs are not recommended for analgesic use in palliative care settings: -Codeine [intolerance common] -Meperidine [neurotoxicity risk] -Propoxyphene [long half-life with toxic accumulations of cardiac and CNS metabolites] |
||||
| ‡Use the following guide to calculate the dose for methadone: -Calculate 24-hour equivalent dose of oral morphine <90 mg, use 4:1 ratio 91 to 300 mg, use 8:1 ratio 301 to 600 mg, use 12:1 ratio |
||||
| Drug† | Oral Dose (mg) | Parenteral Dose (mg) | Duration of Action | Comments |
|---|---|---|---|---|
| Morphine sulfate | 30-60 | 10 | 3 to 4 hr | Active metabolites M6G and M3G can lead to neurotoxicity—especially in renal insufficiency |
| Oxycodone | 20 | NA | 3 to 4 hr | Adverse effects milder than morphine |
| Hydrocodone | 30 | NA | 3 to 4 hr | Not available as single agent |
| Methadone‡ | — | — | 4 to 12 hr | Dosage decreases as opiate substituted for increases—see footnotes |
| Hydromorphone | 7.5-8 | 1.5 | 4 to 5 hr | Opioid of choice for continuous subcutaneous infusions |
| Fentanyl [transdermal] | n/a | 50 mcg/hr = 100 mg po morphine/24 hr | 48 to 72 hr | Caution must be used in handling/disposal of patches |
| Absorption affected by changes in body temperature. Also available in transmucosal delivery for breakthrough pain | ||||
Common Side Effects of Opioids and Their Management Sedation
Sedation usually clears in 1 to 3 days. If sedation persists, patients may benefit from a slight reduction in the opioid dose and an evaluation for the need to add an adjuvant medication or a stimulant such as methylphenidate. Excessive sedation is often a sign that the pain syndrome is opioid resistant. This assumes that an assessment has ruled out the potential contribution of other patient medications to oversedation.
Constipation
This is a common but preventable side effect. Regularly scheduled laxatives are almost always required; surface wetting agents in addition to a gentle laxative such as senna should be considered as a starting regimen. Start a bowel regimen before initiating an around-the-clock analgesic dosing schedule.
Nausea
Persons usually become tolerant to the emetic effect after 3 to 5 days. In the interim, use an antiemetic. Persistent nausea requires further assessment for other etiologies.
Confusion and Excitability
Evaluate for the underlying cause. Eliminate nonessential central nervous system (CNS)–acting medications. If analgesia is satisfactory, reduce the dosage of the opioid by 25%. Neurotoxic effects of opioids can be seen in those patients who have impaired renal function and have been on higher dose or prolonged therapy. Symptoms manifest as delirium, myoclonus, agitation, and hyperalgesia. These symptoms may require a change in opioid (Bruera & Kim, 2003).
Less Common Side Effects
Less common side effects include sweating, pruritus, and urinary retention.
Nonsteroidal Antiinflammatory Drugs
The nonsteroidal antiinflammatory drugs (NSAIDs) are believed to inhibit prostaglandin synthesis, ultimately reducing the patient’s pain perception. NSAIDs are considered nonopioid analgesics and serve as the first line in the management of somatic pain. When an NSAID alone is not sufficient to control the pain, a medication that combines an NSAID with an opioid is the second step of treatment (see Table 32-4). NSAIDs have the potential of producing gastritis, fluid retention, renal failure, and platelet dysfunction (Chang, 2004; Esper & Heidrich, 2005; NCCN, 2005). They are contraindicated in the following situations:
▪ History of gastrointestinal bleeding secondary to NSAIDs
▪ History of peptic ulcer disease
▪ Active gastrointestinal bleeding
▪ Coagulopathies
There are multiple NSAIDs to choose from, and further discussion can be found in many existing resources; some of the more common NSAIDs and dosages are highlighted in Table 32-3.
NSAID choice is determined by availability, cost, patient convenience, adverse effects, and efficacy. The extent of gastrointestinal distress caused by NSAIDs has no relation to the severity of the gastrointestinal symptoms. Concomitant administration of a proton pump inhibitor has been shown to help decrease the risk of gastropathy (American Pain Society, 2003).
Adjuvant Analgesics
An adjuvant analgesic, also called a coanalgesic, is a medication that has a primary indication other than analgesia but has also been found to have analgesic properties (McCaffery & Pasero, 1999). Tricyclic antidepressants and anticonvulsants are the two most commonly used classes of adjuvant medications used to treat neuropathic pain. These medications often require titration, starting with a low dose and titrating to pain relief or intolerable side effects. Explain to the patient that complete pain relief will not begin immediately; it takes time to find the right dose and right medication for each person (Bruera & Kim, 2003). If the first choice of a medication is not effective or not tolerated after careful titration, consider switching to a different adjuvant analgesic. This continuous monitoring of both analgesia and side effects is an acquired skill that requires a working knowledge of the varied patient responses coupled with medication-specific pharmacokinetics.
Table 32-6 highlights some of the commonly used adjuvant analgesics. There is no “cookbook” approach to prescribing adjuvant analgesics. Individuals respond differently to medications based on their underlying pathophysiologic condition, metabolism, organ function, and physical status. It is important that the clinician assess all of the symptoms that the patient is experiencing and tailor adjuvant analgesics specifically to both the pain syndrome and other symptoms (Box 32-1). Many of the adjuvant analgesics used to treat the various pain syndromes prove useful for other symptoms such as dyspnea, depression, and restlessness.
Box 32-1
• Perform a comprehensive patient evaluation.
• Optimize current regimen.
• Select the drug which best addresses pain syndrome.
• Understand the pharmacologic properties of the adjuvant analgesic selected.
• Evaluate risks of polypharmacy.
• Understand individual variability.
• Educate patients on specific role of adjuvant therapy.
• Utilize adjuvant analgesics as part of the entire pain management regimen.
Data from Lussier, D., Huskey, A.G., & Portenoy, R.K. (2004). Adjuvant analgesics in cancer pain management. The Oncologist, 9(4), 571-591.
Corticosteroids
Corticosteroids are useful because of their antiinflammatory effects. They are considered not only for treatment of somatic bone pain but also for both visceral and neuropathic pain syndromes. Corticosteroids are also used in the management of anorexia, depression, nausea, spinal cord compression, superior vena cava syndrome, organ distention, and other conditions (Lussier, Huskey & Portenoy, 2004; National Cancer Institute, 2005). Dosing schedules vary with the disease trajectory and the underlying pathophysiologic condition. The adverse effects of corticosteroids include candidiasis, gastritis, fluid retention, hypertension, hyperglycemia, and mood alterations even to the point of psychosis (Lussier et al., 2004).
Corticosteroid dosage is dependent upon the underlying pathophysiologic condition; doses differ, for example, when treating pain, anorexia, intracranial edema, and spinal cord compression (see individual symptoms). Start with low doses and titrate upward to desired effect.
Bisphosphonates
The bisphosphonate class of medication, although costly, may be considered for the treatment of bone pain due to metastasis when other adjuvants fail. These agents induce apoptosis of osteoclasts, which can lead to tumor shrinkage (Sabino & Mantyh, 2005). The most commonly used bisphosphonates are pamidronate and zolendronic acid. Both must be used with caution in patients with renal impairment. And, due to the potential for the development of osteonecrosis of the jaw, patients should not have invasive dental procedures done while taking these agents (Ruggiero, Mehrotra, Rosenberg et al., 2004).
Radiopharmaceuticals
Under the direction of a radiation oncologist, the use of medications such as strontium 89 may be considered to relieve bone pain in the active patient with a longer prognosis. This medication is absorbed into areas of high bone turnover and can help reduce pain (Nilsson, Strong, Ginman et al., 2005; Reisfield, Silberstein & Wilson, 2005). Be aware that patients often exhibit a short-term “pain flare” when radiopharmaceuticals are started, requiring additional analgesics for several days. Then, analgesics can be titrated down as pain decreases. Never stop opioid medications abruptly.
Invasive Interventions
Tumor Embolization
A novel means of attempting palliation of symptoms performed by interventional radiologists is to localize tumor blood supply under fluoroscopic guidance and inject alcohol, coils, or other agents to occlude those vessels supplying the tumor. When a large percentage of vessels can be embolized, subsequent tumor shrinkage can provide significant improvement in pain. Patients must be educated regarding the potential for increased pain during the first 24 to 72 hours postprocedure (Kent, Forauer, Esper et al., 2004).
Intraspinal Medication Delivery
Pain that responds poorly to standard pharmacologic and conventional modalities may require more invasive measures. Opioids such as morphine may be delivered intrathecally or epidurally and often involve an implantable pump. Intrathecal morphine is currently the most commonly used analgesic administered by pump but remains in the cerebrospinal fluid for extended periods and can spread to the level of the brainstem, possibly inducing respiratory depression. Fentanyl is taken up more readily into the systemic circulation due to its increased lipid solubility and, as a result, has fewer central nervous system depressive effects. Prior to implanting permanent pumps, opioid trials need to be performed to demonstrate that the patient’s pain is opioid responsive. Pumps can be implanted in a permanent fashion if a beneficial response is seen (American Pain Society, 2003; Pappagallo, Dickerson, Varga et al., 2002). Opioids may be administered alone or in combination with other agents such as local anesthetics or clonidine. It is important to consider the variation in systemic absorption between various routes of administration. For example, the approximate equivalent of 30 mg of oral morphine administered on a regular schedule is 10 mg intravenously, 1 mg epidurally, and 100 mcg (0.1 mg) intrathecally (American Pain Society, 2003).
The use of intraspinal delivery systems requires expertise in both insertion and management arenas. It is therefore generally reserved for a select subset of individuals who, under a multidisciplinary review, are deemed appropriate candidates.
Additional Therapeutic Interventions
Radiation Therapy
Tumors involving bone may be exquisitely sensitive to radiation therapy, and the role of this treatment for improving patient functional status and quality of life must be evaluated in correlation to the patient prognosis, anticipated toxicities, and treatment-related burden. It has been reported that radiation therapy can achieve pain relief in this setting for as many as 75% of patients receiving treatment (Rutter & Weissman, 2004). There is good evidence that single-fraction radiation therapy is as effective as multifraction dosing schedules in relieving bone pain (Sze, Shelley, Held et al., 2003).
Neurolytic Blocks
Neurolytic blocks may also provide pain relief in situations where enlarging masses are placing pressure on adjacent structures. The procedure involves the application of a local anesthetic or neurolytic agent as in the case of celiac plexus blocks for the pain associated with pancreatic cancer (NCCN, 2005); Wong, Schroeder, Carns et al., 2004).
At times pain is difficult to treat, even with the means already suggested. Resources for difficult pain management problems should be identified in advance.
Nondrug and Complementary Therapies for Pain
Medications and treatments are not the only effective interventions to alleviate pain and the suffering that may accompany pain. Although not substitutes for analgesics, nondrug and complementary therapies play an important role in managing the total pain experience. Physically, these interventions are believed to decrease stimulation to the sympathetic nervous system, promote muscle relaxation, interfere with pain transmission, and stimulate the release of endogenous pain-relieving substances (e.g., endorphin) (Eshkevari & Heath, 2005). Cognitive and emotional effects of these nondrug interventions include decreased anxiety, restoration of hope, decreased fatigue, improved sleep, and decreased feelings of helplessness and hopelessness. Examples of nondrug and complementary therapies for pain are listed in Table 32-8.
| Type of Intervention | Effects | Examples |
|---|---|---|
| Muscle relaxation | Decreases anxiety | Slow, controlled breathing |
| Promotes vasodilatation | Progressive muscle relaxation | |
| Cold | Reduces inflammation | Ice packs |
| Relieves muscle spasm | Towels soaked in ice water | |
| Heat | Increases blood flow and oxygenation to tissues | Hot water bottles |
| Warm, moist compresses | ||
| Heating pads | ||
| Immersion | ||
| Massage | Aids relaxation | Gentle body massage |
| Increases circulation at site | Therapeutic massage with a licensed therapist | |
| Exercise | Strengthens muscles | Walking |
| Mobilizes stiff joints | Gentle stretching | |
| Immobilization/splinting | Supports painful areas | Canes, walkers |
| Braces, slings, binders | ||
| Touch/Energy therapies | Proposed to affect the human energy system | Healing touch |
| Therapeutic touch | ||
| Reiki therapy | ||
| Relaxation | Alters state of consciousness | Music |
| Increase receptivity to suggestion — often used with imagery | Mindful meditation | |
| Deep breathing | ||
| Decreases anxiety | Hypnosis | |
| Imagery | Aids in relaxation | Many different images may be used |
| Focuses attention away from pain, or to control pain | ||
| Decreases powerlessness | ||
| Distraction | Focuses attention away from pain | Music |
| Pets | ||
| Art | ||
| Humor | ||
| Activities/socialization | ||
| Education | Instructs on appropriate reporting and management of pain | Individual or group education session |
| Addresses fears, concerns, misunderstandings about pain | Written materials | |
| Support | Decreases powerlessness | Structured psychotherapy |
| Improves coping | Facilitated support groups, including those on the internet | |
| Peer support groups, including those on the internet | ||
| Pastoral counseling | Addresses existential issues contributing to the pain experience | Consultation with chaplain or individual’s own clergy |
| Prayer |
Research data on the effects of nondrug and complementary therapies often show conflicting or inconclusive results (Allard, Maunsell, Labbe et al., 2001; Fellowes, Barnes & Wilkinson, 2004; Mundy, DuHamel, & Montgomery, 2003). This may be due to the heterogeneity of the research populations, small sample sizes, lack of randomization, varying techniques in the application of these interventions, and inconsistent control for confounding variables. In a meta-analysis of psychoeducational interventions for pain, Devine (2003) concluded that there is reasonably strong evidence for relaxation-based cognitive-behavioral interventions, education about analgesic use, and supportive counseling. More research on complementary therapies in the palliative care setting is needed.
PAIN MANAGEMENT IN SUBSTANCE ABUSE
Treating pain in patients with either a current or a past history of substance abuse presents complex psychosocial and physical issues to any clinician. These patients fall into three basic categories (Paice & Fine, 2001):
1. Persons who have used or abused drugs or other substances in the past but are no longer using them
3. Persons who are actively abusing drugs
Most clinicians lack even the most basic background in and knowledge of chemical dependency and often struggle with these issues in the treatment of pain in general and certainly in the treatment of pain in patients who have a current or past history of substance abuse. Any patient in a palliative care setting may experience significant pain. The challenge is to offer those who have substance abuse histories the same compassionate, comprehensive care given any patient, recognizing a potential need for some modifications in care and use of additional resources. Many clinicians fear they will be fooled or duped into providing pain medications to individuals who are seeking drugs rather than pain relief. Pain is a subjective symptom, without observable physical signs, and thus in many clinician’s mind, its occurrence cannot be confirmed objectively. Keep in mind that if the goal is to relieve pain, the clinician must accept the patient’s report of pain.
Although additional epidemiologic studies are needed, substance disorders are believed to be present in as many as 6% to 15% of the U.S. population, although researchers believe that the incidence may be a little higher because of underreporting and institutional biases, including a lack of data from primary care centers and from persons alienated from the health care system (Passik & Kirsh, 2005). While the medical use of opioids has increased markedly, reports of abuse have either decreased or remained constant (Joranson, Ryan, Gilson et al., 2000). Thus, treating pain aggressively with opioids does not appear to contribute to increases in the reported health consequences of opioid analgesic abuse.
Treating a patient with a current or past history of substance abuse requires a comprehensive approach that recognizes and considers the interaction of the biologic, chemical, social, and psychologic and psychiatric aspects of substance abuse and addiction (Passik & Kirsh, 2005). Although the principles and guidelines outlined in the discussion that follows provide a framework for treating the patient who is actively abusing a substance, they also apply to those who are substance free but have used them in the past and those in methadone maintenance programs (Passik & Theobald, 2000).
Involve an interdisciplinary team. Reflect on one’s expertise and identify resources that can be accessed for managing this patient and family. A team approach is essential to address the multitude of medical and psychosocial problems presented and to prevent caregiver burnout and fatigue. An ideal team includes a physician with expertise in pain management and palliative care, staff nurses, social workers, and, if possible, a mental health professional versed in addiction medicine.
Set realistic goals for care. Relief of pain and enhancement of quality of life are the goals of care. What is the effect of the patient’s past or current history of substance abuse? Is addiction increasing the patient’s suffering? Are recovery programs aimed at abstinence appropriate? What should be the focus of the patient’s energy and the family’s and caregiver’s intervention? Be mindful that patients who are seeking pain relief and do not receive it may relapse into abuse patterns to alleviate that pain (Passik & Kirsh, 2005).
Define the problem. What often appears as drug-seeking behavior is, rather, pain relief–seeking behavior. Review the definitions of addiction, tolerance, physical dependence, and pseudo-addiction. Review apparently aberrant drug-taking behaviors: Do they suggest addiction or uncontrolled pain? Recommend including most currently accepted definitions—the joint statement would be a great reference.
Evaluate and treat comorbid psychiatric disorders. Substance abuse is accompanied by a high incidence of other psychiatric disorders, including depression and personality and anxiety disorders. Being mindful of the goals of care, obtain assistance in treating these disorders. Successful treatment may enhance patient comfort and quality of life: the goal of care.
Consider the therapeutic effect of tolerance. Tolerance may or not be a factor in treating the pain in persons with a current or past history of substance abuse; it is highly variable. Higher initial doses may be required along with relatively rapid dose escalation to manage pain. What appears as tolerance may indeed be rapidly advancing disease and thus worsening pain. The principle of titrating to effect applies regardless of the underlying cause of the need to increase dosage.
Apply appropriate pharmacologic principles to treat pain. The principles discussed earlier apply equally to the patient with a current or past history of substance abuse. The principle of titration to effect and the individualization of therapy, including providing the right drug at the right dose, however, is often difficult in this population. Be mindful of the goals of care and the definitions of addiction, tolerance, physical dependence, and pseudo-addiction (Passik & Kirsh, 2005). Remember that unrelieved pain can lead to the development of aberrant drug-related behavior, including relapse.
Select drugs and routes of administration for the symptom and setting. Although no data confirm this relationship, among persons with known substance abuse, long-acting medications may not contribute as significantly to aberrant drug-taking behavior as do short-acting drugs (Passik & Kirsh, 2005). Balance the medication choice with the type of pain, goals of care, and patient needs.
Recognize specific drug abuse behavior. Persons with a current or past history of substance abuse, including alcohol abuse, should be observed for actions that suggest substance abuse. If there is a high level of concern about aberrant behavior, the interdisciplinary team may determine and plan a higher level of monitoring, including more frequent visits, interviews with family members, and perhaps urine screening for prescribed and illicit drugs. Keep in mind the goals of care; if more monitoring is indicated, including urine drug screening, patients should be reassured that it will provide a foundation for aggressive symptom-oriented treatment (Passik & Kirsh, 2005).
Use nonpharmacologic approaches as appropriate. Nonpharmacologic approaches should augment pain and symptom management, not replace the use of appropriate pharmacologic therapies. Include educational initiatives (e.g., methods to communicate effectively with staff members about pain and to navigate a complex medical system) and cognitive and behavioral techniques to foster relaxation and enhance coping.
In order to improve the quality of life, and thus the quality of death, clinicians caring for the patient and family should focus on the reduction of suffering—whether the result of the disease process or of the patient’s own acts (Passik & Theobald, 2000). Anyone who provides palliative care must either be familiar with the basic concepts of addiction treatment or have access to readily available resources in this specialty area. Given the impact of either a current or a past substance abuse history on patient suffering and quality of life, identification of resources in advance is essential. Unless a clinician has a background in substance abuse disorders and is conversant with current thought in this area, he or she should not manage such patients and families without the appropriate assistance. The successful outcome of care and the patient’s and family’s quality of life depend on it.
Rapidly Escalating Pain
Rapidly escalating pain needs to be carefully referenced. When pain is severe and escalating rapidly, frequent increases of the dose of opioid may lead to severe side effects. Excitatory toxicity from large opioid doses can include myoclonus, grand mal seizures, delirium, hallucinations, and hyperalgesia. Management may require dose reduction, opioid rotation, hydration, and additional medications to control symptoms, such as midazolam, barbiturates, and baclofen. Take advantage of the resources available to ensure patient comfort.
PATIENT AND FAMILY EDUCATION
The complete plan of pain management and method for alterations should be fully discussed with the patient and family. Potential opioid side effects should be discussed at the time of the first prescription (information should include the assurance that tolerance develops to most of the side effects of opioids except constipation). A plan for a bowel regimen appropriate to the patient should also be agreed upon.
Many patients confuse addiction with the physical dependence that occurs in all patients over time; concerns for addiction need to be addressed. The emphasis of the education must be on alleviating the fear of addiction, as it is extremely rare in patients who are adequately treated for pain. Patients and families should be instructed not to decrease or stop opioids suddenly except under the direction of the health care professionals and should be informed of when to contact the health care provider about the patient’s pain and the frequency of use of as-needed medications. For example, the clinician may tell a patient to call when as-needed medication is used more than three times a day. The following should be provided in written form for the patient and family:
▪ A list of each medication with directions on when to take it
▪ Notification as to whether refills can be called in or require an original prescription
▪ Potential side effects and what to do if they occur
▪ Telephone numbers (24 hours a day, 7 days a week) of a health care professional for assistance with
Problems obtaining or taking medications
New pain or unrelieved pain
Uncontrolled nausea and vomiting
No bowel movement for longer than 2 days
Oversedation, confusion, or other neurologic changes
Any intolerable side effect
EVALUATION AND PLAN FOR FOLLOW-UP
Evaluation intervals are based on the assessment and achievement of pain management goals. The frequency of follow-up is dependent on the level of pain, planned interventions, and patient response. For example, a patient in a pain crisis may need frequent reassessment, including telephone contact between visits. A patient with stable pain may need only periodic evaluations. Gather information on present pain, the worst the pain has been since the last visit, and the most relief the patient has experienced since the last visit. On the basis of the patient’s underlying pathophysiologic condition, the clinician may be able to anticipate and thus assess for new pain syndromes. While caring for a patient with prostate cancer, assessing for the presence of bone pain is wise. Always be mindful of the patient- and family-related barriers to reporting pain and using analgesics discussed at the beginning of the chapter and how they might affect the plan of care.
The management of pain at end-of-life is essential. Uncontrolled pain robs the patient—and family—of quality of life that is so precious and important during these final months.
Mrs. W. is a 78-year-old woman with a history of oral pharyngeal cancer. She is known to have widespread metastatic bone disease. She enters the clinic in a wheelchair accompanied by her daughter, saying she is “absolutely miserable.”
Mrs. W. reports pain in her neck that has been present for over a year and has slowly increased in intensity. It hinders her ability to turn her head. She reports that it is about a 7 on a scale of 0 to 10 and is fairly constant all the time. She describes it as a dull, achy pain that moves from her neck up to her head. Her mobility has markedly decreased in the past 2 to 3 weeks. She has been taking acetaminophen 500 mg with 5 mg of hydrocodone (Vicodin), one tablet every 3 hours, with no real relief: “At first they helped; now they don’t do anything.” She expresses concern about becoming addicted to her pain medication.
She denies constipation. Laboratory values are all within normal range. Recent radiologic examinations reveal widespread increase in disease. Mrs. W. is aware of her advancing disease and expresses a desire to be able to feed her cats, enjoy her children and grandchildren, and be as free of pain as possible. She lives alone and, overall, manages well. She is able to swallow oral medications.
Pharmacologic Choices
There are several options for improving the management of Mrs. W.’s pain. Because it is severe (7 on a 0-to-10 scale), it is appropriate to increase her dose of medication by 50% to 100%. (Note that Mrs. W. is already at the maximum safe dosage of acetaminophen per day, or 4 g, so instructing her to take her Vicodin, two tablets every 4 hours, is not an option.) Consider the following:
▪ Convert Mrs. W. to a sustained-release preparation. Presently Mrs. W. is taking a total of 40 mg of hydrocodone a day. Following the principle of increasing the opioid by 100% because of her severe pain, and using the equianalgesic conversion chart, change her medication to sustained-release morphine, 40 mg orally every 12 hours. Adequate breakthrough medication is also ordered: 10% to 20% of the 24-hour dose, or immediate-release morphine, 12 to 20 mg orally every 1 to 2 hours as needed. If an increase of 50% is more appropriate, the sustained-release morphine dosage would be 30 mg orally every 12 hours with immediate-release morphine, 9 to 15 mg every 1 to 2 hours as needed.
▪ Add an NSAID, if not contraindicated by Mrs. W.’s history, for the pain secondary to metastatic bone disease.
▪ Transdermal fentanyl is not an appropriate choice at this time because her pain is not well controlled. Increases in transdermal fentanyl doses are only appropriate every 72 hours.
Because of the severity of her pain Mrs. W. was switched to sustained-release morphine, 40 mg orally every 12 hours, with immediate-release morphine, 12 to 20 mg orally every 1 to 2 hours as needed for breakthrough pain. Daily contact with Mrs. W. and her family was planned, and she was urged to keep a pain diary. She was a little sleepy the first day but rated her pain as 2 on a 0-to-10 scale, stating, “I can turn my head!” She reported taking no breakthrough medications. By the third day she was alert, sleeping all night, and feeding her cats and reported taking 5 mg of her breakthrough medication only once a day. She was instructed to call if she requires more than three doses of breakthrough medication a day.
REFERENCES
Allard, P.; Maunsell, E.; Labbe, J.; et al., Educational interventions to improve cancer pain control: a systematic review, J Palliat Med 4 (2) ( 2001) 191–203.
American Academy of Pain Medicine; American Pain Society; American Society of Addiction Medicine, Definitions related to the use of opioids for the treatment of pain, Retrieved October 31, 2005, from www.ampainsoc.org/advocacy/opioids2.htm ( 2001).
American Pain Society, Principles of analgesic use in the treatment of acute pain and cancer pain. 5th ed. ( 2003)Author, Glenview, Ill..
Anderson, G.; Vestergaard, K.; Ingeman-Nielsen, M.; et al., Incidence of central post-stroke pain, Pain 61 (1995) 187–193.
Berry, P.H.; Eagan, K.; Eighmy, J.B.; et al., Hospice and palliative nurses practice review. 3rd ed. ( 1999)Kendall/Hunt, Dubuque, Iowa.
Boivie, J., Central pain, In: (Editors: Wall, P.D.; Melzack, R.) Textbook of pain4th ed. ( 1999)Churchill Livingstone, New York, pp. 879–941.
Breitbart, W.; Passik, S.D.; Rosenfeld, H.D., Cancer, mind and spirit, In: (Editors: Wall, P.D.; Melzack, R.) Textbook of pain4th ed. ( 1999)Churchill Livingstone, New York, pp. 1065–1112.
Bruera, E.; Kim, H.N., Cancer pain, JAMA 290 (18) ( 2003) 2476–2479.
Caraceni, A.; Martini, C.; Zecca, E.; et al.Working Group of an IASP Task Force on Cancer Pain, Breakthrough pain characteristics and syndromes in patients with cancer pain: An international study, Palliat Med 18 (3) ( 2004) 177–183.
Chang, H.M., Pain and its management in patients with cancer, Cancer Invest 22 (5) ( 2004) 799–809.
Cherny, N.; Portenoy, R.K., Cancer pain: Principles of assessment and syndromes, In: (Editors: Wall, P.D.; Melzack, R.) Textbook of pain4th ed. ( 1999)Churchill Livingstone, New York, pp. 1017–1064.
Coderre, T.J.; Melzack, R., The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury, J Neurosci 12 (1992) 3671–3675.
Corbett, C.F., Practical management of patients with painful diabetic neuropathy, Diabetes Educ 31 (4) ( 2005) 523–524; 526-528, 530..
Devine, E.C., Meta-analysis of the effect of psychoeducational interventions on pain in adults with cancer, Oncol Nurs Forum 30 (1) ( 2003) 75–89.
Eshkevari, L.; Heath, J., Use of acupuncture for chronic pain: optimizing clinical practice, Holist Nurs Pract 19 (5) ( 2005) 217–221.
Esper, P.; Heidrich, D., Symptom clusters in advanced illness, Semin Oncol Nurs 21 (1) ( 2005) 20–28.
Fellowes, D.; Barnes, K.; Wilkinson, S., Aromatherapy and massage for symptom relief in patients with cancer, Cochrane Database Systematic Reviews ( 2) ( 2004); CD002287..
Fohr, S.A., The double effect of pain medication: Separating myth from reality, Journal of Palliative Medicine 1 (4) ( 1998) 315–328.
Georgesen, J.; Dungan, J.M., Managing spiritual distress in patients with advanced cancer pain, Cancer Nurs 19 (5) ( 1996) 376–383.
Gordon, D.B.; Dahl, J.L.; Miaskowski, C.; et al., American pain society recommendations for improving the quality of acute and cancer pain management: American Pain Society Quality of Care Task Force, Arch Intern Med 165 (14) ( 2005) 1574–1580.
Hudspith, M.J.; Siddall, P.J.; Munglani, R., Physiology of pain, In: (Editors: Hemmings, H.B.; Hopkins, P.M.) Foundations of anesthesia: Basic sciences for clinical practice2nd ed. ( 2006)Mosby, St. Louis, pp. 267–285.
International Association for the Study of Pain, Subcommittee on Taxonomy, Pain terms: A list with definitions and notes on usage, Pain 6 (1979) 249–252.
Joranson, D.E.; Ryan, K.M.; Gilson, A.M.; et al., Trends in medical use and abuse of opioid analgesics, JAMA 283 (2000) 1710–1714.
Kent, E.C.; Forauer, A.; Esper, P.; et al., Palliative embolization of metastases from kidney cancer [Abstract], J Clin Oncol, ASCO Annual Meeting Proceedings (Post-Meeting Edition) 22 (July 15 Suppl) ( 2004) 14S.
Kovach, C.; Weissman, D.; Griffie, J.; et al., Assessment and treatment of discomfort for people with late-stage dementia, J Pain Symptom Manage 18 (1999) 412–419.
Leddy, K.M.; Wolosin, R.J., Patient satisfaction with pain control during hospitalization, Joint Comm J Qual Patient Safety 31 (9) ( 2005) 507–513.
Levenson, J.W.; McCarthy, E.P.; Lynn, J.; et al., J Am Geriatr Soc 48 (5 Suppl) ( 2000) S101–S109.
Levy, M., Pharmacologic management of cancer pain, Semin Oncol 21 (6) ( 1994) 718–739.
Lussier, D.; Huskey, A.G.; Portenoy, R.K., Adjuvant analgesics in cancer pain management, Oncologist 9 (5) ( 2004) 571–591.
McCaffery, M., Nursing practice theories related to cognition, bodily pain, and man-environment interactions. ( 1968)UCLA Student Store, Los Angeles.
McCaffery, M.; Pasero, C., Pain: Clinical manual. 2nd ed. ( 1999)Mosby, St. Louis.
McCarthy, M.; Lay, M.; Addington-Hall, J., Dying from heart disease, J R Coll Physicians Lond 30 (4) ( 1996) 325–328.
Mercadante, W.; Portenoy, R.K., Opioid responsive cancer pain, part 3: Clinical strategies to improve opioid responsiveness, J Pain Symptom Manage 21 (4) ( 2001) 338–354.
Mundy, E.A.; DuHamel, K.N.; Montgomery, G.H., The efficacy of behavioral interventions for cancer treatment-related side effects, Semin Clin Neuropsychiatry 8 (4) ( 2003) 253–275.
National Cancer Institute (NCI), Pain (PDQ)—health professional version, Retrieved August 25, 2005, from www.cancer.gov/cancertopics/pdq/supportivecare/pain/healthprofessional ( 2005).
National Comprehensive Cancer Network (NCCN), NCCN clinical practice guidelines in oncology, Retrieved August 31, 2005, from www.nccn.org/professionals/physician_gls/PDF/pain.pdf.
Nilsson, S.; Strang, P.; Ginman, C.; et al., Palliation of bone pain in prostate cancer using chemotherapy and strontium-89. A randomized phase II study, J Pain Symptom Manage 29 (4) ( 2005) 352–357.
Ogle, K.S.; Hopper, K., End-of-life care for older adults, Primary Care 32 (2005) 811–828.
Page, G.G., Surgery-induced immunosuppression and postoperative pain management, AACN Clin Issues 16 (3) ( 2005) 302–309.
Paice, J.A., Mechanisms and management of neuropathic pain in cancer, J Support Oncol 1 (2) ( 2003) 107–120.
Paice, J.A.; Fine, P.G., Pain at the end of life, In: (Editors: Ferrell, B.R.; Coyle, N.) Textbook of palliative nursing ( 2001)Oxford University Press, New York, pp. 76–90.
Pappagallo, M.; Dickerson, E.D.; Varga, J.; et al., Management of neuropathic pain, In: (Editors: Kuebler, K.K.; Esper, P.) Palliative practices from A to Z for the bedside clinician ( 2002)ONS Press, Pittsburgh, p. 247; 248, 253.
Passik, S.D.; Kirsh, K.L., Managing pain in patients with aberrant drug-taking behaviors, J Support Oncol 3 (1) ( 2005) 83–86.
Passik, S.D.; Theobald, D.E., Managing addiction in the advanced cancer patient: Why bother?J Pain Symptom Manage 19 (2000) 229–234.
Portenoy, R.; Waldman, S., Adjuvant analgesics in pain management: Part 1, J Pain Symptom Manage 9 (6) ( 1994) 390–391.
Portenoy, R.K.; Hagen, N.A., Breakthrough pain: Definition, prevalence, and characteristics, Pain 41 (1990) 273–281.
Reisfield, G.M.; Silberstein, E.B.; Wilson, G.R., Radiopharmaceuticals for the palliation of painful bone metastases, Am J Hospice Palliat Care 22 (1) ( 2005) 41–46.
Ruggiero, S.L.; Mehrotra, B.; Rosenberg, T.J.; et al., Osteonecrosis of the jaws associated with the use of bisphosphonates: A review of 63 cases, J Oral Maxillofac Surg 62 (5) ( 2004) 527–534.
Rutter, C.; Weissman, D.E., Radiation for palliation—part 2, J Palliat Med 7 (6) ( 2004) 866–867.
Sabino, M.A.; Mantyh, P.W., Pathophysiology of bone cancer pain, J Support Oncol 3 (1) ( 2005) 15–24.
Sela, R.A.; Bruera, E.; Conner-Spady, B.; et al., Sensory and affective dimensions of advanced cancer pain, Psycho-Oncology 11 (2002) 23–34.
Serlin, R.C.; Mendoza, T.R.; Nakamura, Y.; et al., When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function, Pain 61 (1995) 277–284.
SUPPORT Study Principal Investigators, A controlled trial to improve care for seriously ill hospitalized patients: A study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT), JAMA 274 (1995) 1591–1598.
Svendsen, K.B.; Andersen, S.; Arnason, S.; et al., Breakthrough pain in malignant and non-malignant diseases: A review of prevalence, characteristics and mechanisms, Eur J Pain 9 (2) ( 2005) 195–206.
Sykes, N.; Thorns, A., The use of opioids and sedatives at the end of life, Lancet Oncol 4 (5) ( 2003) 312–318.
Sze, W.M.; Shelley, M.D.; Held, I.; et al., Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy: A systematic review of randomized trials, Clin Oncol (R Coll Radiol) 15 (6) ( 2003) 345–352.
Ulens, C.; Daenens, P.; Tytgat, J., Norpropoxyphene-induced cardiotoxicity is associated with changes in ion-selectivity and gating of HERG currents, Cardiovasc Res 44 (3) ( 1999) 568–578.
Vachon, M.L., The emotional problems of the patient in palliative medicine, In: (Editors: Doyle, D.; Hanks, G.; Cherny, N.; et al.) Oxford textbook of palliative medicine3rd ed. ( 2004)Oxford University Press, New York.
Ward, S.E.; Goldberg, N.; Miller-McCauley, V.; et al., Patient-related barriers to management of cancer pain, Pain 52 (1993) 319–324.
Warden, V.; Hurley, A.C.; Volicer, L., Development and psychometric evaluation of the Pain Assessment in Advanced Dementia (PAINAD) scale, J Am Med Dir Assoc 4 (1) ( 2003) 9–15.
Wilke, D.J., Neural mechanisms of pain: A foundation for cancer pain assessment and management, In: (Editors: McGuire, D.B.; Yarbro, C.H.; Ferrell, B.R.) Cancer pain management2nd ed. ( 1995)Jones & Bartlett, Boston, pp. 61–87.
Wong, G.Y.; Schroeder, D.R.; Carns, P.E.; et al., Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer, JAMA 291 (9) ( 2004) 1092–1099.
World Health Organization, Cancer pain relief and palliative care (Technical Report Series 804). ( 1990)Author, Geneva.
Zeppetella, G.; O’Doherty, C.A.; Collins, S., Prevalence and characteristics of breakthrough pain in cancer patients admitted to hospice, J Pain Symptom Manage 20 (2) ( 2000) 87–92.
Zeppetella, G.; O’Doherty, C.A.; Collins, S., Prevalence and characteristics of breakthrough pain in patients with non-malignant terminal disease admitted to hospice, Palliat Med 15 (3) ( 2001) 243–246.