CASE 31 Coronary Artery Pseudoaneurysm After Stenting
Case presentation
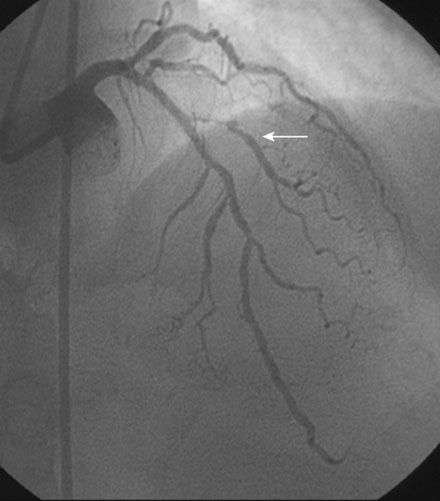
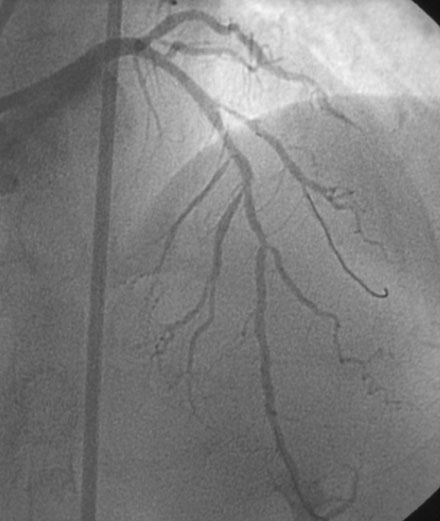
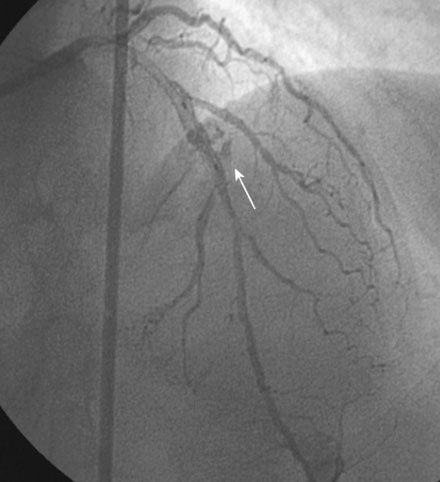
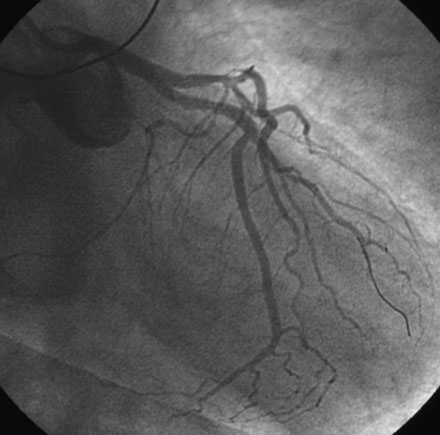
An actively smoking, 65-year-old woman with hypertension, hyperlipidemia, and carotid artery disease presented with dyspnea and chest discomfort radiating to her left arm, occurring at a low workload. A coronary angiogram found severe narrowing of a small caliber diagonal branch and at least moderate narrowing of a heavily calcified segment of the midportion of the left anterior descending artery (Figure 31-1). The circumflex and right coronary arteries appeared normal, and left ventricular function was preserved. Initially treated medically with aspirin, clopidogrel, beta-blockers, and statins, she continued to have persistent angina and was referred for intervention of the diagonal and possibly the left anterior descending artery several weeks later.
Cardiac catheterization
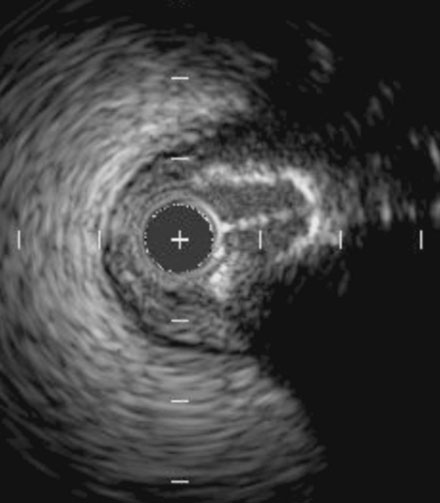
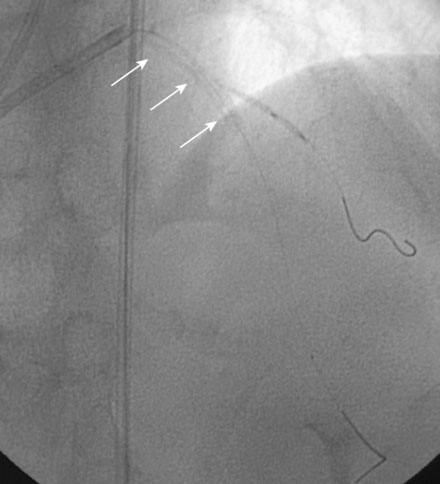
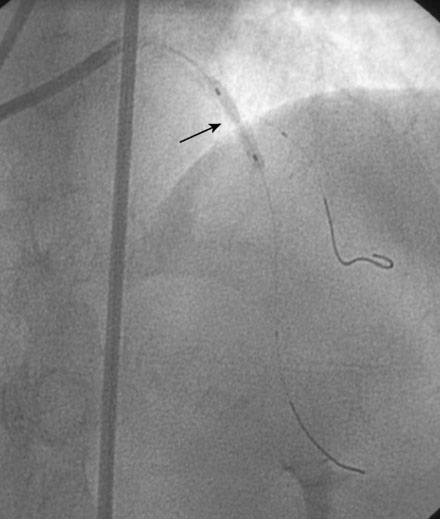
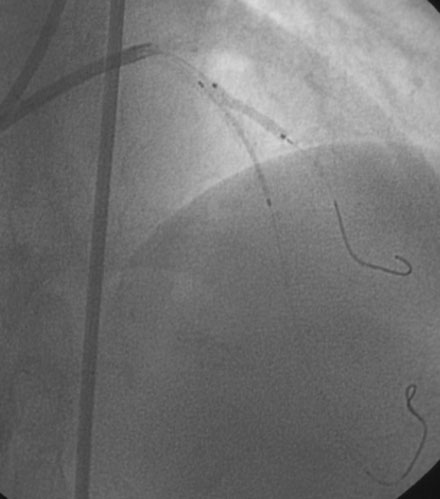
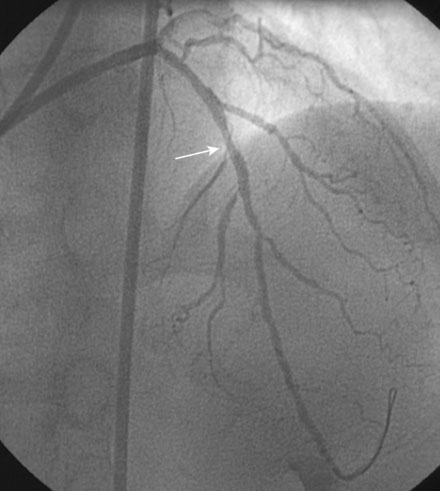
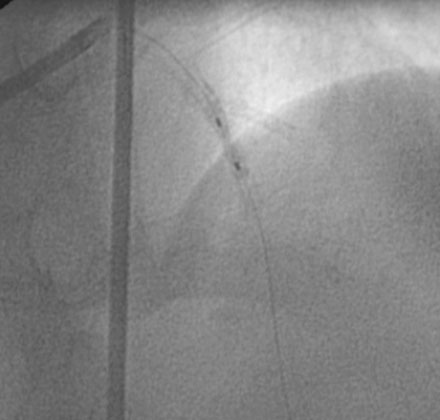
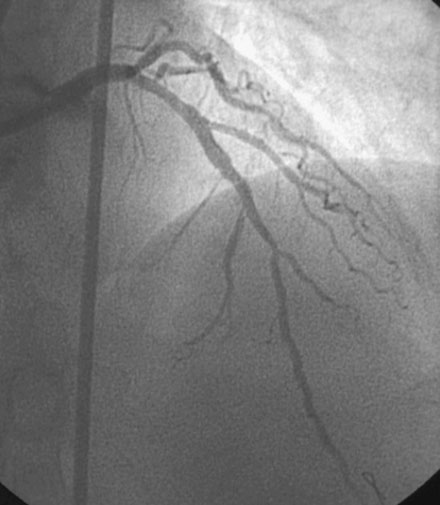
During the second procedure, the operator found it difficult to determine the significance of the disease in the midsegment of the left anterior descending artery and performed intravascular ultrasound. This showed extensive calcification and a minimal luminal area of 1.5 mm2 (Figure 31-2). Based on this data, the operator planned to treat the disease in both the left anterior descending artery and diagonal branch. An 8 French 4.0 left Judkins guide was engaged into the left coronary ostium and 0.014 inch guidewires were positioned distally in both the left anterior descending artery and the diagonal branch; bivalirudin bolus and infusion were used as the procedural anticoagulant. The extensive calcification is shown in Figure 31-3. Balloon angioplasty of the diagonal using a 2.0 mm diameter by 20 mm long compliant balloon (see Figure 31-3) and the left anterior descending artery using a 2.5 mm diameter by 20 mm long compliant balloon (Figure 31-4) was performed. The operator noted that the balloon in the left anterior descending artery did not appear fully expanded at nominal inflation pressures, and, although the luminal appearance improved after angioplasty, a linear dissection in the left anterior descending artery and severe narrowing of the ostium of the diagonal branch were apparent (Figure 31-5 and Video 31-1). At this point, although the operator worried that a stent might not fully expand in the left anterior descending artery, the presence of a significant dissection led to a decision against rotational atherectomy. The operator chose a “crush stent” approach to preserve patency of the diagonal branch using a 2.25 mm diameter by 15 mm long bare-metal stent in the diagonal and a 2.5 mm diameter by 24 mm long paclitaxel-eluting stent in the left anterior descending artery (Figure 31-6). The diagonal stent expanded appropriately, but the operator noted a waist in the balloon used to deploy the left anterior descending artery stent, despite 14 atmospheres inflation pressure. The post-stent angiogram confirmed inadequate stent expansion at this site (Figure 31-7 and Video 31-2). A waist remained in the balloon at the site of persistent narrowing despite the use of a 2.5 mm diameter by 9 mm long noncompliant balloon inflated to 20 atmospheres pressure (Figure 31-8) and caused no change in the angiographic appearance of the lesion. The operator used a 3.0 mm diameter by 15 mm long noncompliant balloon, slowly inflating the balloon until the waist suddenly resolved at 20 atmospheres. With the deflated balloon in place, an angiogram was performed, which revealed contrast extravasation outside of the wall of the artery consistent with a deep dissection or contained perforation (Figure 31-9 and Video 31-3). The bivalirudin infusion was stopped and the patient remained asymptomatic and hemodynamically stable. Five minutes later, another angiogram was performed and showed no evidence of contrast extravasation and an excellent luminal result in both the left anterior descending and diagonal arteries (Figure 31-10 and Video 31-4). She was observed an additional 10 minutes with no change in the angiogram. An echocardiogram showed no pericardial effusion. She was observed overnight and discharged the next morning.
Postprocedural course
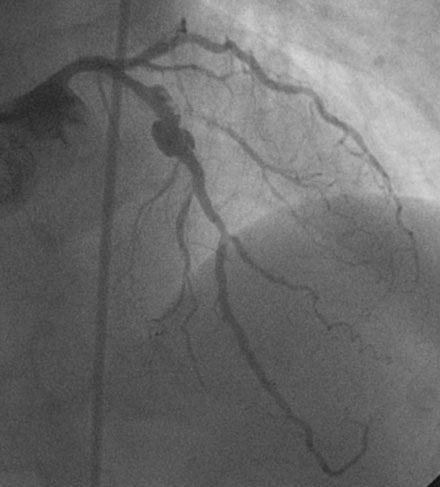
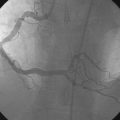
She remained symptom-free for three months and then was admitted to the hospital after developing her typical anginal chest pain intermittently and occurring at rest, lasting up to 10 to 15 minutes and relieved by nitroglycerin. Electrocardiogram and serum biomarkers were negative and she underwent another coronary angiogram. This confirmed severe in-stent restenosis in the diagonal stent. Although the left anterior descending artery stent remained widely patent, the stent site appeared abnormal with contrast appearing outside the confines of the artery wall (Figure 31-11 and Video 31-5). This was felt to represent a pseudoaneurysm. After an extensive discussion regarding the options of medical therapy with close observation, surgery, or deployment of a covered stent, the patient and managing physician chose medical therapy with the plan to reimage the artery in 2 months.
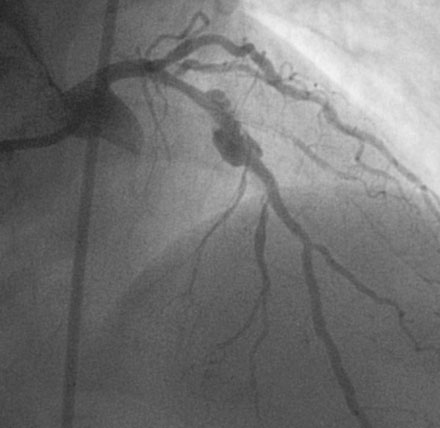
She continued to have angina and, although the diagonal branch had now occluded, the repeat angiogram showed no change in the pseudoaneurysm 2 months later (Figure 31-12 and Videos 31-6, 31-7). Again, after extensive discussions, she was referred for surgery. The surgeon removed the stent and ligated and excluded the aneurysmal portion of the vessel using a left internal mammary artery graft to bypass the segment. She made an uneventful recovery and remained free of angina 1 year later.
Discussion
Coronary aneurysms developing after percutaneous intervention are very rare. They were observed in 1.25% of patients in a recent study of 1197 patients undergoing routine angiography after drug-eluting stent implantation.1 Aneurysms are most commonly caused after intervention from abnormal healing of a deep dissection, contained perforation, or vessel rupture, as observed in this case. Proper healing after these events may be further impaired by drug-eluting stents.2
The natural history of coronary aneurysms after intervention is not well-known. They may be incidental findings with no consequences, and many resolve spontaneously.1,3 However, they have the potential for serious complications, including expansion and rupture of pseudoaneurysms, representing a potential source of coronary embolization; or acting as a nidus for stent thrombosis. Distinguishing a true aneurysm from a pseudoaneurysm can be accomplished by intravascular ultrasound and is based on the ability to identify an intact vessel wall in the case of true aneurysms, and disruption of the vessel wall with damage to the adventia in the event of a pseudoaneurysm.4
There is no consensus on optimal management of coronary aneurysms and pseudoaneurysms. Individual case reports have described success with conservative management, PTFE-covered stents, and coil embolization.5–7 Surgical options, as described in the present case, include ligation and resection of the aneurysm with distal bypass grafting.
1 Alfonso F., Perez-Vizcayno M.J., Ruiz M., Suarez A., Cazares M., Hernandez R., Escaned J., Banuelos C., Jiminez-Quevedo P., Macaya C. Coronary aneurysms after drug-eluting stent implantation. Clinical, angiographic, and intravascular ultrasound findings. J Am Coll Cardiol. 2009;53:2053-2060.
2 Aoki J., Kirtane A., Leon M.B., Dangas G. Coronary artery aneurysm after drug eluting stent implantation. J Am Coll Cardiol Interv. 2008;1:14-21.
3 Mikhail B., Brewer R.J., Clark V.L. Spontaneous closure of a perforation-induced coronary artery pseudoaneurysm. J Interv Cardiol. 2002;14:282-284.
4 Maehara A., Mintz G.S., Ahmed J.M., Fuchs S., Castagna M.T., Pichard A.D., Satler L.F., Waksman R., Suddath W.O., Kent K.M., Weissman N.J. An intravascular ultrasound classification of angiographic coronary artery aneurysms. Am J Cardiol. 2001;88:365-370.
5 Szalat A., Durst R., Cohen A., Lotan C. Use of polytetrafluoroethylene-covered stent for treatment of coronary artery aneurysm. Catheter Cardiovasc Interv. 2005;66:203-208.
6 Maroo A., Rasmussen P.A., Masaryk T.J., Ellis S.G., Lincoff M., Kapadia S. Stent-assisted detachable coil embolization of pseudoaneurysms in the coronary circulation. Catheter Cardiovasc Interv. 2006;68:409-415.
7 Brasselet C., Perotin S., Fuzellier J.F. Subacute development of a coronary artery pseudoaneurysm after primary angioplasty and stenting for acute myocardial infarction. J Interv Cardiol. 2003;15:168-170.