Section 30 Toxinology
30.1 Snakebite
Epidemiology
The commonest clinical manifestation is coagulopathy which occurs in about three-quarters of envenomed cases, about half from brown snakes and half from the tiger snake group. Neurotoxicity and myotoxicity are now uncommon and mechanical ventilation is rarely required for treatment. The types of snakes causing major envenoming differ across Australia. Exotic snakebite remains a problem from snakes in zoos and an unknown number of illegally kept snakes, but hospital presentations are rare.
Clinical features and toxinology
Envenoming results when venom is injected subcutaneously and reaches the systemic circulation. Whether or not a snakebite results in envenoming depends on a number of factors including fang length, average venom yield of the snake, effectiveness of the bite and bite site. Recent studies have suggested that only a small amount of the injected venom actually reaches the systemic circulation.1
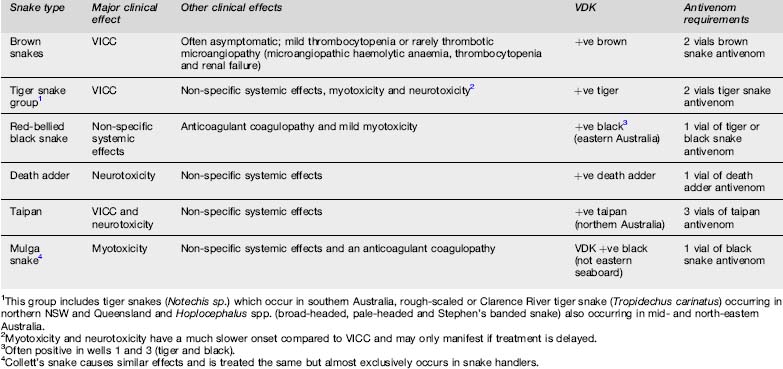
The medically important Australian snakes and their associated clinical effects are listed in Table 30.1.1.
Table 30.1.1 Summary of clinical effects, diagnosis and antivenom requirements for envenoming by major Australian snakes
Coagulopathy
Venom-induced consumption coagulopathy (VICC)
This is the commonest and most important clinical effect in Australian snake envenoming. Venom-induced consumption coagulopathy (VICC) results from a prothrombin activator in the snake venom converting prothrombin (factor II) to thrombin which leads to consumption of fibrinogen, massive increases in fibrinogen degradation products and consumption of factors V and VIII due to thrombin activation. Most dangerous Australian snakes contain such a prothrombin activator including brown snakes, snakes in the tiger snake group and taipans. Venom-induced consumption coagulopathy develops rapidly within 15 to 60 min and the onset may coincide with the initial collapse seen with major envenoming by brown snakes and taipans. Recovery usually takes 12 to 18 h.2
Anticoagulant coagulopathy
This occurs with Mulga and Collett’s snake, and in about half of red-bellied black snake envenomings. It is unlikely to result in haemorrhage and of itself is rarely of clinical importance. However, anticoagulant coagulopathy is a marker of significant envenoming and is rapidly reversed with antivenom.
Treatment
First aid
Australian snake venoms appear to be absorbed via the lymphatic system so absorption is likely to be increased by movement and exercise. The aim of first aid is to minimize movement of venom to the systemic circulation. This is achieved by a pressure bandage (elastic bandage such as ACE®) being applied over the bite site and then covering the whole limb with a similar pressure to that used for a limb sprain. The bitten limb must be immobilized as well as the whole patient, or the first aid is ineffective.3 Immobilization consists of splinting and complete prevention of movement or exercise of the bitten part. It has been shown that movement of all limbs, not just the affected one, needs to be minimized for optimal effect.4 Transport should be brought to the patient and walking must be avoided. Prompt, properly applied first aid probably prevents significant absorption of venom for many hours although there is only anecdotal evidence to support this. Pressure bandaging is clearly impractical for bites that are not on the limbs but direct pressure with a pad and immobilization may be useful. The bite site should not be washed so that it can be swabbed for venom detection.
Initial assessment and treatment
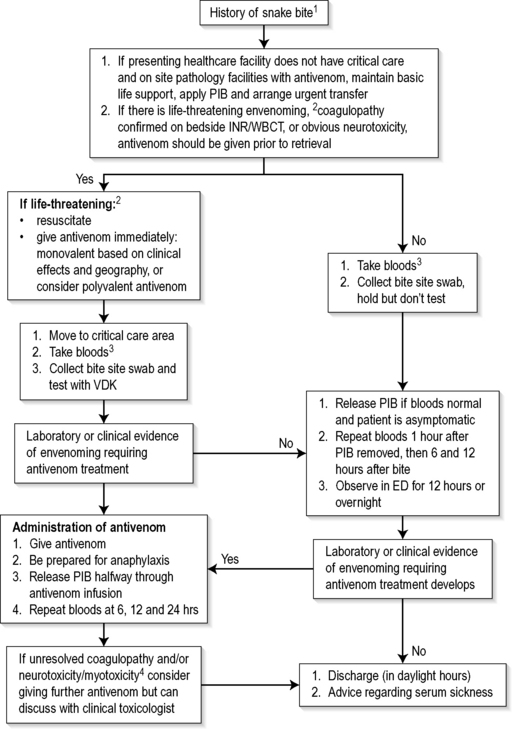
Figure 30.1.1 provides a simple approach to the management of suspected and envenomed snakebite patients. The patient is managed in an area with full resuscitation facilities. Assessment and management proceed simultaneously. The airway, breathing and circulation are assessed and stabilized. The majority of patients are not critically unwell and can have a focused neurological examination for early signs of paralysis (e.g. ptosis, drooling), examination of draining lymph nodes and general examination for signs of bleeding (oozing from the bite site, gum bleeds). Intravenous access should be established and intravenous fluids commenced.
Further management
Two major diagnostic and risk assessment issues exist for snakebite:
If there is no evidence of envenoming after clinical assessment and initial laboratory testing the first-aid bandage can be removed. The patient requires ongoing close observation including repeated investigations one hour after bandage removal, and at 6 and 12 h after the bite (see Figure 30.1.1).
In the majority of cases a combination of these three factors allows determination of the correct monovalent snake antivenom required. If it is unclear which snake is involved then one vial of polyvalent antivenom should be administered. This contains sufficient antivenom for an initial dose for all types of snakes. In some parts of Australia, such as Victoria and Perth, a combination of brown and tiger snake antivenom can be administered in preference to polyvalent. In Tasmania only tiger snake antivenom is required.
Administration of antivenom
The initial dose of antivenom for children is the same as for adults and is provided in Table 30.1.1. Further doses are usually not required and recovery is determined by the reversibility of effects and the time it takes for recovery once venom is neutralized. The benefits of antivenom are listed in Table 30.1.2, which underlines the importance of waiting for recovery after antivenom administration, particularly with VICC.5 Although there is controversy over the dose of antivenom, recent studies have demonstrated that previously recommended large doses are not required. For brown snake envenoming an initial dose of two vials binds all venom and sufficient time must then be allowed for resynthesis of clotting factors.1,2
Table 30.1.2 The benefits of antivenom for different effects of envenoming (Modified from Therapeutic Guidelines Emergency Medicine, March 2008 with permission)
| Clinical effect | Benefit |
|---|---|
| Venom-induced consumption coagulopathy (VICC) | Appears to neutralize toxin effect allowing clotting factors to be resynthesized and clotting to recover over 6 to 18 hours |
| Anticoagulant coagulopathy | Neutralizes a toxin inhibitor of coagulation with immediate improvement in coagulation studies |
| Neurotoxicity | Neutralizes toxin in the intravascular compartment and will prevent further development of neurotoxicity but will not reverse neurotoxic effects already present |
| Myolysis/rhabdomyolysis | Neutralizes myotoxins and will prevent further muscle injury but will not reverse effects |
| Local effects | Unlikely to reverse any already developed local effects |
| Renal damage | Unlikely to have an effect |
Generalized systemic effects: nausea, vomiting, headache, abdominal pain, diarrhoea and diaphoresis.
Rapidly reverses non-specific effects and is a useful indication of antivenom binding venom components.
Premedication for snake antivenom administration has previously been controversial but is no longer recommended in Australia. One randomized controlled trial suggested that adrenaline was an effective premedication for snake antivenom.6 However, the trial was for an antivenom with a much higher reaction rate and was too small to assess the safety of adrenaline.7 Promethazine has been shown to be ineffective as a premedication,8 and often leads to drowsiness that makes neurological assessment difficult. Immediate-type hypersensitivity reactions occur in about a quarter of antivenom administrations in Australia, but are only severe (mainly hypotension) in 5% of administrations.9 Reactions are more common with tiger snake antivenom and polyvalent antivenom compared to brown snake antivenom. Antivenom should always be administered in a critical care area with readily available adrenaline, intravenous fluids and resuscitation equipment.
Clinical investigation
Snake venom detection kit
In non-envenomed patients the SVDK has a high false positive rate, especially in the brown snake well. This is even more problematic for urine testing so a bite site swab is preferred if available. A positive VDK on urine does not indicate systemic toxicity and in asymptomatic patients with normal laboratory studies this is most likely a false positive. The test should not be done on blood. If the snake responsible for the bite accompanies the patient, a swab of the fangs can be tested except that this may require considerable dilution because it will be too concentrated and overwhelms the SVDK with all wells changing colour.
Disposition
Patients with suspected snakebite but no evidence of envenoming one hour after the removal of first aid may be admitted to an observation area. Blood tests including coagulation studies and a creatine kinase (CK) should be repeated at 1 and 6 h after first aid is removed and be observed for 12 h or overnight (Fig. 30.1.1). Envenomed patients requiring ventilatory support should have continued management in ICU, but patients with coagulopathy only are commonly managed in ED observation wards.
1 Isbister GK, O’Leary MA, Schneider JJ, et al. Efficacy of antivenom against the procoagulant effect of Australian brown snake (Pseudonaja sp.) venom: In vivo and in vitro studies. Toxicon. 2007;49:57-67.
2 Isbister GK, Williams V, Brown SG, et al. Clinically applicable laboratory end-points for treating snakebite coagulopathy. Pathology. 2006;38:568-572.
3 Sutherland SK, Coulter AR, Harris RD. Rationalisation of first-aid measures for elapid snake bite. Lancet. 1979;1:183-186.
4 Howarth DM, Southee AE, Whyte IM. Lymphatic flow rates and first-aid in simulated peripheral snake or spider envenomation. Medical Journal. 1994;161:695-700.
5 Isbister GK. Snake bite: a current approach to management. Australian Prescribing. 2006;29:125-129.
6 Premawardhena AP, de Silva CE, Fonseka MMD, et al. Low dose subcutaneous adrenaline to prevent acute adverse reactions to antivenom serum in people bitten by snakes: randomised, placebo controlled trial. British Medical Journal. 1999;318:1041-1043.
7 Lalloo DG, Theakston RD. Snake antivenoms. Journal of Toxicology: Clinical Toxicology. 2003;41:277-290.
8 Fan HW, Marcopito LF, Cardoso JL, et al. Sequential randomised and double blind trial of promethazine prophylaxis against early anaphylactic reactions to antivenom for bothrops snake bites. British Medical Journal. 1999;318:1451-1452.
9 Isbister GK, Brown SG, MacDonald E, et al. Australian Snakebite Project Investigators. Current use of Australian snake antivenoms and frequency of immediate-type hypersensitivity reactions and anaphylaxis. Medical Journal Australia. 2008;188(8):473-476. Apr 21
30.2 Spider bite
Introduction
Australasia is home to a variety of spiders as well as some species that have been introduced, probably including the redback spider. The majority of spiders have small jaws which are too small to penetrate human skin. Larger spiders with toxic venom, and habits and distribution that promote human encounters, can cause medically significant envenoming. Although there are over 2000 characterized species of spiders in Australia, a much smaller group is responsible for the majority of human bites with six families of spiders being responsible for over 80% of bites.1 Spiders commonly responsible for human bites include huntsman spiders (Sparassidae), orb weaving spiders (Araneidae), white-tail spiders (Lampona spp.), redback spiders and the closely related Steatoda spp. (Theridiidae), wolf spiders (Lycosidae) and jumping spiders (Salticidae).1 Fatalities have occurred in Australia after being bitten by the redback and the funnel web spider (FWS).2
Redback spider (Latrodectus hasselti)
Venom
The components toxic to humans in the venom of widow spiders are α-latrotoxins that cause massive release of neurotransmitters and deplete synaptic vesicles at nerve endings. Recent work based on in vitro effects suggests that all widow spiders have a similar toxin.3 However, although the effects of the toxin are well understood at the cellular level it remains unclear how it produces the clinical syndrome.
Clinical features
The majority of patients bitten develop some effects from redback spider bites with pain being the most common and important symptom. Systemic effects occur in about a third of cases. Initially the bite may be painless, or may feel like a pinprick or a burning sensation. The pain then increases over the first hour and may radiate proximally to the regional lymph nodes or the chest or abdomen. Localized sweating and less commonly piloerection may occur and are virtually pathognomonic of latrodectism. Regional and distant sweating is also common, and bilateral below knee sweating can occur. Systemic effects include malaise, lethargy, nausea and vomiting and headache. A summary of the clinical effects is listed in Table 30.2.1, including less common effects. Pain and systemic effects persist for 1 to 4 days.4 Delayed effects or effects persisting for days to weeks have been reported but it is unclear in many cases whether the effects are a consequence of the spider bite.
Table 30.2.1 Clinical features of redback spider bite (Reproduced with permission from Therapeutic Guidelines Emergency Medicine 2008)
| Local and regional effects |
Treatment
Antivenom therapy
Antivenom is regarded as the primary treatment for redback spider bite. Despite it conventionally being given by the intramuscular (i.m.) route there has been increased use by the intravenous (i.v.) route. Two randomized controlled trials have shown no difference between i.v. and i.m. antivenom administration and the median dose used in both trials was two vials.5 Fears that i.v. antivenom results in a higher rate of reactions are not founded yet and the reaction rate with diluted i.v. administration is similar to that with i.m. antivenom.6 It is therefore reasonable to administer redback antivenom by either i.m. injection or slow i.v. infusion (diluted in 100 mL normal saline and given over 15 min). The initial dose should be two vials and premedication is not recommended. Repeat doses of antivenom are sometimes used and reassessment is appropriate after a period of 2 h. However, it is important that ongoing analgesia is also given to the patient. As for all antivenoms, the dose for children is the same as for adults.
Steatoda species (Cupboard or button spiders)
There have been a number of reports of bites by Steatoda spp., mainly in Australia.7 They appear to cause a similar syndrome to latrodectism with persistent local pain but fewer systemic features. In vitro studies of these spiders’ venom demonstrate that they cause similar but far less potent effects compared to α-latrotoxin. These studies also demonstrate in vitro neutralization of Steatoda venom with redback antivenom.8 The majority of bites by this group of spiders cause only minor effects, although the patient may have annoying pain for a period of hours.7 Uncommonly, they can cause more severe and persistent pain, similar to widow spiders. In the latter case it is postulated that redback antivenom may be an appropriate treatment.7
Funnel web spider (Atrax and Hadronyche Species)
Distribution and taxonomy
At least 39 species of funnel web spiders (FWS) occur along the east coast of Australia, including Tasmania and Adelaide. However, only six species occurring from Southern NSW to Southern Queensland have been associated with significant envenoming – Sydney FWS (Atrax robustus), the Southern Tree FWS (Hadronyche cerberea), the Northern Tree FWS (H. formidabilis), the Blue Mountains FWS (H. versuta), the Toowoomba or Darling Downs FWS (H. infensa) and the Port Macquarie FWS (H. sp 14).9 Historical records suggest there is an increase in bites by other species in the last few decades compared to most bites being due to the Sydney FWS in the past. This may be due to increasing population density in the area of distribution of Hadronyche species. Funnel web spiders are burrowing spiders and most encounters with humans occur when males are out looking for mates.
Venom
The males have a more potent venom than the females and only males have been reported to cause significant illness in humans.10 The important toxins in human envenoming appears to be δ-atracotoxins which have been isolated from the venom of a number of species of FWS.10 These are low-molecular-weight neurotoxins that prevent inactivation of sodium channels. The main effect of the neurotoxin is an autonomic storm that can be predominantly sympathetic or parasympathetic, or mixed in effect, associated with initial excitation at neuromuscular junctions, followed by paralysis.10
Epidemiology
Although there are a large number of suspected FWS bites each year, severe envenoming is rare and only 5 to 10 cases requiring antivenom occur annually.9 Many definite bites by FWS do not result in envenoming (dry bites) and the frequency of non-envenoming varies between species.9 In addition, many cases are a result of other big black spiders that appear to be FWS and are not collected or identified.
Clinical features
The initial bite is painful due to the size of the fangs and fang marks are usually present. Severe envenoming develops rapidly and usually occurs within 30 min.9 Initial effects include paraesthesia (local, distal extremities and perioral), local fasciculations, tongue fasciculations and non-specific systemic effects (nausea, vomiting and abdominal pain). Autonomic features are typical of systemic envenoming with hypersalivation, lacrimation and generalized sweating. Other autonomic features can include miosis, mydriasis, tachycardia or bradycardia and hypertension. Initially, the patient is usually agitated and anxious with decreased level of consciousness and coma developing as late signs.9 Non-cardiogenic pulmonary oedema may develop, and is thought to result from venom-induced capillary leakage. Prior to antivenom treatment this occurred early, but is now more commonly reported as a delayed effect.
Treatment
Antivenom therapy
Definitive treatment is venom neutralization with specific FWS antivenom. The antivenom is derived from rabbit serum and appears to be less antigenic to humans than horse serum antivenoms with a low reaction rate (<2%).9 Premedication is not recommended but antivenom must be administered in a critical care area with adrenaline available. Antivenom is indicated for systemic envenoming as defined above. Initially, two vials should be given (four if severe), and repeated every 15 min if there is no improvement. Delayed serum sickness reactions have been reported in at least one case.9
Mouse spiders (Missulena spp.)
Another group of spiders, the mouse spiders (Missulena spp.), are rarely reported to cause similar effects to FWSs.11 These spiders belong to the family Actinopodidae, and occur in most parts of Australia. Most bites are by wandering male spiders and do not cause any major effects. The initial bite causes pain and fang marks, due to the size of the fangs. There is one report of a bite by the Eastern mouse spider (Missulena bradleyi) that caused a syndrome similar to funnel web envenoming in a 19-month-old child.11 Recent work on the venom of the Eastern mouse spider has demonstrated that the venom causes similar effects in vitro to FWS venom, and it is neutralized in vitro by funnel web antivenom.12 However, all other reported cases have caused only local effects and less commonly local neurotoxic effects and/or mild non-specific systemic effects.11
Other Australasian spiders
There are a number of other Australian spiders that can and do cause human bites. In the majority of cases they cause only minor effects and symptomatic treatment is all that is required. In a large study of definite spider bites there were no cases of necrotic lesions or allergic reactions, suggesting these effects are either rare or do not occur.1 The incidence of secondary infection is also low, and occurred in less than 1% of cases in the same study.1
Necrotic arachnidism
Necrotic arachnidism is generally defined as necrotic lesions or ulcers that occur following a spider bite and are a result of venom effects. Significant skin necrosis following bites from recluse spiders (Loxosceles species) is well reported in many parts of the world.13Loxosceles rufescens has been introduced to South Australia and has been responsible for a few bites, but there is no evidence that it has spread beyond this distribution.
The white-tailed spider (Lampona cylindrata/murina group) has been implicated in the development of necrotic arachnidism. However, recent studies show that this is not the case, with 130 definite bites by these spiders causing no cases of necrotic lesions.14 Other spiders have been implicated in this condition, including wolf spiders, sac spiders and the black house spiders, but there is similarly little evidence to support this and prospective cases of definite bites by these groups of spiders have not demonstrated necrotic lesions.1Table 30.2.2 provides an approach to the patient with a skin ulcer attributed to a spider bite.
Table 30.2.2 An approach to the investigation and diagnosis of necrotic skin ulcers presenting as suspected spider bites (From: Isbister, GK. Spider bite. Australian Doctor 2004 with permission)
| A. Establish whether or not there is a history of spider bite |
An approach to the patient with spider bite
The first step is to take a careful history so as to determine whether the case is a definite spider bite or only a suspected spider bite. The diagnosis of definite spider bite requires sighting of the spider at the time of the bite and usually some initial symptoms such as local pain. If there is no history of bite or no spider was seen, then other diagnoses must be excluded. This is particularly important in persons presenting with ulcers or skin lesions with suspected spider bites (Table 30.2.2). It is important in these cases that appropriate investigations are done and the case treated as a necrotic ulcer of unknown aetiology. In the majority of these cases an infective cause is found, although less commonly they are a result of pyoderma gangrenosum or a vasculitis.15
The third group includes all other spiders. Despite previous concerns about particular spiders, such as the badged huntsman (Neosparassus spp.) and white-tail spiders, all other spiders are very unlikely to cause more than minor effects.1 Patients can be reassured, their tetanus status confirmed and updated, if required, and symptomatic treatment with ice and analgesia can be offered. These patients do not need to be observed in hospital.
1 Isbister GK, Gray MR. A prospective study of 750 definite spider bites, with expert spider identification. Quarterly Journal of Medicine. 2002;95:723-731.
2 Isbister GK. Spider bite: a current approach to management. Australian Prescribing. 2006;29:154-156.
3 Graudins A, Padula M, Broady K, et al. Red-back spider (Latrodectus hasselti) antivenom prevents the toxicity of widow spider venoms. Annals of Emergency Medicine. 2001;37:154-160.
4 Isbister GK, Gray MR. Latrodectism: a prospective cohort study of bites by formally identified redback spiders. Medical Journal of Australia. 2003;179:88-91.
5 Ellis RM, Sprivulis PC, Jelinek GA, et al. A double-blind, randomized trial of intravenous versus intramuscular antivenom for Red-back spider envenoming. Emergency Medicine Australasia. 2005;17:152-156.
6 Isbister GK. Safety of i.v. administration of redback spider antivenom. Internal Medicine Journal. 2007;37:820-822.
7 Isbister GK, Gray MR. Effects of envenoming by comb-footed spiders of the genera Steatoda and Achaearanea (family Theridiidae: Araneae) in Australia. Journal of Toxicology: Clinical Toxicology. 2003;41:809-819.
8 Graudins A, Gunja N, Broady KW, et al. Clinical and in vitro evidence for the efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of envenomation by a Cupboard spider (Steatoda grossa). Toxicon. 2002;40:767-775.
9 Isbister GK, Gray MR, Balit CR, et al. Funnel-web spider bite: a systematic review of recorded clinical cases. Medical Journal of Australia. 2005;182:407-411.
10 Nicholson GM, Graudins A. Spiders of medical importance in the Asia-Pacific: Atracotoxin, latrotoxin and related spider neurotoxins. Clinical and Experimental Pharmacology and Physiology. 2002;29:785-794.
11 Isbister GK. Mouse spider bites (Missulena spp.) and their medical importance. A systematic review. Medical Journal of Australia. 2004;80:225-227.
12 Rash LD, Birinyi-Strachan LC, Nicholson GM, et al. Neurotoxic activity of venom from the Australian Eastern mouse spider (Missulena bradleyi) involves modulation of sodium channel gating. British Journal of Pharmacology. 2000;130:1817-1824.
13 Swanson DL, Vetter RS. Bites of brown recluse spiders and suspected necrotic arachnidism. New England Journal of Medicine. 2005;352:700-707.
14 Isbister GK, Gray MR. White-tail spider bite: a prospective study of 130 definite bites by Lampona species. Medical Journal of Australia. 2003;179:199-202.
15 Isbister GK, Whyte IM. Suspected white-tail spider bite and necrotic ulcers. Internal Medicine Journal. 2004;34:38-44.
30.3 Marine envenoming and poisoning
Jellyfish envenoming
Every summer many thousands of people are stung by jellyfish, resulting in painful stings. Fortunately, only the box jellyfish (Chironex fleckeri) and the species of jellyfish responsible for Irukandji syndrome have been documented to cause deaths. Approximately 70 deaths have been attributed to Chironex in Australian waters, and children are particularly prone to a fatal outcome.1 The last ten Chironex fleckeri deaths in the Northern Territory have been children. Chiropsalmus quadrigatus is closely related to the box jellyfish, and although no deaths have been recorded, serious envenoming may occur and recommended management is as for Chironex.
Two deaths have been attributed to the Irukandji syndrome in North Queensland.
First aid
First aid for all non-tropical Australian jellyfish stings consists of removing the tentacles.2 Vinegar is not recommended due to concerns that it may increase firing of undischarged nematocysts. For non-tropical blue bottle (Physalia sp.) stings hot water has been demonstrated to reduce pain3. It should be applied as hot as can be tolerated, ensuring the patients do not burn themselves. For other non-tropical jellyfish stings, ice is recommended as first aid, although the evidence for this is minimal.2
First aid for jellyfish stings in tropical Australia involves local application of vinegar to inactivate undischarged nematocysts. However, vinegar will not inactivate venom from discharged nematocysts and will not reduce pain.1,4 Vinegar should be applied liberally for at least 30 s. Pressure immobilization bandages are no longer recommended.2
Box jellyfish envenoming
The box jellyfish, also known as the sea wasp (Chironex fleckeri), is found in the tropical, particularly shallow coastal and estuarine, waters of northern Australia, predominantly between November and April. In this environment the jellyfish may be extremely difficult to see and the sudden severe pain of a sting may be the first indication of its presence. Tentacles are often still adherent to the victim’s skin on removal from the water. These tentacles have a typical banded, ladder appearance and leave similar marks on the skin. The venom has numerous effects, including cardiotoxicity and dermatonecrosis. Shock and loss of consciousness from cardiorespiratory depression may occur and victims, especially children, have died within minutes of being stung. However, in a prospective study of jellyfish stings presenting to the Royal Darwin Hospital over a 12-month period, of 23 patients with nematocyst proven Chironex fleckeri sting, only one required parenteral analgesia and none received antivenom.5
Treatment
Box jellyfish antivenom is preferably given diluted 1 in 10 in normal saline by slow intravenous (i.v.) injection, but may also be given by paramedics or surf lifesavers by intramuscular (i.m.) injection (3 ampoules). In cardiac arrest the use of up to 6 ampoules given consecutively undiluted has been advocated.4 Premedication is not recommended because allergic reactions are uncommon.
Irukandji syndrome
The Irukandji jellyfish (Carukia barnesi) consists of a bell measuring only 2 cm across, but with tentacles up to 75 cm in length. It is found in waters north of Geraldton, Western Australia and Mackay, Queensland. This jellyfish is almost invisible in the water. It was first captured in 1961 in Cairns by Dr Jack Barnes, who proved this jellyfish was responsible for the Irukandji syndrome by reproducing the symptoms by stinging himself, his 9-year-old son and the local lifeguard.7 All three were taken to hospital for treatment! Evidence is emerging that more than one jellyfish species may be responsible for this syndrome.
Patients with Irukandji syndrome often have minimal symptoms at the time of the sting. After a latent period of approximately half an hour the ‘Irukandji syndrome’ may develop, with clinical features that include vomiting, abdominal, chest and back pain, sweats, blood pressure lability, and tachycardia.8,9 Occasionally these cases go on to develop pulmonary oedema. Pulmonary oedema usually occurs within 10 h of a sting but can occur within 3 h. All patients developing cardiac dysfunction have ongoing pain. There have now been two deaths from Irukandji syndrome, although these were as a result of cerebral haemorrhage rather than pulmonary oedema.10
Treatment
Vinegar is recommended as first aid.2 These patients are in pain and may require large doses of opioids to relieve their symptoms. In a review of 62 cases of Irukandji syndrome presenting to Cairns hospitals in one year, 38 required parenteral analgesia.8 Fentanyl has been recommended. Patients should be observed in hospital for 6 h after their last dose of opioid and, if asymptomatic, may then be discharged. Those patients who go on to develop pulmonary oedema usually require intensive care admission for supportive care of ventilation and blood pressure. Global cardiac hypokinesis has been documented in this situation and inotropes may be necessary. Intravenous magnesium has been advocated in assisting the management of patients with ongoing symptoms.11 There is no available antivenom.
Sea snake envenoming
Sea snakes are readily distinguished from terrestrial snakes by their flat oar-like tail. It is important to remember that terrestrial snakes may also take to the water, but swim on the surface. Sea snakes, like terrestrial snakes, are air-breathing reptiles and in Australia are found in tropical or temperate waters. They do not survive long out of water but bites have been recorded from handling animals that have been washed ashore.
The bite of a sea snake typically causes minimal pain,1,9 in contrast to fish stings which tend to cause intense pain. Symptoms include progressive muscle pain and tenderness, with pain and stiffness on passive movement. Neuromuscular paralysis and rhabdomyolysis are common, but coagulopathy is rare.9 Fortunately, as for terrestrial snakes, most bitten victims are not envenomed.
Treatment
Note: CSL venom detection kit (VDK) does not detect sea snake venom.
Blue-ringed octopus envenoming
This small octopus, which may weigh only 10–100 g and measure 12–20 cm across the tentacles, is common along the Australian coastline. It is normally brown in colour, but the characteristic bright blue rings become apparent when the animal is agitated. Humans are at risk of envenoming when they disturb the animal. There are two documented deaths from the blue-ringed octopus (Hapalochlaena maculosa) in Australia,1 and several other cases of potentially fatal envenoming that were successfully managed. The active component of the venom, maculotoxin, is similar or identical to tetrodotoxin. It acts as a paralyzing agent by preventing conduction in motor nerves via sodium channel blockade.9 Death occurs from respiratory failure due to paralysis. The bite is typically painless, but a small lesion with bleeding may occasionally be visible. There is a spectrum of envenoming, from no symptoms to localized neurology to rapid onset of paralysis, during which time the patient remains conscious until succumbing to the effects of hypoxia.
Cone-shell envenoming
Of the many species of cone shell, about 18 have been implicated in human envenoming. The sole Australian fatality reported was caused by Conus geographus in 1936.12 The cone shell animal injects venom from a radular tooth harpoon carried on a proboscis that protrudes from the narrow end of the shell. The venom consists of peptitoxins called conotoxins. Pain is usually felt at the site of the bite and, in serious envenoming, evidence of muscular weakness may rapidly develop and occasionally progress to respiratory paralysis.
Stonefish envenoming
Although the stonefish (Synanceia sp.) has caused deaths elsewhere, there are no confirmed Australian fatalities,1 although Sutherland reports the death of an Army doctor on Thursday Island in 1915.9 The stonefish possesses 13 dorsal fin spines, each with paired venom glands. These spines become erect when the fish is trodden on and venom is discharged deep into the wound. The venom has neurotoxic, myotoxic, vascular and myocardial effects. Because of their excellent camouflage stonefish are not often seen, and the first indication of their presence may be the excruciating pain of a sting.
Treatment
Management of other venomous fish stings
The following generalizations may apply to the management of any fish spine wound:
Stingray injury
Stingrays possess a barbed spine with an enveloping integumentary sheath and associated venom glands on the tail. Human injury usually occurs from slashing of the tail when the animal is trodden on. Three deaths have been documented in Australia as a result of stingray injury, the last occurring in 2006. All died from penetrating cardiac wounds.1,13 In one of these cases, cardiac tamponade occurred 5 days post injury, secondary to myonecrosis of myocardium at the site of the wound. Part of the spine is not infrequently left in the wound. Injury occurs from both direct trauma and envenoming. Treatment is symptomatic and as for any fish spine injury, no antivenom is available.
Ciguatera poisoning
Poisoning is characterized by an acute gastrointestinal illness and a subsequent neurological illness classically involving reversal of heat and cold sensation.9 Gastrointestinal and neurological symptoms usually begin within 1 and 24 h of eating contaminated fish. Gastrointestinal symptoms include nausea, vomiting, abdominal pain and diarrhoea, and neurological symptoms include myalgia, paraesthesiae, a burning sensation on contact with cold, mood disorders and disturbance of balance. Alcohol classically exacerbates symptoms, which may recur on eating contaminated fish. Treatment is supportive. A recent randomized controlled trial has demonstrated no benefit in using mannitol.14
Scombroid poisoning
Scombroid poisoning is an allergic-type reaction to a toxin that develops in the flesh of fish after they have been landed. It typically occurs from eating the flesh of mackerel, bonito and tuna. If the fish is not immediately refrigerated upon being caught, micro-organisms may break down histidine in the flesh to form histamine-like substances. Following the ingestion of fish containing this toxin, the features of histamine ingestion appear, including an urticarial rash with associated weakness and lethargy. Associated features may include bronchospasm, diarrhoea and vomiting. Less severe reactions may be treated with antihistamines but more severe reactions should be treated as for anaphylaxis.15
Paralytic shellfish poisoning
Paralytic shellfish poisoning is similar to ciguatera in that it occurs as a result of the concentration of a toxin produced by a dynoflagellate microorganism. The toxin, known as saxitoxin, becomes concentrated in the flesh of bivalve molluscs and has similar effects to tetrodotoxin. Treatment is supportive. Death may result from respiratory failure in untreated cases.9
Tetrodotoxin poisoning
Tetrodotoxin (TTX) is a paralytic toxin that occurs in the flesh, skin and viscera of puffer fish. Tetrodotoxin acts by selectively blocking voltage-sensitive sodium channels and thus preventing conduction in motor and sensory nerves. In Australia, TTX poisoning is rare and usually occurs in those who do not know the puffer fish to be poisonous. In Japan, where such fish are a delicacy called ‘fugu’, numerous cases and deaths occur every year. Toxicity usually manifests soon after eating the fish, with typical features including perioral paraesthesiae followed by numbness of the mouth, tongue and face, and then widespread paralysis. Severe cases can progress to death from respiratory failure. Management is supportive as there is no antidote.1,9 All cases should be admitted for observation until peak clinical effects have passed. It is extremely unlikely that life-threatening effects will occur after 24 h in patients who have not already developed severe effects.16
1 Williamson JA, Fenner PJ, Burnett JW, Rifkin JF, editors. Venomous and Poisonous Marine Animals a Medical and Biological Handbook. Sydney: University of New South Wales Press, 1996.
2 Australian Resuscitation Council guideline 8.9.6: Envenomation Jellyfish Stings. 2008. http://www.resus.org. au (Accessed 31st January
3 Loten C, Stokes B, Worsley D, et al. A randomised trial of hot water (45°C) immersion versus ice packs for pain relief in bluebottle stings. Medical Journal of Australia. 2006;184:329-330.
4 White J. CSL Antivenom Handbook. Melbourne: CSL Ltd, 1995.
5 O’Rielly G, Isbister GK, Lawrie PM, et al. Prospective study of jellyfish stings from tropical Australia, including the major box jellyfish Chironex fleckeri. Medical Journal of Australia. 2001;175:652-655.
6 Ramasamy S, Isbister GK, Seymour JE, et al. The in vivo cardiovascular effects of box jellyfish Chironex fleckeri venom in rats: efficacy of pre treatment with antivenom, verapamil and magnesium sulphate. Toxicon. 2004;43:685-690.
7 Barnes J. Cause and effect in Irukandji stingings. Medical Journal of Australia. 1964;177:654-655.
8 Little M, Mulcahy R. A year’s experience of Irukandji envenoming in far north Queensland. Medical Journal of Australia. 1998;169:638-641.
9 Sutherland SK, Tibballs J, editors. Australian Animal Toxins, 2nd edn, Melbourne: Oxford University Press, 2001.
10 Fenner PJ, Hadok JC. Fatal envenomation by jellyfish causing Irukandji syndrome. Medical Journal of Australia. 2002;177:362-363.
11 Corkeron M, Pereira P, MacKrocanis C. Early experience with magnesium administration in Irukandji Syndrome. Anaesthetic Intensive Care. 2004;32:666-669.
12 Flecker H. Cone shell mollusc poisoning with report of a fatal case. Medical Journal of Australia. 1936;1:464-466.
13 Fenner PJ, Williamson JA, Skinner RA. Fatal and non-fatal stingray envenomation. Medical Journal of Australia. 1989;151:621-625.
14 Schnorf H, Tauarii M, Cundy T. Ciguatera fish poisoning: a double blinded randomised trial of mannitol therapy. Neurology. 2002;58:873-880.
15 Smart DR. Scombroid poisoning: a report of seven cases involving the Western Australian salmon Arripis truttaceus. Medical Journal of Australia. 1992;157:748-751.
16 Isbister GK, Son J, Wang F, et al. Puffer fish poisoning: a potentially life-threatening condition. Medical Journal of Australia. 2002;177:650-653.
17 Carrette TJ, Cullen P, Little M, et al. Temperature effects on box jellyfish venom: a possible treatment for envenomed patients? Medical Journal of Australia. 2002;177:654-655.